Simultaneous Heterotrophic Nitrification and Aerobic Denitrification of Water after Sludge Dewatering in Two Sequential Moving Bed Biofilm Reactors (MBBR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Setup
2.2. Reject Water Characteristics and Chemical Analysis
2.3. Reject Water Element Analysis
2.4. Biomass Growth on Carriers
2.5. Microbial Analysis
2.5.1. Microbiome DNA Extraction
2.5.2. Microbiome Gene Amplification by Polymerase Chain Reaction (PCR)
2.6. Data Analysis
3. Results
3.1. Ammonium Transformation in MBBR Reactors
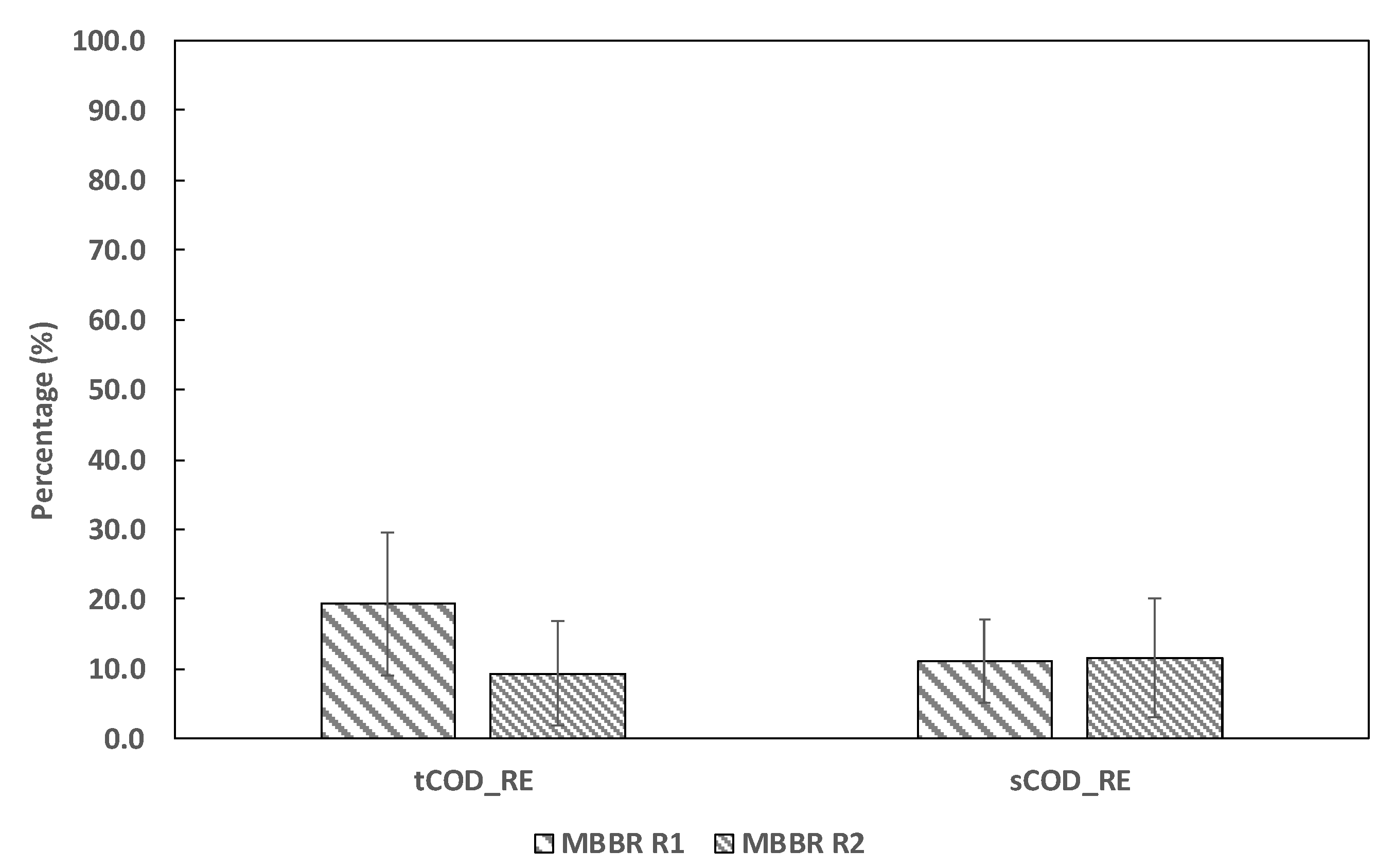
3.2. Chemical Oxygen Demand (COD)
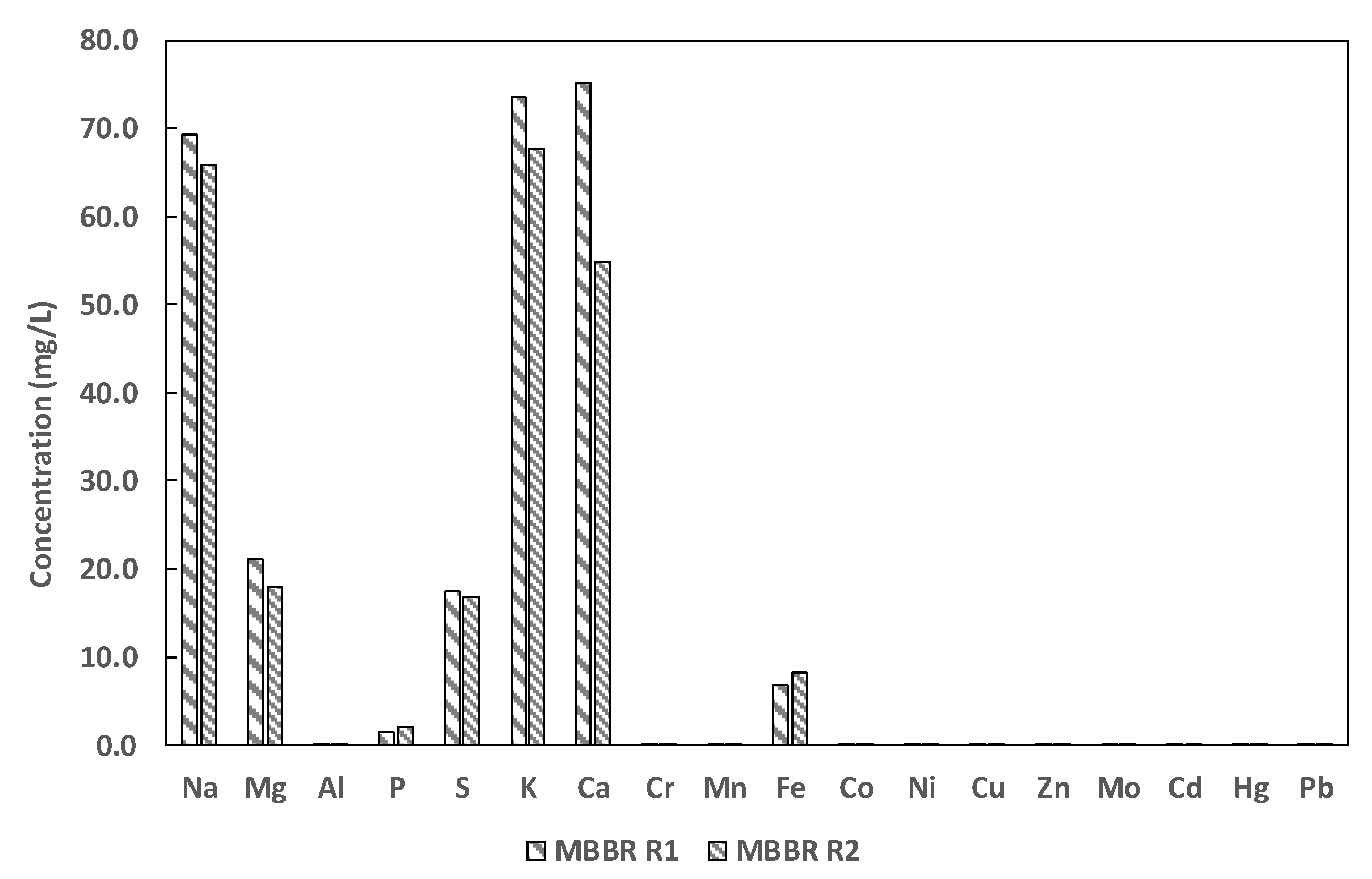
3.3. The Reject Water Element Composition
3.4. Biomass on the Carriers
3.5. Microbial Community in the Reactors
4. Discussion
4.1. Ammonium Transformation to Nitrite and Nitrate
4.2. Aerobic Bacterial Denitrification
4.3. Biomass Growth and Metallic Metallic Elements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janus, H.M.; van der Roest, H.F. Don’t reject the idea of treating reject water. Water Sci. Technol. 1997, 35, 27–34. [Google Scholar] [CrossRef]
- Sivalingam, V.; Dinamarca, C.; Janka, E.; Kukankov, S.; Wang, S.; Bakke, R. Effect of Intermittent Aeration in a Hybrid Vertical Anaerobic Biofilm Reactor (HyVAB) for Reject Water Treatment. Water 2020, 12, 1151. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ni, J. Heterotrophic nitrification–aerobic denitrification by novel isolated bacteria. J. Ind. Microbiol. Biotechnol. 2011, 38, 1305–1310. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- Kundu, P.; Pramanik, A.; Dasgupta, A.; Mukherjee, S.; Mukherjee, J. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by Chryseobacterium sp. R31 Isolated from Abattoir Wastewater. BioMed Res. Int. 2014, 2014, 436056. [Google Scholar] [CrossRef] [Green Version]
- Kalniņš, M.; Bērziņš, A.; Gudrā, D.; Megnis, K.; Fridmanis, D.; Danilko, P.; Muter, O. Selective enrichment of heterotrophic nitrifiers Alcaligenaceae and Alcanivorax spp. from industrial wastewaters. AIMS Microbiol. 2020, 6, 32–42. [Google Scholar] [CrossRef]
- Grunditz, C.; Dalhammar, G. Development of nitrification inhibition assays using pure cultures of Nitrosomonas and Nitrobacter. Water Res. 2001, 35, 433–440. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, D.; Lee, D.S.; Park, J.M. Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J. Hazard. Mater. 2008, 152, 915–921. [Google Scholar] [CrossRef]
- Piculell, M.; Suarez, C.; Li, C.; Christensson, M.; Persson, F.; Wagner, M.; Hermansson, M.; Jönsson, K.; Welander, T. The inhibitory effects of reject water on nitrifying populations grown at different biofilm thickness. Water Res. 2016, 104, 292–302. [Google Scholar] [CrossRef]
- Velusamy, K.; Krishnani, K.K. Heterotrophic nitrifying and oxygen tolerant denitrifying bacteria from greenwater system of coastal aquaculture. Appl. Biochem. Biotechnol. 2013, 169, 1978–1992. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, Q.; Zhao, C.; Wen, D.; Tang, X. Aerobic degradation of pyridine by a new bacterial strain, Shinella zoogloeoides BC026. J. Ind. Microbiol. Biotechnol. 2009, 36, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.B.; Gupta, S.K. Simultaneous carbon and nitrogen removal from high strength domestic wastewater in an aerobic RBC biofilm. Water Res. 2001, 35, 1714–1722. [Google Scholar] [CrossRef]
- Joo, H.S.; Hirai, M.; Shoda, M. Improvement in ammonium removal efficiency in wastewater treatment by mixed culture of Alcaligenes faecalis no. 4 and L1. J Biosci. Bioeng. 2007, 103, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef]
- Sakai, K.; Nisijima, H.; Ikenaga, Y.; Wakayama, M.; Moriguchi, M. Purification and Characterization of Nitrite-oxidizing Enzyme from Heterotrophic Bacillus badius I-73, with Special Concern to Catalase. Biosci. Biotechnol. Biochem. 2000, 64, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ni, J. Ammonium removal by Agrobacterium sp. LAD9 capable of heterotrophic nitrification-aerobic denitrification. J. Biosci. Bioeng. 2012, 113, 619–623. [Google Scholar] [CrossRef]
- Ren, Y.-X.; Yang, L.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Zhao, B.; He, Y.L.; Hughes, J.; Zhang, X.F. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour. Technol. 2010, 101, 5194–5200. [Google Scholar] [CrossRef]
- Gupta, A.B. Thiosphaera pantotropha: A sulphur bacterium capable of simultaneous heterotrophic nitrification and aerobic denitrification. Enzym. Microb. Technol. 1997, 21, 589–595. [Google Scholar] [CrossRef]
- Liu, T.; Mao, Y.-j.; Shi, Y.-p.; Quan, X. Start-up and bacterial community compositions of partial nitrification in moving bed biofilm reactor. Appl. Microbiol. Biotechnol. 2017, 101, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater/Prepared and Publlished Jointly by American Public Health Association, American Water Works Association and Water Environment Federation, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Yang, Q.; Liu, X.; Wu, W.; Liu, Z.; Gu, P. Achieving partial denitrification-anammox in biofilter for advanced wastewater treatment. Environ. Int. 2020, 138, 105612. [Google Scholar] [CrossRef]
- Duan, S.; Zhang, Y.; Zheng, S. Heterotrophic nitrifying bacteria in wastewater biological nitrogen removal systems: A review. Crit. Rev. Environ. Sci. Technol. 2021, 1–37. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. Adv. Appl. Microbiol. 2019, 106, 113–192. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-H. Sustainable nitrogen elimination biotechnologies: A review. Process Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- Wang, X.J.; Xia, S.Q.; Chen, L.; Zhao, J.F.; Renault, N.J.; Chovelon, J.M. Nutrients removal from municipal wastewater by chemical precipitation in a moving bed biofilm reactor. Process Biochem. 2006, 41, 824–828. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Pi, S.; Cui, D.; Ma, F.; Zhao, H.-p.; Li, A. A critical review of aerobic denitrification: Insights into the intracellular electron transfer. Sci. Total Environ. 2020, 731, 139080. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Tomasek, A.; Staley, C.; Wang, P.; Kaiser, T.; Lurndahl, N.; Kozarek, J.L.; Hondzo, M.; Sadowsky, M.J. Increased Denitrification Rates Associated with Shifts in Prokaryotic Community Composition Caused by Varying Hydrologic Connectivity. Front. Microbiol. 2017, 8, 2304. [Google Scholar] [CrossRef]
- Lai, E.; Hess, M.; Mitloehner, F.M. Profiling of the Microbiome Associated With Nitrogen Removal During Vermifiltration of Wastewater From a Commercial Dairy. Front. Microbiol. 2018, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, X.; Zhang, X.; Sun, D. Influence of surface modification of polyethylene biocarriers on biofilm properties and wastewater treatment efficiency in moving-bed biofilm reactors. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2012, 65, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, S.; Park, S.; Hayashi, H.; Jung, J.; Hirata, A. Enhancement of nitrifying biofilm formation using selected EPS produced by heterotrophic bacteria. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2001, 43, 197–204. [Google Scholar] [CrossRef]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced Biofilm Formation by Ferrous and Ferric Iron Through Oxidative Stress in Campylobacter jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivalingam, V.; Ibrahim, O.; Kukankov, S.; Omodara, B.; Janka, E.; Wang, S.; Dinamarca, C.; HH, H.; Bakke, R. Chemical equilibrium model to investigate scaling in moving bed biofilm reactors (MBBR). In Proceedings of the SIMS 2019, Västeräs, Sweden, 13–16 August 2019. [Google Scholar]




| Units | MBBR R1 | MBBR R2 | |
|---|---|---|---|
| Reactor volume | L | 67.4 | 67.4 |
| Temperature | °C | 30 (±2) | 30 (±2) |
| Water depth | m | 0.61 | 0.57 |
| Type of media | -- | BWT S® | BWT S® |
| Surface area of carriers | m2/m3 | 650 | 650 |
| Total projection surface area | m2 | 26.3 | 26.3 |
| Filling rate | % | 60 | 60 |
| HRT | day | 1.2 | 1.2 |
| COD loading | kg/m3·d | 2.4 (±0.4) | 1.9 (±0.2) |
| NH4-N loading | kg/m3·d | 0.48 (±0.1) | 0.25 (±0.1) |
| Dissolved oxygen (DO) | mg/L | 3.8 (±2.4) | 3.8 (±2.4) |
| Reactors | Specific Nitritation Rate (SNR) (mg NO2/m2·d) | Specific Denitrification Rate (SDR) (mg N2/m2·d) |
|---|---|---|
| MBBR R1 | 468.7 (±137.8) | 55.0 (±101.8) |
| MBBR R2 | 157.3 (±105.1) | 73.1 (±81.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janka, E.; Pathak, S.; Rasti, A.; Gyawali, S.; Wang, S. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification of Water after Sludge Dewatering in Two Sequential Moving Bed Biofilm Reactors (MBBR). Int. J. Environ. Res. Public Health 2022, 19, 1841. https://doi.org/10.3390/ijerph19031841
Janka E, Pathak S, Rasti A, Gyawali S, Wang S. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification of Water after Sludge Dewatering in Two Sequential Moving Bed Biofilm Reactors (MBBR). International Journal of Environmental Research and Public Health. 2022; 19(3):1841. https://doi.org/10.3390/ijerph19031841
Chicago/Turabian StyleJanka, Eshetu, Sabin Pathak, Alireza Rasti, Sandeep Gyawali, and Shuai Wang. 2022. "Simultaneous Heterotrophic Nitrification and Aerobic Denitrification of Water after Sludge Dewatering in Two Sequential Moving Bed Biofilm Reactors (MBBR)" International Journal of Environmental Research and Public Health 19, no. 3: 1841. https://doi.org/10.3390/ijerph19031841
APA StyleJanka, E., Pathak, S., Rasti, A., Gyawali, S., & Wang, S. (2022). Simultaneous Heterotrophic Nitrification and Aerobic Denitrification of Water after Sludge Dewatering in Two Sequential Moving Bed Biofilm Reactors (MBBR). International Journal of Environmental Research and Public Health, 19(3), 1841. https://doi.org/10.3390/ijerph19031841







