Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments
Abstract
:1. Introduction
2. Materials and Methods
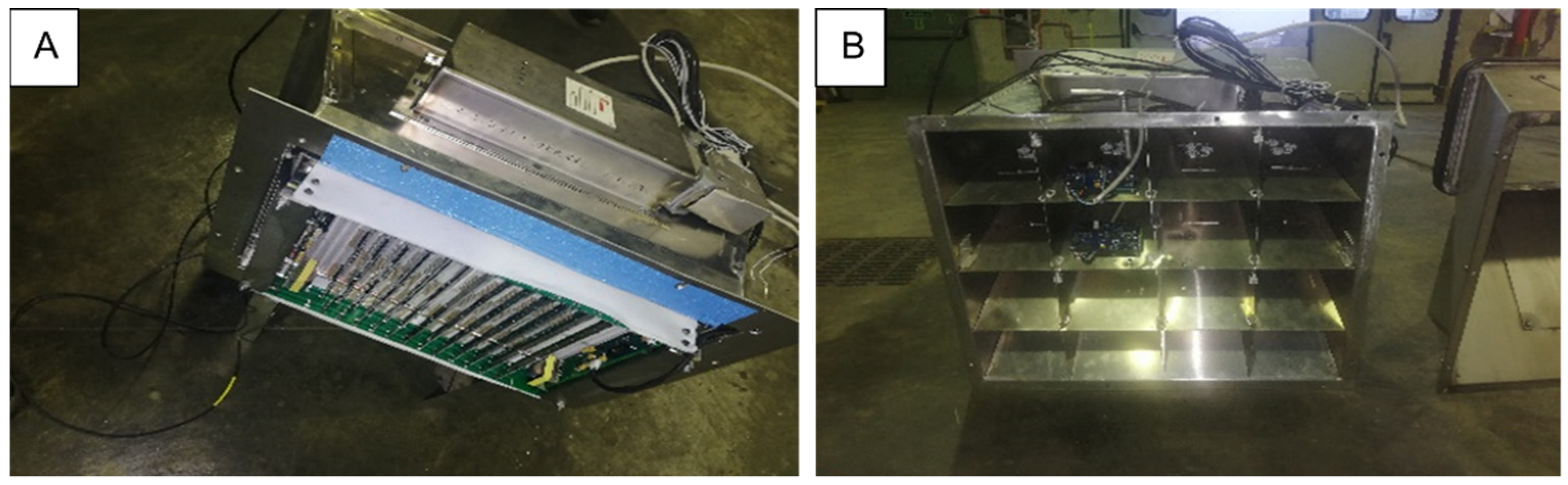
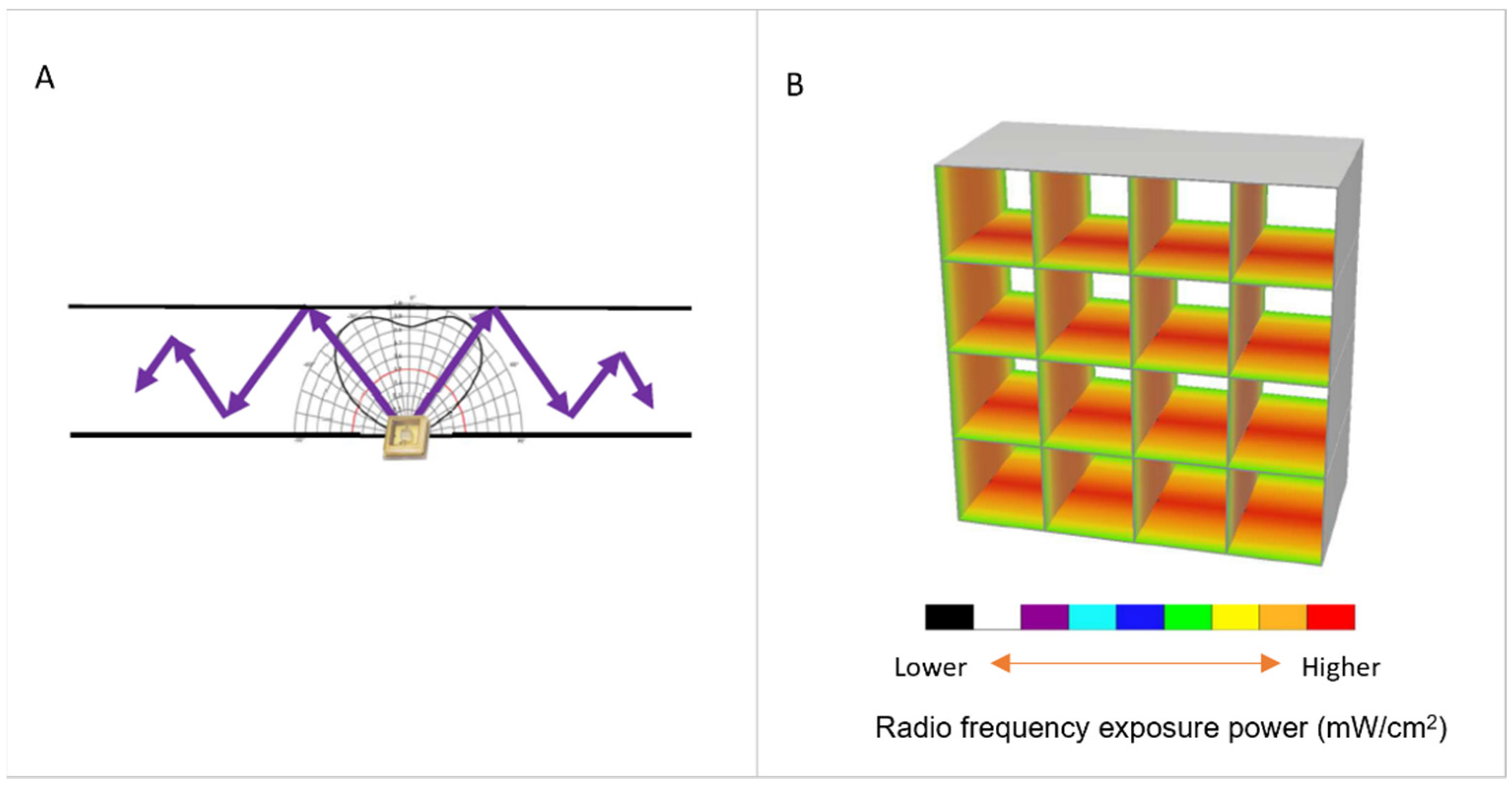
2.1. Continuous Sanitation Air (CSA) System
2.2. Test Microorganism Suspension Preparation
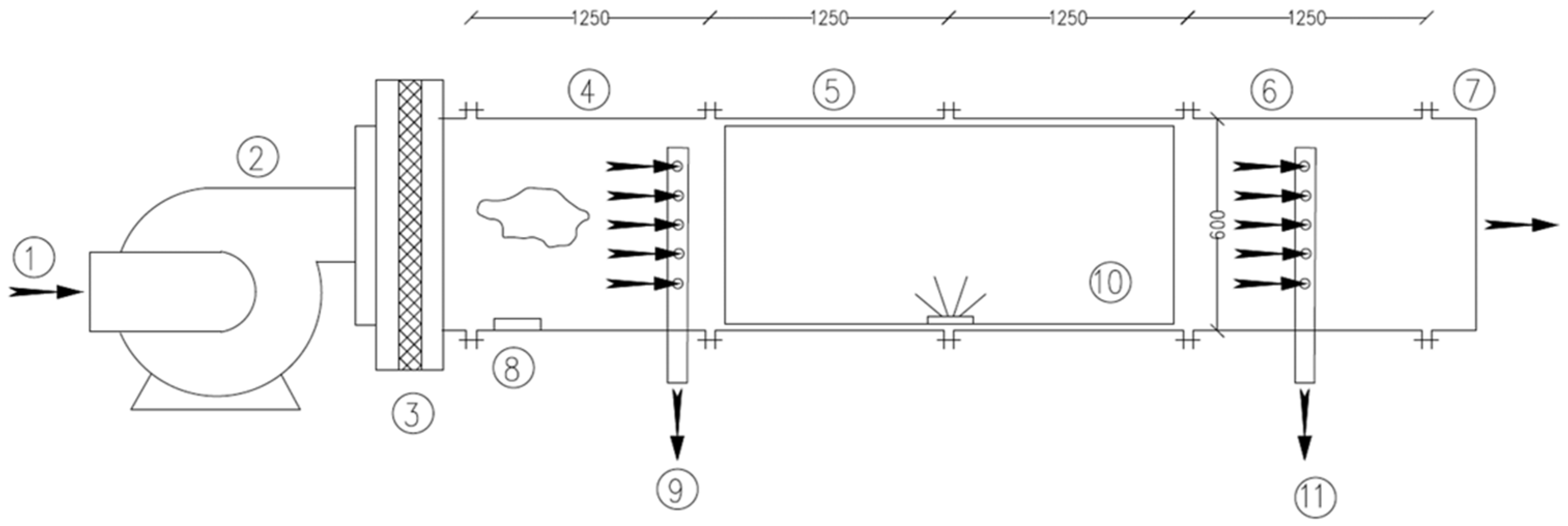
2.3. Test Rig Configuration
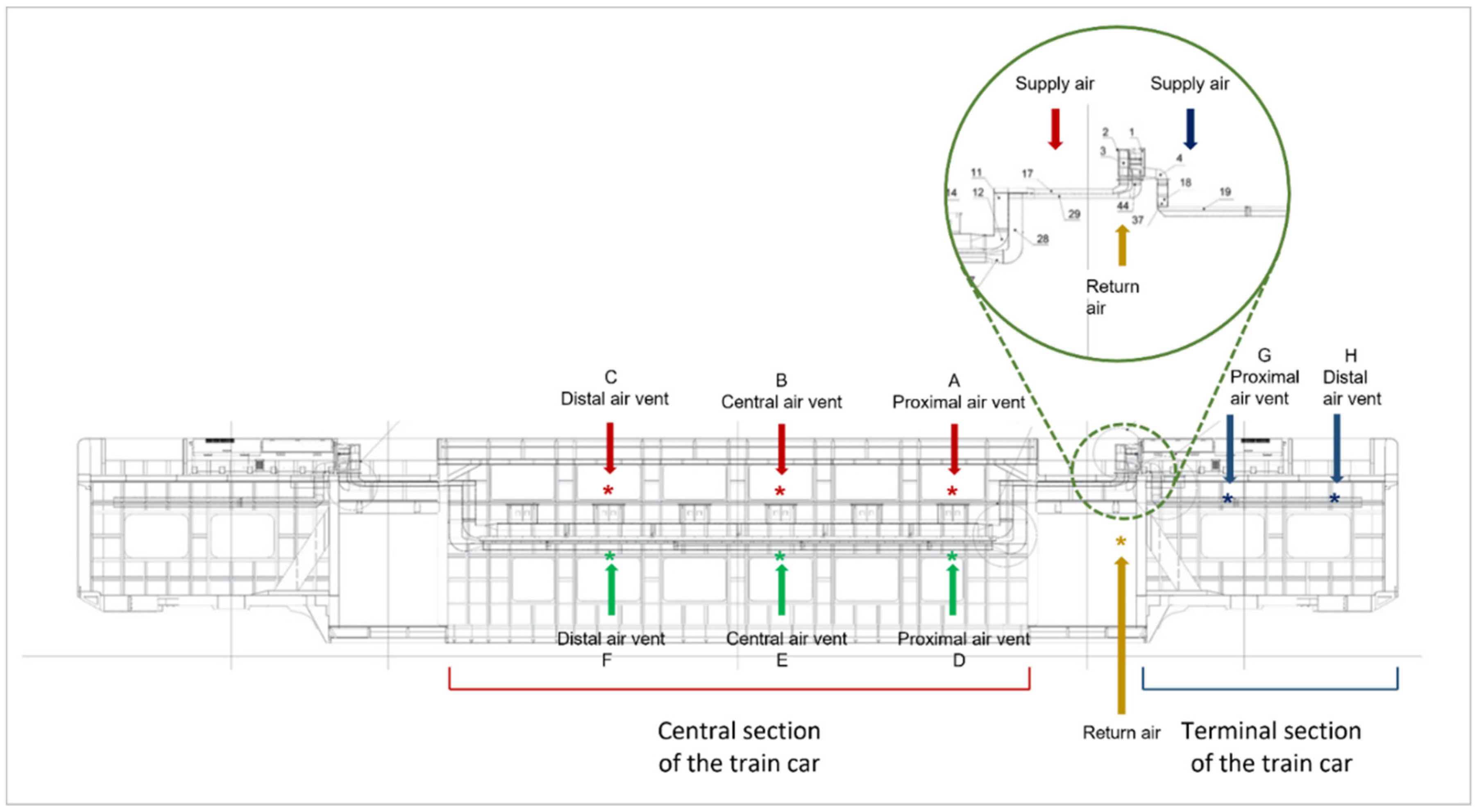
2.4. Air Sampling
2.5. Assessment of CSA System Sanitation Efficiency in the Test Rig
2.6. Assessment of CSA System Sanitation Efficiency in the Train Setting
2.7. Inactivation Rate
2.8. Determination of UV-C Dose Required for the Inactivation of the Microbial Species
3. Results
3.1. Microclimatic Parameters
3.2. Microbial Inactivation Rates in the Test Rig Configuration
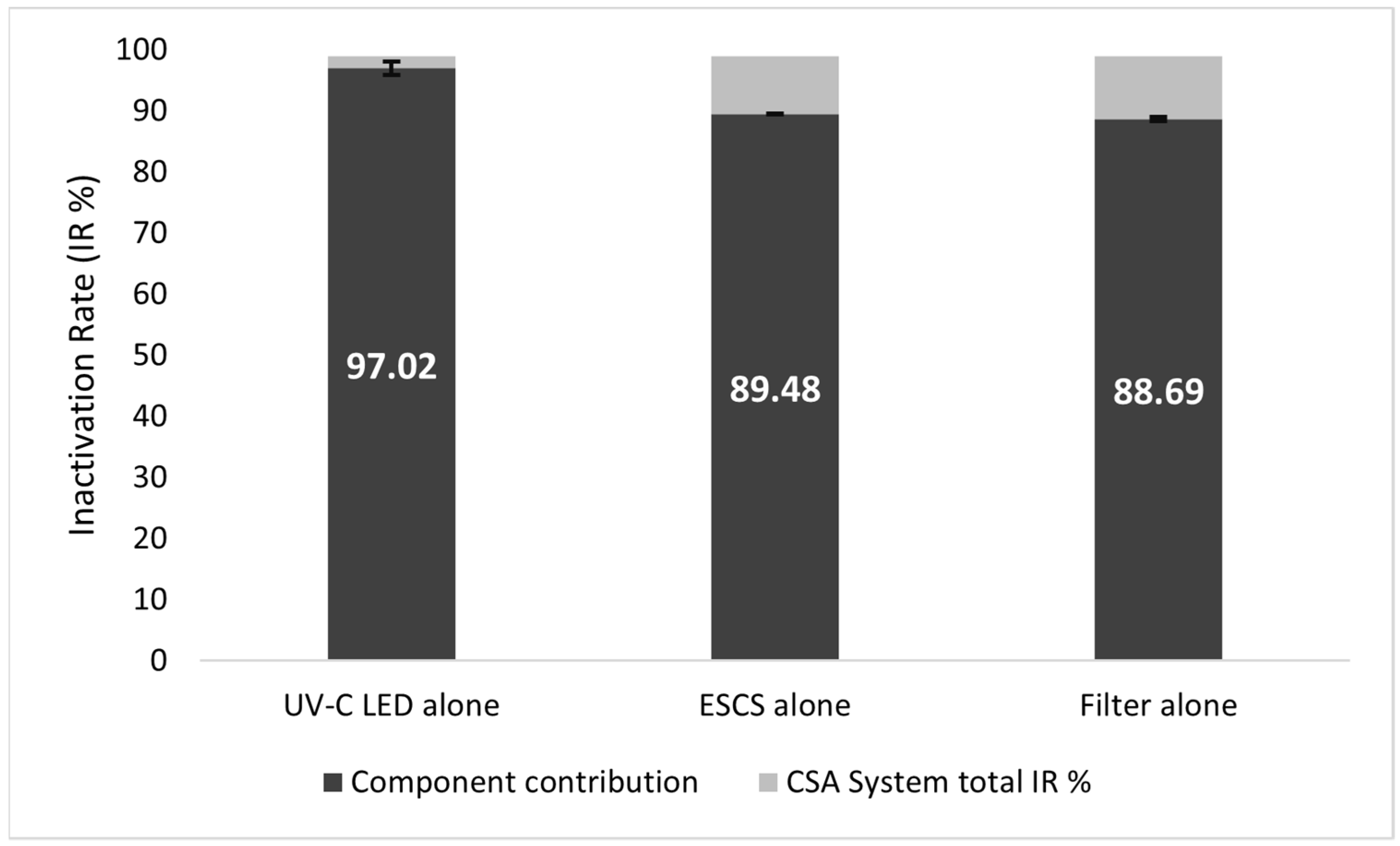
3.3. Assessment of Component Contribution on Microbial Inactivation Rates in the Test Rig Configuration
3.4. Microbial Inactivation Rates in the Train Setting
3.5. Determination of UV Dose Required for the Inactivation of the Microbial Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Meadow, J.F.; Altrichter, A.E.; Kembel, S.W.; Kline, J.; Mhuireach, G.; Moriyama, M.; Northcutt, D.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air. 2014, 24, 41–48. [Google Scholar] [CrossRef]
- Prussin Ii, A.; Marr, L. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 2009, 6 (Suppl. 6), S737–S746. [Google Scholar] [CrossRef] [Green Version]
- Mandal, J.; Brandl, H. Bioaerosols in Indoor Environment—A Review with Special Reference to Residential and Occupational Locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar]
- Dannemiller, K.C.; Gent, J.F.; Leaderer, B.P.; Peccia, J. Influence of housing characteristics on bacterial and fungal communities in homes of asthmatic children. Indoor Air. 2016, 26, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Montagna, M.; Cristina, M.L.; Giglio, O.D.; Spagnolo, A.M.; Napoli, C.; Cannova, L.; Deriu, M.G.; Delia, S.T.; Giuliano, A.; Guida, M.; et al. Serological and molecular identification of Legionella spp. isolated from water and surrounding air samples in Italian healthcare facilities. Environ. Res. 2015, 146, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection Sources of a Common Non-tuberculous Mycobacterial Pathogen, Mycobacterium avium Complex. Front. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, M.C. Aerosol Transmission of SARS-CoV-2: Physical Principles and Implications. Public Health Front. 2020, 8, 590041. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Mosso, S.; Sampò, S.; Elisabetta, C. Assessment of Microbiological Indoor Air Quality in an Italian Office Building Equipped with an HVAC System. Environ. Monit. Assess. 2009, 161, 473–483. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, C. Airborne microbiological characteristics in public buildings of Korea. Build. Environ. 2007, 42, 2188–2196. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, C.H.; Hsu, K.L. Size and seasonal distributions of airborne bioaerosols in commuting trains. Atmos. Environ. 2010, 44, 4331–4338. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Stobnicka-Kupiec, A.; Górny, R.; Cyprowski, M.; Ławniczek-Wałczyk, A. Microbial Air Quality in Municipal Buses Before and After Disinfection of their Air-Conditioning Systems. Ecol. Eng. 2019, 20, 189–194. [Google Scholar] [CrossRef]
- European Union Agency for Railways Ventilation in Railway Vehicles. COVID-19 Information Bulletin. 2020. Available online: https://www.era.europa.eu/content/era-publishes-covid-19-information-bulletin-ventilation-railway-vehicles_en (accessed on 18 November 2021).
- Moccia, G.; De Caro, F.; Pironti, C.; Boccia, G.; Capunzo, M.; Borrelli, A.; Motta, O. Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent. Medicina 2020, 56, 578. [Google Scholar] [CrossRef] [PubMed]
- Pastuszka, J.S.; Mucha, W.; Wlazło, A.; Lis, D.; Marchwińska-Wyrwał, E.; Mainka, A. The Study of the Sterilization of the Indoor Air in Hospital/Clinic Rooms by Using the Electron Wind Generator. Int. J. Environ. Res. Public Health 2019, 16, 4935. [Google Scholar] [CrossRef] [Green Version]
- Curtis, G.L.; Faour, M.; Jawad, M.; Klika, A.K.; Barsoum, W.K.; Higuera, C.A. Reduction of Particles in the Operating Room Using Ultraviolet Air Disinfection and Recirculation Units. J. Arthroplast. 2018, 33, S196–S200. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Wang, M.; Hao, X.; He, S.; Zhou, R. Design of an air isolation and purification (AIP) desk for medical use and characterization of its efficacy in ambient air isolation and purification. Biosaf. Health 2020, 2, 169–176. [Google Scholar] [CrossRef]
- Banche, G.; Iannantuoni, M.; Musumeci, A.; Allizond, V.; Cuffini, A. Use of Negative and Positive Ions for Reducing Bacterial Pathogens to Control Infections. Acta Sci. Microbiol. 2019, 2, 35–38. [Google Scholar] [CrossRef]
- Comini, S.; Mandras, N.; Iannantuoni, M.R.; Menotti, F.; Musumeci, A.G.; Piersigilli, G.; Allizond, V.; Banche, G.; Cuffini, A.M. Positive and Negative Ions Potently Inhibit the Viability of Airborne Gram-Positive and Gram-Negative Bacteria. Microbiol. Spectr. 2021, 9, e0065121. [Google Scholar] [CrossRef]
- Rudnick, S.N.; First, M.W.; Vincent, R.L.; Brickner, P.W. In-Place Testing of In-Duct Ultraviolet Germicidal Irradiation. HVAC R Res. 2009, 15, 525–535. [Google Scholar] [CrossRef]
- Ryan, K.; McCabe, K.; Clements, N.; Hernandez, M.; Miller, S.L. Inactivation of Airborne Microorganisms Using Novel Ultraviolet Radiation Sources in Reflective Flow-Through Control Devices. Aerosol Sci. Technol. 2010, 44, 541–550. [Google Scholar] [CrossRef]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics. 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef] [PubMed]
- Brickner, P.W.; Vincent, R.L.; First, M.; Nardell, E.; Murray, M.; Kaufman, W. The application of ultraviolet germicidal irradiation to control transmission of airborne disease: Bioterrorism countermeasure. Public Health Rep. 2003, 118, 99–114. [Google Scholar] [CrossRef]
- Method of Evaluating the UV Dose to Airborne Microorganisms Transiting In-Duct Ultraviolet Germicidal Irradiation Devices. Available online: https://www.iso.org/standard/67814.html (accessed on 31 July 2019).
- Air Filters for General Ventilation—Part 1: Technical Specifications, Requirements and Classification System Based upon Particulate Matter Efficiency (ePM). Available online: https://www.iso.org/standard/57864.html (accessed on 31 December 2016).
- Lin, C.Y.; Li, C.S. Control Effectiveness of Ultraviolet Germicidal Irradiation on Bioaerosols. Aerosol Sci. Technol. 2002, 36, 474–478. [Google Scholar] [CrossRef]
- VanOsdell, D.; Foarde, K. Defining the Effectiveness of UV Lamps Installed in Circulating Air Ductwork, report, November 30, 2002. Available online: https://digital.library.unt.edu/ark:/67531/metadc736724/ (accessed on 18 November 2021).
- Katara, G.; Hemvani, N.; Chitnis, S.; Chitnis, V.; Chitnis, D.S. Surface disinfection by exposure to germicidal UV light. Indian J. Med. Microbiol. 2008, 26, 241–242. [Google Scholar] [CrossRef]
- Fletcher, L.A.; Gaunt, L.F.; Beggs, C.B.; Shepherd, S.J.; Sleigh, P.A.; Noakes, C.J.; Kerr, K.G. Bactericidal action of positive and negative ions in air. BMC Microbiol. 2007, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jin, X.; Nunayon, S.S.; Lai, A.C.K. Disinfection by in-duct ultraviolet lamps under different environmental conditions in turbulent airflows. Indoor Air. 2020, 30, 500–511. [Google Scholar] [CrossRef]
- Forthomme, A.; Joubert, A.; Andres, Y.; Simon, X.; Duquenne, P.; Bemer, D.; Coq, L. Microbial aerosol filtration: Growth and release of a bacteria-fungi consortium collected by fibrous filters in different operating conditions. J. Aerosol Sci. 2014, 72, 32–46. [Google Scholar] [CrossRef]
- Al-Abdalall, A.; Al-dakheel, S.; Al-Abkari, H. Impact of Air-Conditioning Filters on Microbial Growth and Indoor Air Pollution. In Low-Temperature Technologies; Morosuk, T., Sultan, M., Eds.; IntechOpen: London, UK, 2019; pp. 179–206. [Google Scholar] [CrossRef] [Green Version]
- Jankowska, E.; Reponen, T.; Willeke, K.; Grinshpun, S.; Choi, K.-J. Collection of fungal spores on air filters and spore reentrainment from filters into air. J. Aerosol Sci. 2000, 31, 969–978. [Google Scholar] [CrossRef]
- Inagaki, H.; Saito, A.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg. Microbes Infect. 2020, 9, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- CIE—Commission Internationale de l’Eclairage (International Commission on Illumination). Position Statement on Ultraviolet (UV) Radiation to Manage the Risk of COVID-19 Transmission. Available online: http://cie.co.at/publications/cie-position-statement-use-ultraviolet-uv-radiation-manage-risk-covid-19-transmission (accessed on 18 November 2021).
| Airflow Rates (m3/h) | 1000 | 2000 | 3000 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Off * | On * | % IR | Off * | On * | % IR | Off * | On * | % IR | |
| E. coli ATCC25922 | 5220 ± 11 | 36 ± 19 | 99.31 ± 0.5 | 4203 ± 55 | 36 ± 19 | 99.14 ± 0.4 | 4023 ± 145 | 42 ± 25 | 98.96 ± 0.6 |
| B. subtilis ATCC6633 | 971 ± 59 | 0 | 100 | 714 ± 92 | 0 | 100 | 533 ± 11 | 3 ± 2.78 | 99.44 ± 0.5 |
| Cladosporium spp. | 1090 ± 21 | 333 ± 12 | 69.45 ± 0.5 | 875 ± 25 | 281 ± 30 | 67.89 ± 2.5 | 822 ± 11 | 264 ± 14 | 67.88 ± 1.3 |
| Nebulization Time (Min) | 2 | ||
|---|---|---|---|
| Sampling Sites | Off * | On * | % IR |
| A | 364.00 ± 25.00 | 19.45 ± 2.75 | 94.58 ± 1.13 |
| B | 513.50 ± 39.50 | 19.45 ± 2.75 | 96.07 ± 1.07 |
| C | 475.00 ± 47.00 | 19.45 ± 8.35 | 95.69 ± 2.18 |
| D | 472.50 ± 83.50 | 38.85 ± 5.55 | 91.73 ± 0.29 |
| E | 453.00 ± 103.00 | 24.55 ± 13.45 | 93.57 ± 4.43 |
| F | 333.50 ± 44.50 | 22.20 ± 11.10 | 92.77 ± 4.29 |
| G | 350.00 ± 17.00 | 27.75 ± 5.55 | 92.13 ± 1.12 |
| H | 491.50 ± 97.50 | 27.80 | 94.11 ± 1.17 |
| Microbial Species | Airflow Rate (m3/h) | D (J/m2) * |
|---|---|---|
| E. coli ATCC25922 | 1000 | 13.10 |
| 2000 | 12.53 | |
| 3000 | 12.01 | |
| B. subtilis ATCC6633 | 1000 | n.d. |
| 2000 | n.d. | |
| 3000 | 30.73 | |
| Cladosporium spp. | 1000 | 564.66 |
| 2000 | 540.89 | |
| 3000 | 540.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldelli, G.; Aliano, M.P.; Amagliani, G.; Magnani, M.; Brandi, G.; Pennino, C.; Schiavano, G.F. Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments. Int. J. Environ. Res. Public Health 2022, 19, 1559. https://doi.org/10.3390/ijerph19031559
Baldelli G, Aliano MP, Amagliani G, Magnani M, Brandi G, Pennino C, Schiavano GF. Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments. International Journal of Environmental Research and Public Health. 2022; 19(3):1559. https://doi.org/10.3390/ijerph19031559
Chicago/Turabian StyleBaldelli, Giulia, Mattia Paolo Aliano, Giulia Amagliani, Mauro Magnani, Giorgio Brandi, Carmelo Pennino, and Giuditta Fiorella Schiavano. 2022. "Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments" International Journal of Environmental Research and Public Health 19, no. 3: 1559. https://doi.org/10.3390/ijerph19031559
APA StyleBaldelli, G., Aliano, M. P., Amagliani, G., Magnani, M., Brandi, G., Pennino, C., & Schiavano, G. F. (2022). Airborne Microorganism Inactivation by a UV-C LED and Ionizer-Based Continuous Sanitation Air (CSA) System in Train Environments. International Journal of Environmental Research and Public Health, 19(3), 1559. https://doi.org/10.3390/ijerph19031559






