The Contribution of Frailty to Participation of Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Tools
2.3. Statistical Analysis
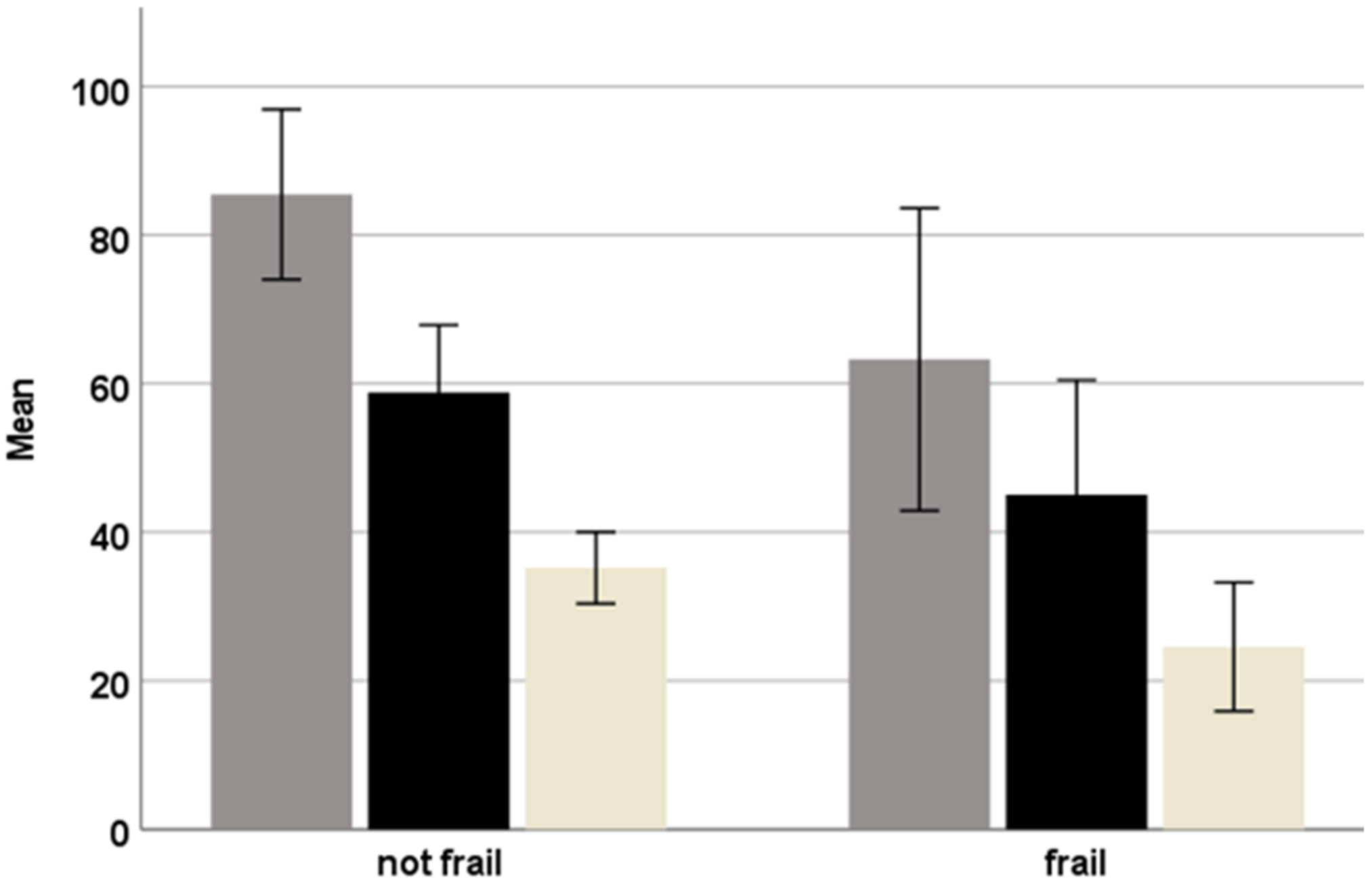
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, L.L.; De Leon, C.F.M.; Wilson, R.S.; Bienias, J.L.; Evans, D.A. Social resources and cognitive decline in a population of older African Americans and Whites. Neurol 2004, 63, 2322–2326. [Google Scholar] [CrossRef] [PubMed]
- Keysor, J.J. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am. J. Prev. Med. 2003, 25, 129–136. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cauley, J.A.; Stone, K.; Thompson, T.J.; Bauer, D.C.; Cummings, S.R.; Ensrud, K.E.; Study of Osteoporotic Fractures Research Group. Study of Osteoporotic Fractures Research Group. Relationship of changes in physical activity and mortality among older women. JAMA 2003, 289, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Anaby, D.; Miller, W.C.; Eng, J.J.; Noreau, L. Participation and well-being among older adults living with chronic conditions. Soc. Indic. Res. 2011, 100, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stav, W.B.; Hallenen, T.; Lane, J.; Arbesman, M. Systematic review of occupational engagement and health outcomes among community-dwelling older adults. Am. J. Occup. Ther. 2012, 66, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). International Classification of Functioning, Disability and Health: ICF; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Reed, K.; Hocking, C.; Smythe, L. The interconnected meanings of occupation: The call, being-with, possibilities. J. Occup. Sci. 2010, 17, 140–149. [Google Scholar] [CrossRef]
- Desrosiers, J.; Noreau, L.; Rochette, A.; Bourbonnais, D.; Bravo, G.; Bourget, A. Predictors of long-term participation after stroke. Disabil. Rehabil. 2006, 28, 221–230. [Google Scholar] [CrossRef]
- Edward, D.; Christiansen, C. Occupational development. In Occupational Therapy: Performance, Participation and Well-Being; Christiansen, C., Baum, C.M., Eds.; Slack: San Francisco, CA, USA, 2005; pp. 42–63. [Google Scholar]
- Liu, J.Y.W. The severity and associated factors of participation restriction among community-dwelling frail older people: An application of the International Classification of Functioning, Disability and Health (WHO-ICF). BMC Geriatr. 2017, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Anaby, D.; Miller, W.C.; Eng, J.J.; Jarus, T.; Noreau, L.; PACC Research Group. Can personal and environmental factors explain participation of older adults? Disabil. Rehabil. 2009, 31, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Weiss, D.; Sourial, N.; Karunananthan, S.; Quail, J.M.; Wolfson, C.; Bergman, H. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: A cross-sectional study. Aging Clin. Exp. Res. 2010, 22, 54–62. [Google Scholar] [CrossRef]
- Desrosiers, J.; Robichaud, L.; Demers, L.; Gélinas, I.; Noreau, L.; Durand, D. Comparison and correlates of participation in older adults without disabilities. Arch. Gerontol. Geriatr. 2009, 49, 397–403. [Google Scholar] [CrossRef]
- De Albuquerque Sousa, A.C.P.; Dias, R.C.; Maciel, Á.C.C.; Guerra, R.O. Frailty syndrome and associated factors in community-dwelling elderly in Northeast Brazil. Arch. Gerontol. Geriatr. 2012, 54, e95–e101. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, J.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwee, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study Group. Frailty and the prediction of negative health outcomes: A meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. White paper: “walking speed: The sixth vital sign”. J. Geriatr. Phys. Ther. 2009, 32, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Bortone, I.; Sardone, R.; Lampignano, L.; Castellana, F.; Zupo, R.; Lozupone, M.; Moretti, B.; Giannelli, G.; Panza, F. How gait influences frailty models and health-related outcomes in clinical-based and population-based studies: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 274–297. [Google Scholar] [CrossRef]
- Warren, M.; Ganley, K.J.; Pohl, P.S. The association between social participation and lower extremity muscle strength, balance, and gait speed in US adults. Prev. Med. Rep. 2016, 4, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, J.; Neyens, J.C.; Van Rossum, E.; Spreeuwenberg, M.D.; De Witte, L.P. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: A systematic review. BMC Geriatr. 2011, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Al Snih, S.; Graham, J.E.; Ray, L.A.; Samper-Ternent, R.; Markides, K.S.; Ottenbacher, K.J. Frailty and incidence of activities of daily living disability among older Mexican Americans. J. Rehabil. Med. 2009, 41, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairhall, N.; Sherrington, C.; Kurrle, S.E.; Lord, S.R.; Cameron, I.D. ICF participation restriction is common in frail, community-dwelling older people: An observational cross-sectional study. Physiotherapy 2011, 97, 26–32. [Google Scholar] [CrossRef]
- Maruta, M.; Makizako, H.; Ikeda, Y.; Han, G.; Shimokihara, S.; Miyata, H.; Nakamura, A.; Tokuda, K.; Kubozono, T.; Ohishi, M.; et al. Characteristics of meaningful activities in community-dwelling Japanese older adults with pre-frailty and frailty. Arch. Gerontol. Geriatr. 2021, 28, 104616. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Kawai, H.; Fujiwara, Y.; Watanabe, Y.; Hirano, H.; Kim, H.; Ihara, K.; Ejiri, M.; Ishii, K.; Oka, K.; et al. Association between activity diversity and frailty among community-dwelling older Japanese: A cross-sectional study. Arch. Gerontol. Geriatr. 2021, 95, 104377. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Nofuji, Y.; Seino, S.; Murayama, H.; Yoshida, Y.; Tanigaki, T.; Yokoyama, Y.; Narita, M.; Nishi, M.; Kitamura, A.; et al. Healthy lifestyle behaviors and transitions in frailty status among independent community-dwelling older adults: The Yabu cohort study. Maturitas 2020, 136, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Wood-Dauphinee, S.L.; Opzoomer, M.A.; Williams, J.I.; Marchand, B.; Spitzer, W.O. Assessment of global function: The Reintegration to Normal Living Index. Arch. Phys. Med. Rehabil. 1988, 69, 583–590. [Google Scholar] [PubMed]
- Miller, A.; Clemson, L.; Lannin, N. Measurement properties of a modified Reintegration to Normal Living Index in a community-dwelling adult rehabilitation population. Disabil. Rehabil. 2011, 33, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.; Lau, R.W.; Yeung, P.K.; Liao, L.R.; Chung, R.C. Development and validation of the Chinese version of the Reintegration to Normal Living Index for use with stroke patients. J. Rehabil. Med. 2011, 43, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Bourget, N.; Deblock-Bellamy, A.; Blanchette, A.K.; Batcho, C.S. Use and psychometric properties of the reintegration to normal living index in rehabilitation: A systematic review. Ann. Phys. Rehabil. Med. 2018, 61, 262–269. [Google Scholar] [CrossRef]
- Liu, J.Y.W.; Ma, K.W. The psychometric properties of the Chinese version—Reintegration to normal living index (C-RNLI) for identifying participation restriction among community-dwelling frail older people. BMC Geriatr. 2017, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Raîche, M.; Hébert, R.; Dubois, M.F. PRISMA-7: A case-finding tool to identify older adults with moderate to severe disabilities. Arch. Geronotol. Geriatr. 2008, 47, 9–18. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Costello, M.; Small, C.; Spooner, L.; Flannery, A.; O’Reilly, L.; Heffernan, L.; Mannion, E.; Maughan, A.; Joyce, A.; et al. Comparison of frailty screening instruments in the emergency department. Int. J. Environ. Res. Public Health 2019, 16, 3626. [Google Scholar] [CrossRef] [Green Version]
- Clegg, A.; Rogers, L.; Young, J. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: A systematic review. Age Ageing 2014, 44, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Saenger, A.L.F.; Caldas, C.P.; Raîche, M.; Da Motta, L.B. Identifying the loss of functional independence of older people residing in the community: Validation of the PRISMA-7 instrument in Brazil. Arch. Geronotol. Geriatr. 2018, 74, 62–67. [Google Scholar] [CrossRef]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar]
- Masedo, A.I.; Hanley, M.; Jensen, M.P.; Ehde, D.; Cardenas, D.D. Reliability and validity of a self-report FIM™(FIM-SR) in persons with amputation or spinal cord injury and chronic pain. Am. J. Phys. Med. Rehabil. 1005, 84, 167–176. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Tang, F.; Chi, I.; Zhang, W.; Dong, X. Activity engagement and cognitive function: Findings from a community-dwelling US Chinese aging population study. Gerontol. Geriatr. Med. 2018, 4, 2333721418778180. [Google Scholar] [CrossRef] [Green Version]
- Desrosiers, J. Participation and occupation. Can. J. Occup. Ther. 2005, 72, 195–203. [Google Scholar] [CrossRef]
- Hwang, T.J.; Rabheru, K.; Peisah, C.; Reichman, W.; Ikeda, M. Loneliness and social isolation during the COVID-19 pandemic. International psychogeriatrics. Int. Psychogeriatr. 2020, 32, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Law, M. Participation in the occupations of everyday life. Am. J. Occup. Ther. 2006, 56, 640–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Gruenewald, T.L.; Seeman, T.E.; Sarkisian, C.A. Productive activities and development of frailty in older adults. J. Gernotol. B Psychol. Sci. Soc. Sci. 2010, 65B, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.C.; Chang, W.C. Trajectories of frailty and related factors of the older people in Taiwan. Exp. Aging Res. 2015, 41, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Y.; Li, C.; Larbi, A.; Feng, L.; Shen, Q.; Chong, M.S.; Lim, W.S.; Feng, L. Associations of lifestyle activities and a heathy diet with frailty in old age: A community-based study in Singapore. Aging 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, I.; Desrosiers, J.; Gosselin, S. Predictors of reintegration to normal living in older adults discharged from an intensive rehabilitation program. Int. J. Rehabil. Res. 2008, 31, 267–274. [Google Scholar] [CrossRef] [PubMed]
- McGilton, K.S.; Omar, A.; Stewart, S.S.; Chu, C.H.; Blodgett, M.B.; Bethell, J.; Davis, A.M. Factors that influence the reintegration to normal living for older adults 2 years post hip fracture. J. Appl. Gernotol. 2020, 39, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Søvde, B.E.; Sandvoll, A.M.; Natvik, E.; Drageset, J. In the borderland of the body: How home-dwelling older people experience frailty. Scand. J. Caring Sci. 2021. [Google Scholar] [CrossRef]
| N (%) | ||
|---|---|---|
| Sex | Male Female | 61 (50.4) 60 (49.6) |
| Living Arrangement | Alone With partner/children | 32 (26.4) 89 (73.5) |
| Education | 0–8 years 9–12 years 13+ years | 29 (24.0) 61 (50.4) 31 (25.6) |
| Work/Volunteer | Yes No | 24 (19.8) 97 (80.1) |
| Tools | Mean (SD) | Min–Max | |
|---|---|---|---|
| Participation | RNL-I total (10–100) | 78.2 (18.0) | 14.5–100 |
| RNL-I—participation in physical activities (4–40) | 31.7 (7.9) | 7–40 | |
| RNL-I—participation in social events (7–70) | 54.3 (13.1) | 8–70 | |
| Frailty | PRISMA-7 (0–7) | 2.9 (1.4) | 0–6 |
| Cognition | MoCA (0–30) | 21.2 (3.2) | 16–28 |
| BADL | FIM (18–126) | 116.4 (9.2) | 79–126 |
| IADL | IADLq (0–23) | 19.4 (3.7) | 8–23 |
| Participation | |||
|---|---|---|---|
| Total RNL-I | RNL-I Physical Activities | RNL-I Social Events | |
| Age | −0.115 | −0.084 | −0.210 * |
| Sex | 0.05 | −0.021 | 0.112 |
| Cognition | 0.276 ** | 0.158 | 0.232 * |
| Frailty | −0.634 ** | −0.657 ** | −0.587 ** |
| BADL | 0.600 ** | 0.694 ** | 0.575 ** |
| IADL | 0.633 ** | 0.693 ** | 0.582 ** |
| Adjusted R2 | Unstandardized B (SE) | Standardized Beta | Sig. F Change | |
|---|---|---|---|---|
| Age | 0.015 | 0.024 (0.416) | 0.004 | 0.953 |
| Cognition | 0.08 | 0.379 (0.422) | 0.068 | 0.371 |
| Frailty | 0.315 | −5.21 (1.32) | −0.402 | 0.000 |
| BADL | 0.056 | 0.593 (0.178) | 0.319 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rand, D.; Sternberg, S.A.; Gasner Winograd, R.; Buckman, Z.; Bentur, N. The Contribution of Frailty to Participation of Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 1616. https://doi.org/10.3390/ijerph19031616
Rand D, Sternberg SA, Gasner Winograd R, Buckman Z, Bentur N. The Contribution of Frailty to Participation of Older Adults. International Journal of Environmental Research and Public Health. 2022; 19(3):1616. https://doi.org/10.3390/ijerph19031616
Chicago/Turabian StyleRand, Debbie, Shelley A. Sternberg, Reut Gasner Winograd, Zvi Buckman, and Netta Bentur. 2022. "The Contribution of Frailty to Participation of Older Adults" International Journal of Environmental Research and Public Health 19, no. 3: 1616. https://doi.org/10.3390/ijerph19031616
APA StyleRand, D., Sternberg, S. A., Gasner Winograd, R., Buckman, Z., & Bentur, N. (2022). The Contribution of Frailty to Participation of Older Adults. International Journal of Environmental Research and Public Health, 19(3), 1616. https://doi.org/10.3390/ijerph19031616






