Complementary and Alternative Medicine Use in Hospitalized Cancer Patients—Study from Silesia, Poland
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
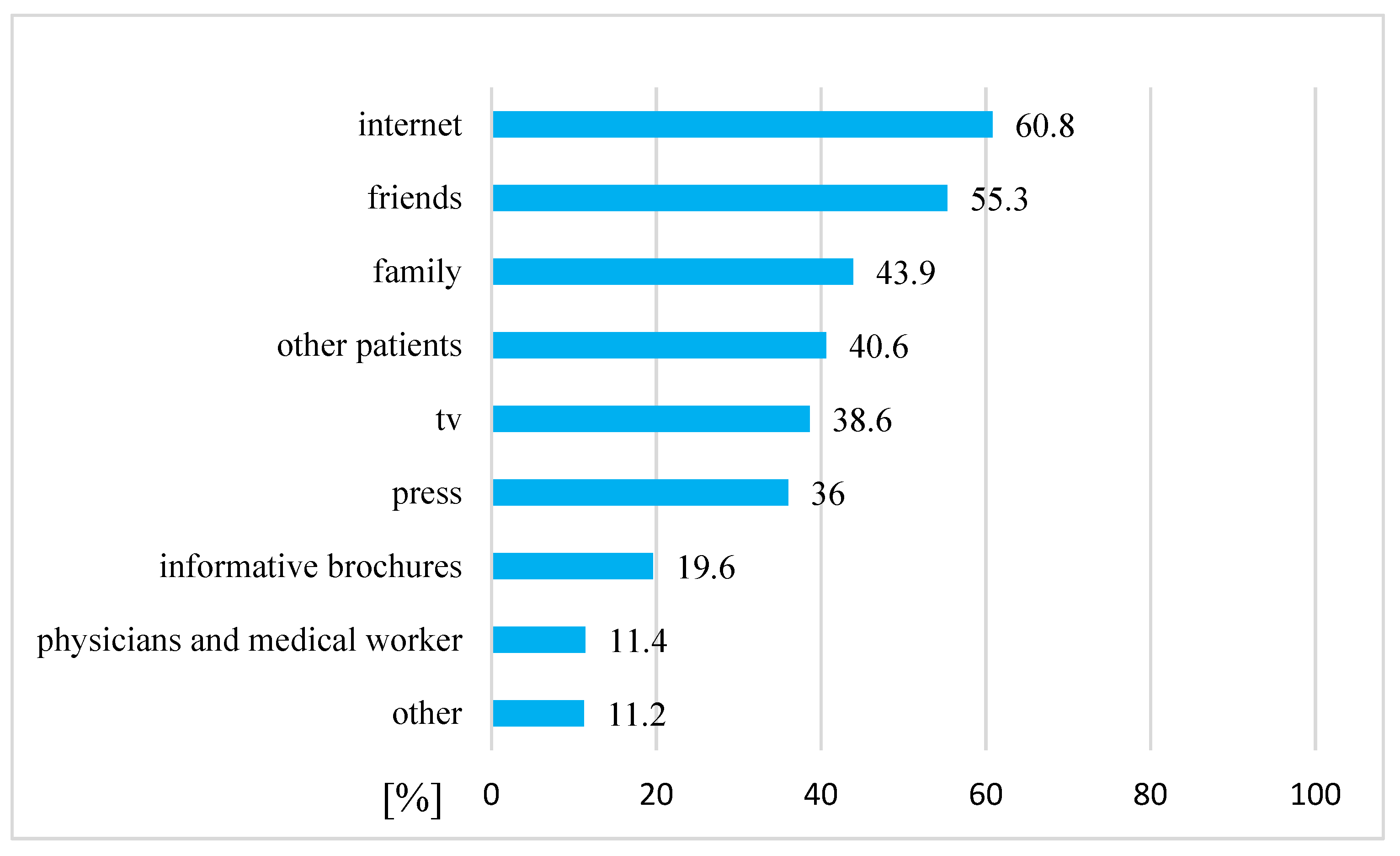
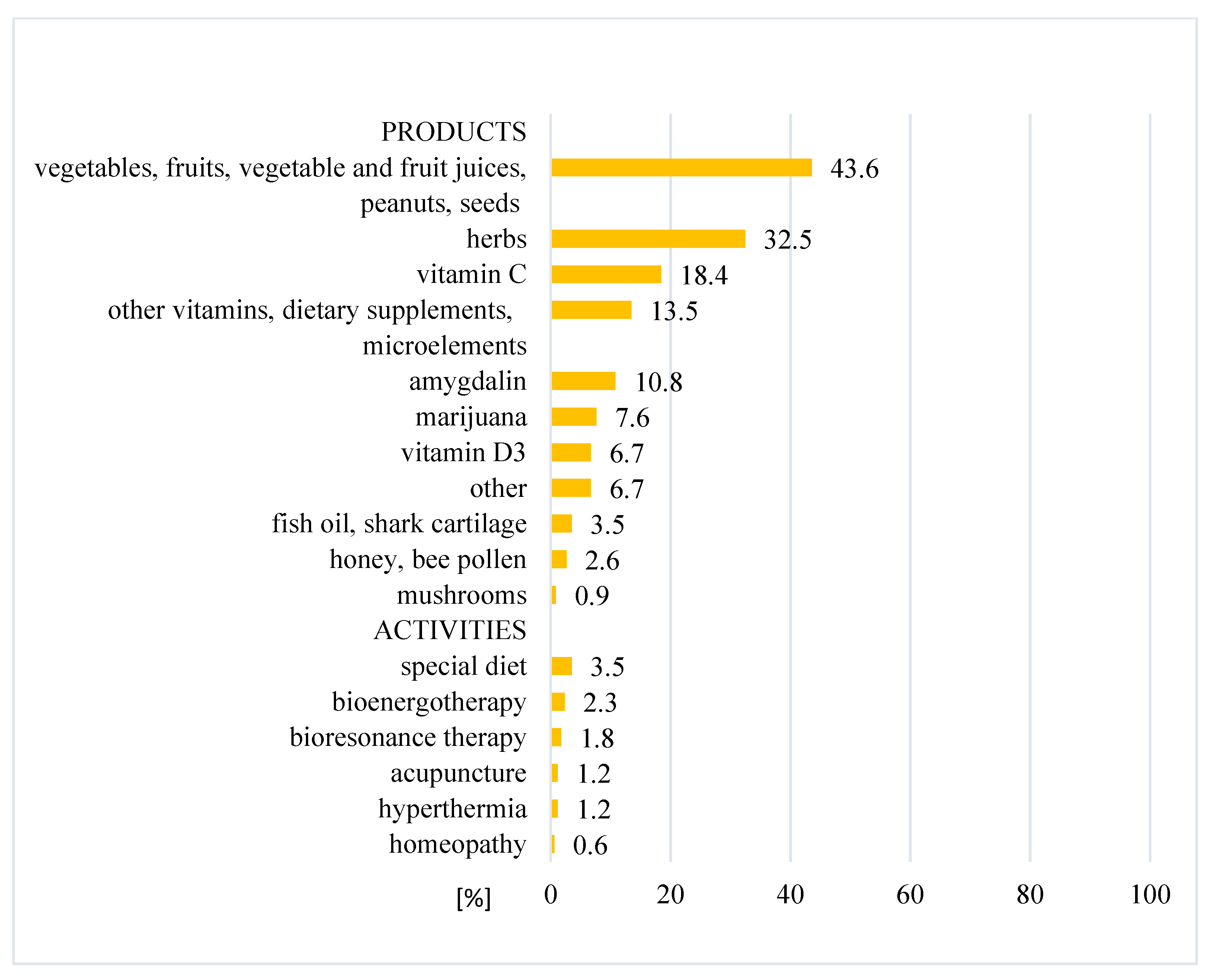
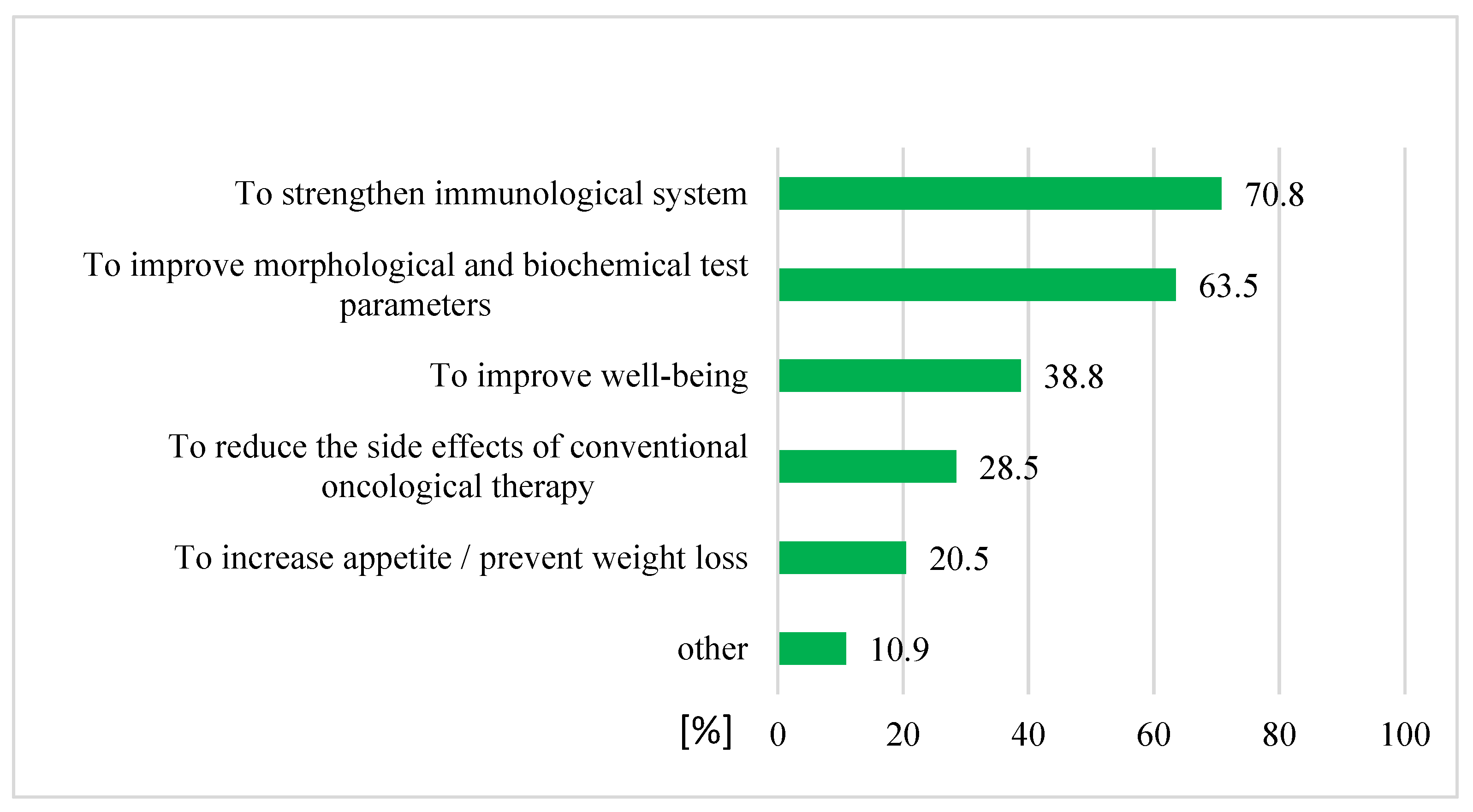
3. Results
4. Discussion
4.1. Prevalence and Reasons for CAM Use
4.2. Predictors of CAM Usage
4.3. Types of CAM
4.4. Sources of Information about CAM
4.5. Experiences and Recommendation of CAM Use
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Center for Complementary and Integrative Health. Available online: https://www.nccih.nih.gov/ (accessed on 1 January 2018).
- Davis, E.L.; Oh, B.; Butow, P.N.; Mullan, B.A.; Clarke, S. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: A systematic review. Oncologist 2012, 17, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- Horneber, M.; Bueschel, G.; Dennert, G.; Less, D.; Ritter, E.; Zwahlen, M. How many cancer patients use complementary and alternative medicine: A systematic review and metaanalysis. Integr. Cancer Ther. 2012, 11, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Angell, M.; Kassirer, J.P. Alternative medicine-the risks of untested and unregulated remedies. N. Engl. J. Med. 1998, 339, 839–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.Y.; Glissmeyer, M.; Tonnes, S.; Hudson, T.; Johnson, N. Outcomes of breast cancer in patients who use alternative therapies as primary treatment. Am. J. Surg. 2006, 192, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Coppes, M.J.; Anderson, R.A.; Egeler, R.M.; Wolff, J.E.A. Alternative therapies for the treatment of childhood cancer. N. Engl. J. Med. 1998, 339, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Intangible risks of complementary and alternative medicine. J. Clin. Oncol. 2001, 19, 2365–2366. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Johnson, N.; DelaMelena, T.; Glissmeyer, M.; Steinbocket, K. Alternative therapy used as primary treatment for breast cancer negatively impacts outcomes. Ann. Surg. Oncol. 2011, 18, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.; Vrouwe, S.; Kamruzzaman, A.; Balbaid, A.; Fenton, D.; Berendt, R.; Yu, A.; Tai, P. Outcome analysis of breast cancer patients who declined evidence-based treatment. World J. Surg. Oncol. 2012, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Saquib, J.; Parker, B.A.; Natarajan, L.; Madlensky, L.; Saquib, N.; Patterson, R.E.; Newman, V.A.; Pierce, J.P. Prognosis following the use of complementary and alternative medicine in women diagnosed with breast cancer. Complement. Ther. Med. 2012, 20, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aż 75 Proc. Pacjentów Onkologicznych Stosuje Jakieś Terapie Niekonwencjonalne. Available online: https://wyborcza.pl/TylkoZdrowie/7,137474,21436975,az-75-proc-pacjentow-onkologicznych-stosuje-jakies-terapie.html (accessed on 3 March 2017).
- Czy da Się Alternatywnie Wyleczyć Raka? Available online: https://www.polityka.pl/tygodnikpolityka/nauka/1706436,1,czy-da-sie-alternatywnie-wyleczyc-raka.read (accessed on 30 May 2017).
- Burgess, T.F. A General Introduction to the Design of Questionnaires for Survey Research. In Guide to the Design of Questionnaires; University of Leeds: Leeds, UK, 2001. [Google Scholar]
- Boparai, J.K.; Singh, S.; Kathuria, P. How to Design and Validate a Questionnaire: A Guide. Curr. Clin. Pharmacol. 2018, 13, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fernádez-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, I.; Amano, N.; Eguchi, K.; Narabayashi, M.; Imanishi, J.; Hirai, M.; Nakano, T.; Takashima, S. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J. Clin. Oncol. 2005, 23, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr. Oncol. 2018, 25, e275–e281. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.A.; Sanders, T.; Palmer, J.L.; Greisinger, A.; Singletary, S.E. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J. Clin. Oncol. 2000, 18, 2505–2514. [Google Scholar] [CrossRef]
- Patterson, R.E.; Neuhouser, M.L.; Hedderson, M.M.; Schwartz, S.M.; Standish, L.J.; Bowen, D.J.; Marshall, L.M. Types of alternative medicine used by patients with breast, colon, or prostate cancer: Predictors, motives, and costs. J. Altern. Complement. Med. 2002, 8, 477–485. [Google Scholar] [CrossRef]
- Labidi, S.; Ennouri, S.; Rachdi, H.; El Benna, H.; Mejri, N.; Daoud, N.; Berrazaga, Y.; Boussen, H. Use of complementary and alternative medicine in cancer: A Tunisian single-center experience. Bull. Cancer 2020, 107, 209–214. [Google Scholar] [CrossRef]
- Cui, Y.; Shu, X.O.; Gao, Y.; Wen, W.; Ruan, Z.X.; Jin, F.; Zheng, W. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Res. Treat. 2004, 85, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Abuelgasim, K.A.; Alsharhan, Y.; Alenzi, T.; Alhazzani, A.; Ali, Y.Z.; Jazieh, A.R. The use of complementary and alternative medicine by patients with cancer: A cross-sectional survey in Saudi Arabia. BMC Complement. Altern. Med. 2018, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Gras, M.; Vallard, A.; Brosse, C.; Beneton, A.; Sotton, S.; Guyotat, D.; Fournel, P.; Daguenet, E.; Magné, N.; Morisson, S. Use of Complementary and Alternative Medicines among Cancer Patients: A Single-Center Study. Oncology 2019, 97, 18–25. [Google Scholar] [CrossRef]
- Berretta, M.; Della Pepa, C.; Tralongo, P.; Fulvi, A.; Martellotta, F.; Lleshi, A.; Nasti, G.; Fisichella, R.; Romano, C.; De Divitiis, C.; et al. Use of complementary and alternative medicine (cam) in cancer patients: An Italian multicenter survey. Oncotarget 2017, 8, 24401–24414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtacki, J.; Pawlowski, L.; Pawlowska, I.; Lichodziejewska-Niemierko, M. Complementary and alternative medicine (CAM) use among patients with cancer undergoing palliative care: A pilot study of a single institution in Poland. J. Clin. Oncol. 2017, 35, 178. [Google Scholar] [CrossRef]
- Wode, K.; Henriksson, R.; Sharp, L.; Stoltenberg, A.; Hök Nordberg, J. Cancer patients’ use of complementary and alternative medicine in Sweden: A cross-sectional study. BMC Complement. Altern. Med. 2019, 19, 62. [Google Scholar] [CrossRef] [Green Version]
- Tascilar, M.; de Jong, F.A.; Verweij, J.; Mathijssen, R.H.J. Complementary and alternative medicine during cancer treatment: Beyond innocence. Oncologist 2006, 11, 732–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dy, G.K.; Bekele, L.; Hanson, L.J.; Furth, A.; Mandrekar, S.; Sloan, J.A.; Adjei, A.A. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J. Clin. Oncol. 2004, 22, 4810–4815. [Google Scholar] [CrossRef]
- Judson, P.L.; Abdallah, R.; Xiong, Y.; Ebbert, J.; Lancaster, J.M. Complementary and alternative medicine use in individuals presenting for care at a comprehensive cancer center. Integr. Cancer Ther. 2017, 16, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Gansler, T.; Kaw, C.; Crammer, C.; Smith, T. A population-based study of prevalence of complementary methods use by cancer survivors: A report from the American Cancer Society’s studies of cancer survivors. Cancer 2008, 113, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vega, D.; Cabanillas, F.; Rivera, N.; Sollivan, P.; Pardo, W.; Rivera, C.; Hernandez, M. Prevalence of complementary/alternative medicine use in cancer patients in a tertiary hospital in Puerto Rico. P. R. Health Sci. J. 2020, 39, 294–299. [Google Scholar] [PubMed]
- Song, S.; Cohen, A.J.; Lui, H.; Mmonu, N.A.; Brody, H.; Patino, G.; Liaw, A.; Butler, C.; Fergus, K.B.; Mena, J.; et al. Use of GoFundMe® to crowdfund complementary and alternative medicine treatments for cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 1857–1865. [Google Scholar] [CrossRef]
- Michalczyk, K.; Pawlik, J.; Czekawy, I.; Kozłowski, M.; Cymbaluk-Płoska, A. Complementary methods in cancer treatment—Cure or curse? Int. J. Environ. Res. Public Health 2021, 18, 356. [Google Scholar] [CrossRef]
- Ezeome, E.R.; Anarado, A.N. Use of complementary and alternative medicine by cancer patients at the University of Nigeria Teaching Hospital, Enugu, Nigeria. BMC Complement. Altern. Med. 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quandt, S.A.; Chen, H.; Grzywacz, J.G.; Bell, R.A.; Lang, W.; Arcury, T.A. Use of complementary and alternative medicine by persons with arthritis: Results of the national health interview survey. Arthritis Rheum. 2005, 53, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Hamed Abdalla, M.E.A.; Ali, A.M.; Loong, L. The use of complementary and alternative medicine (CAM) among cancer patients at a tertiary hospital in Malaysia. Complement. Ther. Med. 2020, 50, 102343. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Rainie, L. Vital Decisions: How Internet Users Decide What Information to Trust When They or Their Loved Ones Are Sick; Pew Internet and American Life Project: Washington, DC, USA, 2002. [Google Scholar]
- Shelley, B.M.; Sussman, A.L.; Williams, R.L.; Segal, A.R.; Crabtree, B.F.; Rios Net Clinicians. ‘They don’t ask me so I don’t tell them’: Patient-clinician communication about traditional, complementary, and alternative medicine. Ann. Fam. Med. 2009, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.M.; Kessler, R.C.; Van Rompay, M.I.; Kaptchuk, T.J.; Wilkey, S.A.; Appel, S.; Davis, R.B. Perceptions about complementary therapies relative to conventional therapies among adults who use both: Results from a national survey. Ann. Intern. Med. 2001, 135, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.S.; Dean-Clower, E. Integrative medicine in hematology/oncology: Benefits, ethical considerations, and controversies. Hematol. Am. Soc. Hematol. Educ. Program 2005, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Mehling, W.E.; Jacobs, B.; Acree, M.; Wilson, L.; Bostrom, A.; West, J.; Acquah, J.; Burns, B.; Chapman, J.; Hecht, F.M. Symptom management with massage and acupuncture in postoperative cancer patients: A randomized controlled trial. J. Pain Symptom Manag. 2007, 33, 258–266. [Google Scholar] [CrossRef]
- Brooks, S.L.; Rowan, G.; Michael, M. Potential issues with complementary medicines commonly used in the cancer population: A retrospective review of a tertiary cancer center’s experience. Asia Pac. J. Clin. Oncol. 2018, 14, e535–e542. [Google Scholar] [CrossRef]
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Use of alternative medicine for cancer and its impact on survival. J. Natl. Cancer Inst. 2018, 110. [Google Scholar] [CrossRef]
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018, 4, 1375–1381. [Google Scholar] [CrossRef]
- Deng, G.E.; Frenkel, M.; Cohen, L.; Cassileth, B.R.; Abrams, D.I.; Capodice, J.L.; Courneya, K.S.; Dryden, T.; Hanser, S.; Kumar, N.; et al. Evidence-based clinical practice guidelines for integrative oncology: Complementary therapies and botanicals. J. Soc. Integr. Oncol. 2009, 7, 85–120. [Google Scholar] [CrossRef] [PubMed]
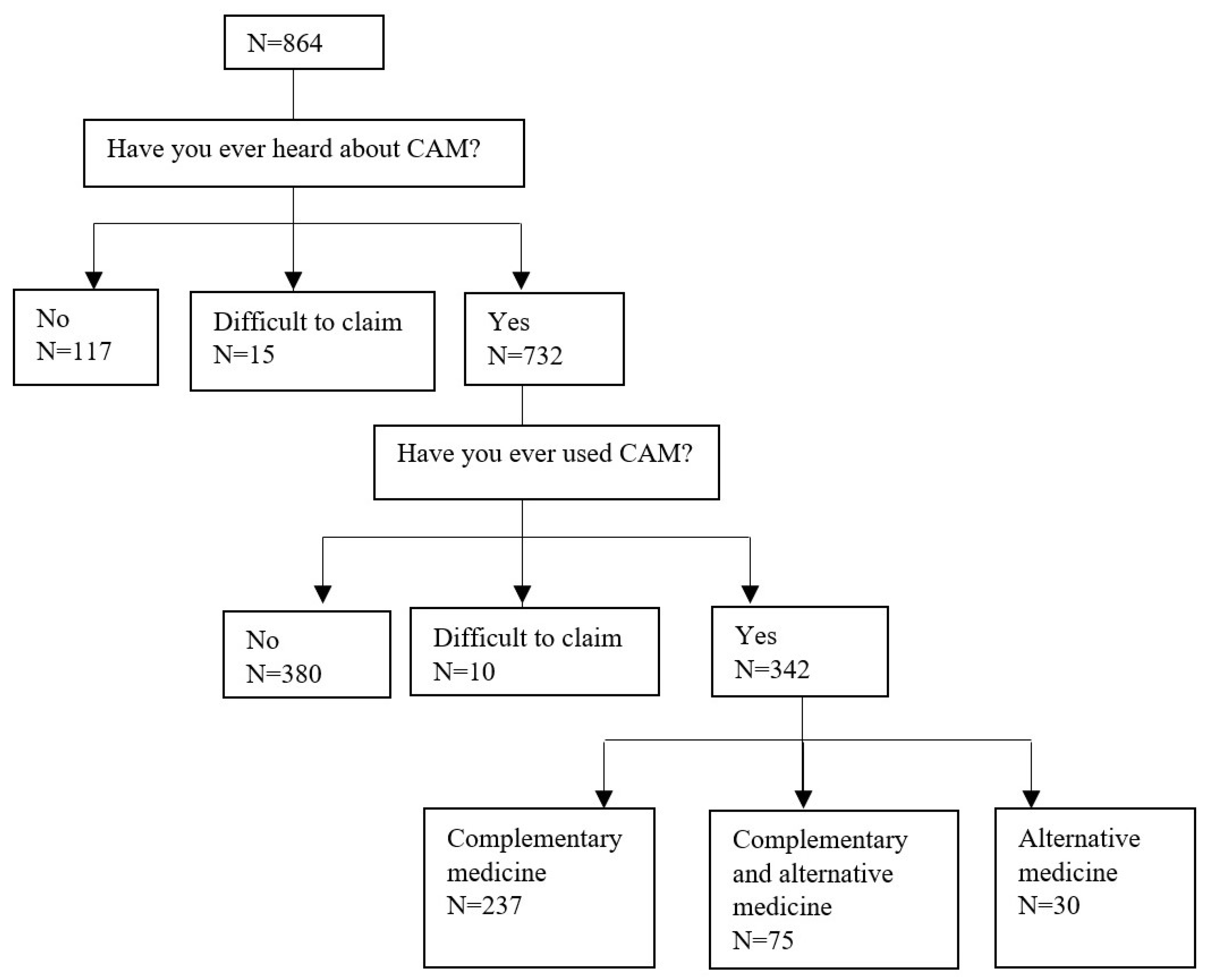
| Variables | All | CAM Users | CAM Non-Users | OR of CAM Usage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 732 | (%) | N = 342 | (%) | N = 380 | (%) | p * | OR | (95% CI) | p ** | OR | (95% CI) | p *** | ||
| Age median (IQR) | 62 (55–68) | 61 (54–67) | 63 (56–69) | 0.009 | 0.99 | (0.97–1.00) | 0.02 | 0.99 | (0.98–1.00) | 0.07 | ||||
| Gender | ||||||||||||||
| Female | 407 | (55.6) | 199 | (58.2) | 200 | (52.6) | 0.13 | 1.00 | Reference | |||||
| Male | 325 | (44.4) | 143 | (41.8) | 180 | (47.4) | 1.25 | (0.93–1.68) | 0.13 | |||||
| Education | ||||||||||||||
| Primary | 48 | (6.6) | 18 | (5.3) | 30 | (7.9) | 0.04 | 1.00 | Reference | 1.00 | Reference | |||
| Vocational | 194 | (26.5) | 76 | (22.2) | 112 | (29.5) | 1.13 | (0.59–2.17) | 0.71 | 1.01 | (0.52–1.97) | 0.97 | ||
| Secondary | 304 | (41.5) | 153 | (44.7) | 147 | (38.7) | 1.73 | (0.93–3.25) | 0.08 | 1.47 | (0.78–2.80) | 0.24 | ||
| Higher | 186 | (25.4) | 95 | (27.8) | 91 | (23.9) | 1.74 | (0.91–3.34) | 0.10 | 1.49 | (0.76–2.91) | 0.25 | ||
| Place of residence | ||||||||||||||
| Village | 113 | (15.4) | 61 | (17.8) | 50 | (13.2) | 0.17 | 1.00 | Reference | 1.00 | Reference | |||
| City < 50,000 inhabitants | 145 | (19.8) | 67 | (19.6) | 75 | (19.7) | 0.73 | (0.44–1.21) | 0.22 | 0.72 | (0.43–1.19) | 0.20 | ||
| City 50–200,000 inhabitants | 310 | (42.4) | 133 | (38.9) | 174 | (45.8) | 0.63 | (0.40–0.97) | 0.04 | 0.64 | (0.41–1.00) | 0.05 | ||
| City >200,000 inhabitants | 164 | (22.4) | 81 | (23.7) | 81 | (21.3) | 0.82 | (0.50–1.33) | 0.42 | 0.84 | (0.51–1.38) | 0.49 | ||
| Professional activity | ||||||||||||||
| Employed | 299 | (40.9) | 164 | (48.0) | 130 | (34.2) | <0.001 | 1.00 | Reference | |||||
| Pensioner | 424 | (57.9) | 175 | (51.1) | 244 | (64.2) | 2.52 | (0.62–10.28) | 0.20 | |||||
| Unemployed | 9 | (1.2) | 3 | (0.9) | 6 | (1.6) | 1.43 | (0.35–5.81) | 0.61 | |||||
| Cancer localization | ||||||||||||||
| Colon | 170 | (23.2) | 80 | (23.4) | 90 | (23.7) | 1.00 | (0.37–2.72) | 1.00 | |||||
| Upper digestive system | 113 | (15.5) | 46 | (13.5) | 64 | (16.8) | 0.38 | 0.81 | (0.29–2.25) | 0.68 | ||||
| Lung | 89 | (12.2) | 49 | (14.3) | 39 | (10.3) | 1.41 | (0.50–4.00) | 0.51 | |||||
| Breast | 89 | (12.2) | 49 | (14.3) | 38 | (10.0) | 1.45 | (0.51–4.11) | 0.48 | |||||
| Gynecological | 64 | (8.7) | 29 | (8.5) | 31 | (8.2) | 1.05 | (0.36–3.09) | 0.93 | |||||
| Head and neck | 42 | (5.7) | 21 | (6.1) | 21 | (5.5) | 1.13 | (0.36–3.48) | 0.84 | |||||
| Urinary system—without prostate | 37 | (5.1) | 16 | (4.7) | 21 | (5.5) | 0.86 | (0.27–2.72) | 0.79 | |||||
| Brain | 31 | (4.2) | 12 | (3.5) | 19 | (5.0) | 0.71 | (0.21–2.35) | 0.58 | |||||
| Prostate | 17 | (2.3) | 8 | (2.3) | 9 | (2.4) | 1.00 | Reference | ||||||
| Other | 80 | (10.9) | 32 | (9.4) | 48 | (12.6) | 0.75 | (0.26–2.15) | 0.59 | |||||
| Time since diagnosis (years) median (IQR) | 0.62 (0.33–1.54) | 0.70 (0.41–1.71) | 0.53 (0.28–1.44) | 0.002 | 1.00 | (0.99–1.00) | 0.13 | |||||||
| Intention of planned treatment | ||||||||||||||
| Curative | 370 | (50.6) | 171 | (50.0) | 196 | (51.6) | 0.83 | 1.00 | Reference | |||||
| Palliative | 167 | (22.8) | 81 | (23.7) | 83 | (21.8) | 1.12 | (0.77–1.62) | 0.55 | |||||
| Difficult to evaluate | 195 | (26.6) | 90 | (26.3) | 101 | (26.6) | 1.02 | (0.72–1.45) | 0.91 | |||||
| Stage of disease | ||||||||||||||
| Early | 163 | (22.3) | 62 | (18.1) | 101 | (26.6) | 0.02 | 1.00 | Reference | 1.00 | Reference | |||
| Local and/or regional development | 238 | (32.5) | 120 | (35.1) | 111 | (29.2) | 1.76 | (1.17–2.65) | 0.006 | 1.69 | (1.12–2.56) | 0.01 | ||
| Metastatic | 194 | (26.5) | 101 | (29.5) | 92 | (24.2) | 1.79 | (1.17–2.73) | 0.007 | 1.69 | (1.10–2.60) | 0.02 | ||
| Difficult to evaluate | 137 | (18.7) | 59 | (17.3) | 76 | (20.0) | 1.26 | (0.79–2.01) | 0.32 | 1.25 | (0.78–2.01) | 0.35 | ||
| N | (%) | ||
|---|---|---|---|
| Form of alternative medicine: | |||
| Used as the only treatment instead of traditional treatment. | 1 | (3.3) | |
| Used as a supportive therapy of traditional cancer treatment. | 28 | (93.4) | |
| Used as therapy after completion of traditional treatment. | 1 | (3.3) | |
| Informed oncologist about used alternative medicine: | |||
| Yes | 10 | (33.3) | |
| No | 18 | (60.0) | |
| Difficult to claim | 2 | (6.7) | |
| Expectations of using alternative medicine: * | |||
| Complete recovery | 3 | (10.0) | |
| Increasing effectiveness of conventional cancer treatment | 17 | (56.7) | |
| No precise expectations—using every possible treatment | 11 | (36.7) | |
| Other | 4 | (13.3) | |
| Timing of decision about using alternative medicine: | |||
| Before traditional cancer treatment | 11 | (36.7) | |
| During traditional cancer treatment | 18 | (60.0) | |
| When traditional cancer treatment was completed or without expected results | 0 | (0.0) | |
| Other | 1 | (3.3) | |
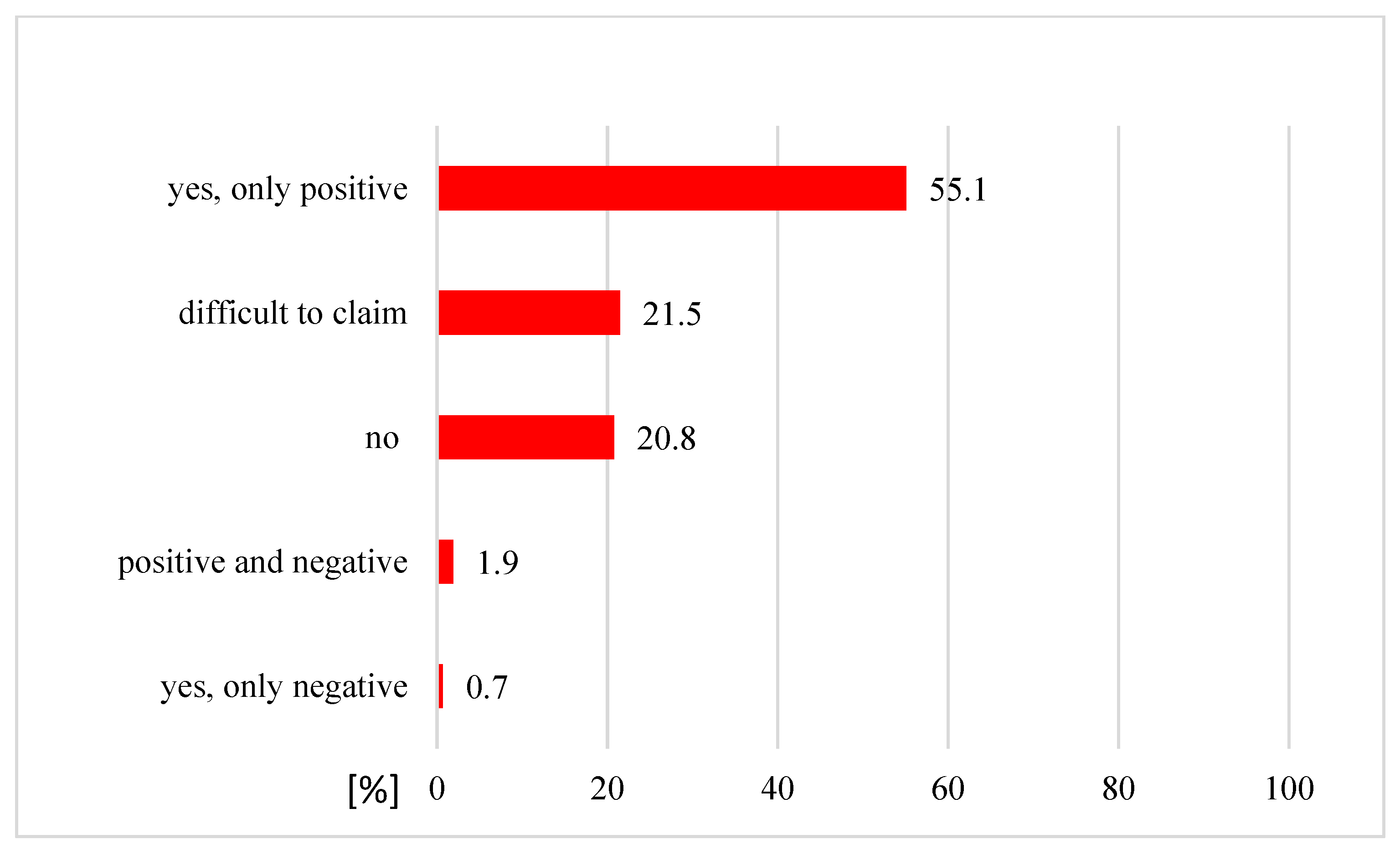
| Perceived positive effects of alternative medicine: | |||
| Yes | 5 | (16.7) | |
| No | 17 | (56.7) | |
| Difficult to claim | 8 | (26.6) | |
| Perceived negative effects of alternative medicine: | |||
| Yes | 2 | (6.7) | |
| No | 27 | (90.0) | |
| Difficult to claim | 1 | (3.3) | |
| Considering withdrawal from conventional cancer treatment to use alternative medicine: | |||
| Yes | 1 | (3.3) | |
| No | 28 | (93.4) | |
| Difficult to claim | 1 | (3.3) | |
| Recommendation of alternative medicine to other patients: | |||
| Yes | 12 | (40.0) | |
| No | 9 | (30.0) | |
| Difficult to claim | 9 | (30.0) | |
| Have you still used alternative methods? | |||
| Yes | 9 | (30.0) | |
| No | 15 | (50.0) | |
| Some of them yes, some of them no | 6 | (20.0) | |
| Reasons for resigning from alternative medicine: * | |||
| No positive effects | 7 | (23.3) | |
| Negative effects and/or complications | 2 | (6.7) | |
| Encouraged to resign use by a doctor/other medical professional | 1 | (3.3) | |
| Encouraged to resign use by family/friends | 0 | (0.0) | |
| Negative information about using method | 0 | (0.0) | |
| Running out the funds to continue the therapy | 5 | (16.7) | |
| Other | 9 | (30.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzycka, K.; Kurzawa, M.; Kucharz, M.; Godawska, M.; Oleksa, M.; Stawowy, M.; Slupinska-Borowka, K.; Sznek, W.; Gisterek, I.; Boratyn-Nowicka, A.; et al. Complementary and Alternative Medicine Use in Hospitalized Cancer Patients—Study from Silesia, Poland. Int. J. Environ. Res. Public Health 2022, 19, 1600. https://doi.org/10.3390/ijerph19031600
Kasprzycka K, Kurzawa M, Kucharz M, Godawska M, Oleksa M, Stawowy M, Slupinska-Borowka K, Sznek W, Gisterek I, Boratyn-Nowicka A, et al. Complementary and Alternative Medicine Use in Hospitalized Cancer Patients—Study from Silesia, Poland. International Journal of Environmental Research and Public Health. 2022; 19(3):1600. https://doi.org/10.3390/ijerph19031600
Chicago/Turabian StyleKasprzycka, Karolina, Marta Kurzawa, Malgorzata Kucharz, Monika Godawska, Marta Oleksa, Marta Stawowy, Katarzyna Slupinska-Borowka, Wiktoria Sznek, Iwona Gisterek, Agnieszka Boratyn-Nowicka, and et al. 2022. "Complementary and Alternative Medicine Use in Hospitalized Cancer Patients—Study from Silesia, Poland" International Journal of Environmental Research and Public Health 19, no. 3: 1600. https://doi.org/10.3390/ijerph19031600
APA StyleKasprzycka, K., Kurzawa, M., Kucharz, M., Godawska, M., Oleksa, M., Stawowy, M., Slupinska-Borowka, K., Sznek, W., Gisterek, I., Boratyn-Nowicka, A., Rucinska, M., Osowiecka, K., & Nawrocki, S. (2022). Complementary and Alternative Medicine Use in Hospitalized Cancer Patients—Study from Silesia, Poland. International Journal of Environmental Research and Public Health, 19(3), 1600. https://doi.org/10.3390/ijerph19031600







