The Prevalence of Antimicrobial Resistant Neisseria gonorrhoeae in Papua New Guinea: A Systematic Review and Meta-Analysis
Abstract
:1. Background
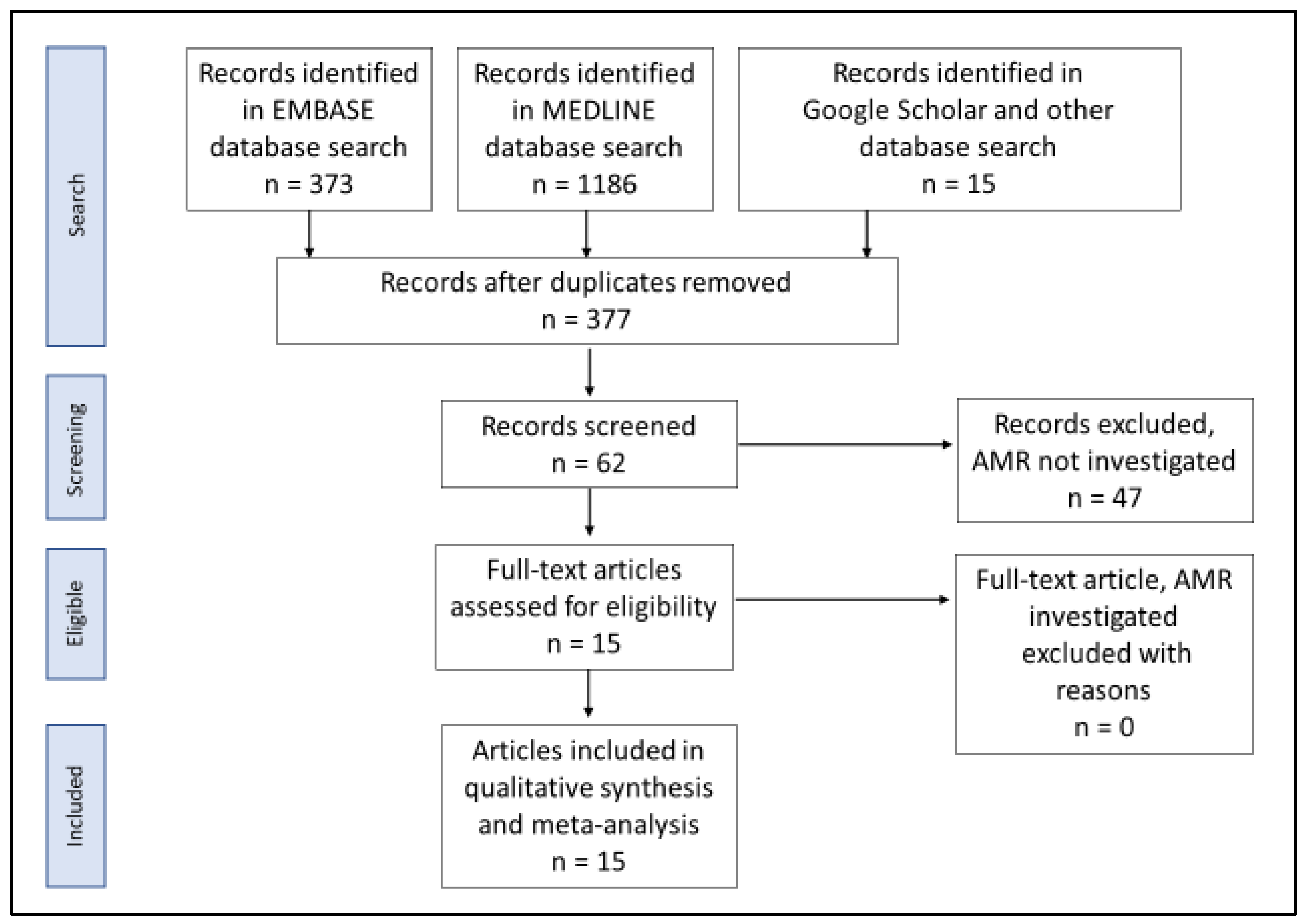
2. Materials and Methods
2.1. Literature Search
- -
- Original study that investigated NG AMR in humans from PNG;
- -
- Peer-reviewed articles published between January 1980 and October 2020. The cut-off year was set at 1980 and after was to ensure that the reported prevalence in the primary studies were generated using the Australia Gonococcal Surveillance Program (AGSP) and/or the WHO Gonococcal Antimicrobial Surveillance Program (GASP) methodology. These two programs use similar standardized methods for NG AMR testing and result interpretation;
- -
- The total number of samples or isolates analyzed and the resistance prevalence described and stated. This was to ensure that we have the exact numerator (number of sample or isolates that were resistant to the antibiotic tested against) and denominator (total number of sample or isolates analyzed) to enable accurate proportion estimations in the meta-analysis; and
- -
- Abstract and full text available in English.
2.2. Data Extraction and Analysis
3. Results
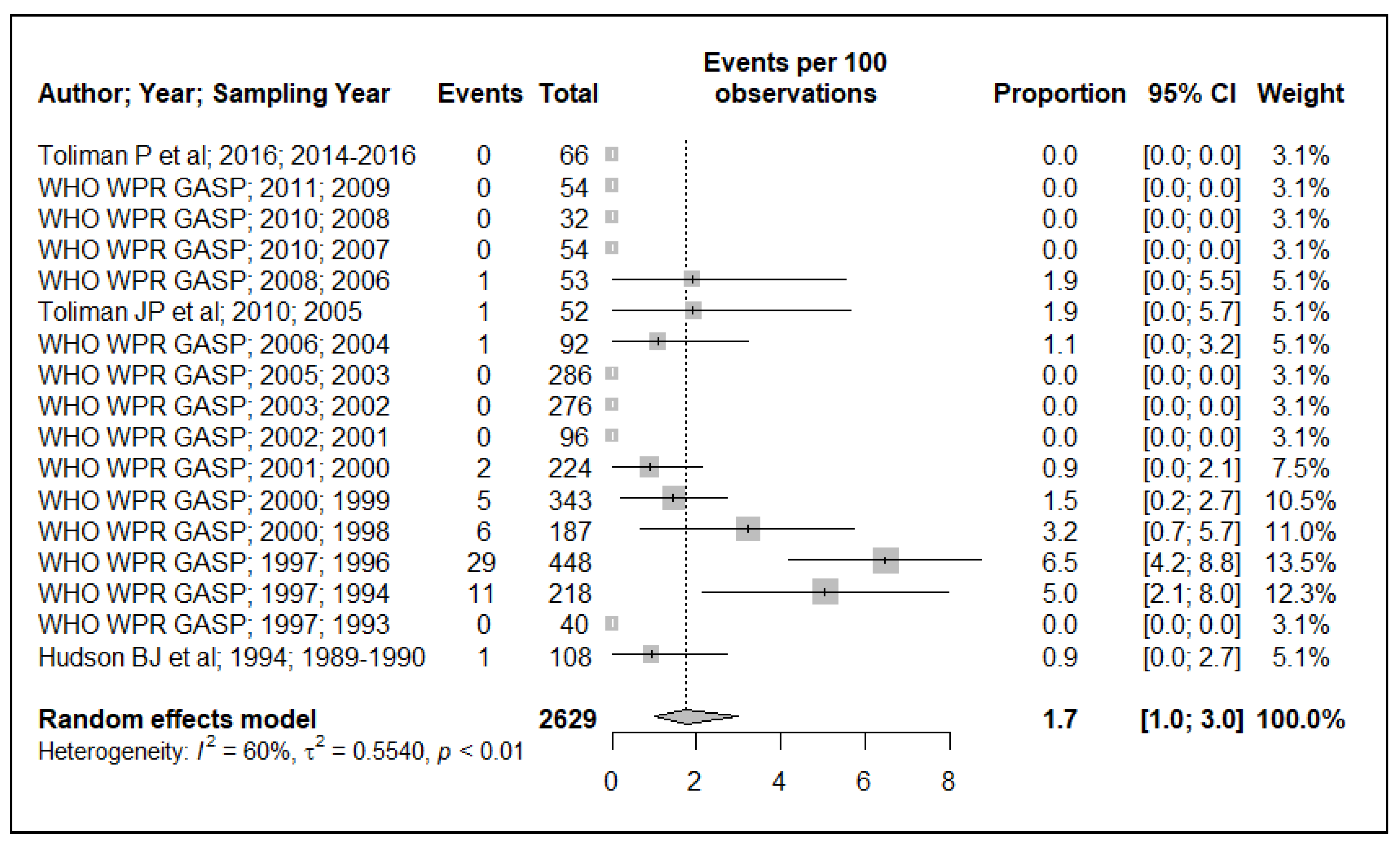
3.1. Resistance Prevalence Estimates
3.1.1. Penicillinase-Producing N. gonorrhoeae (PPNG)
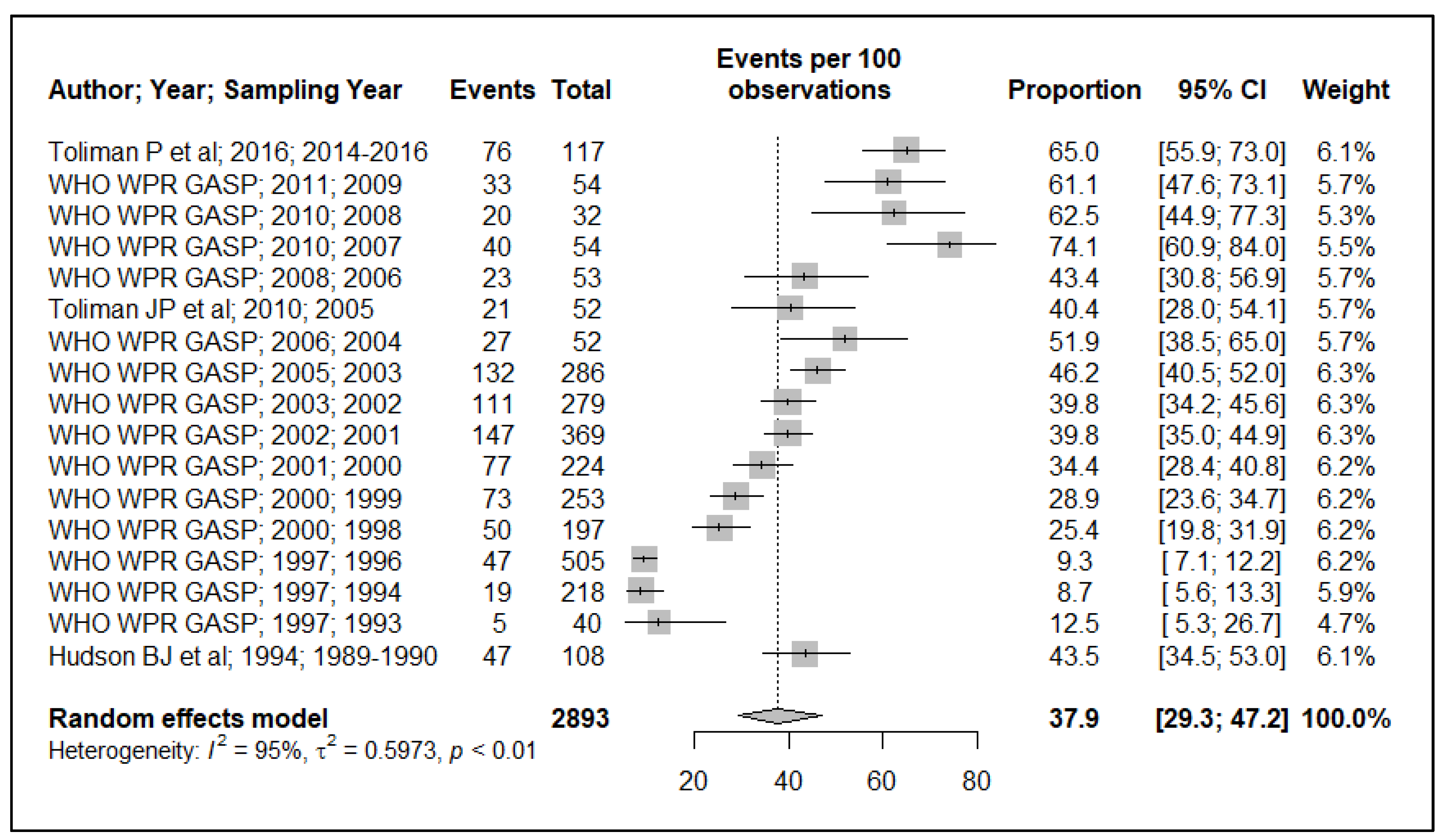
3.1.2. Chromosomally-Mediated Penicillin Resistance
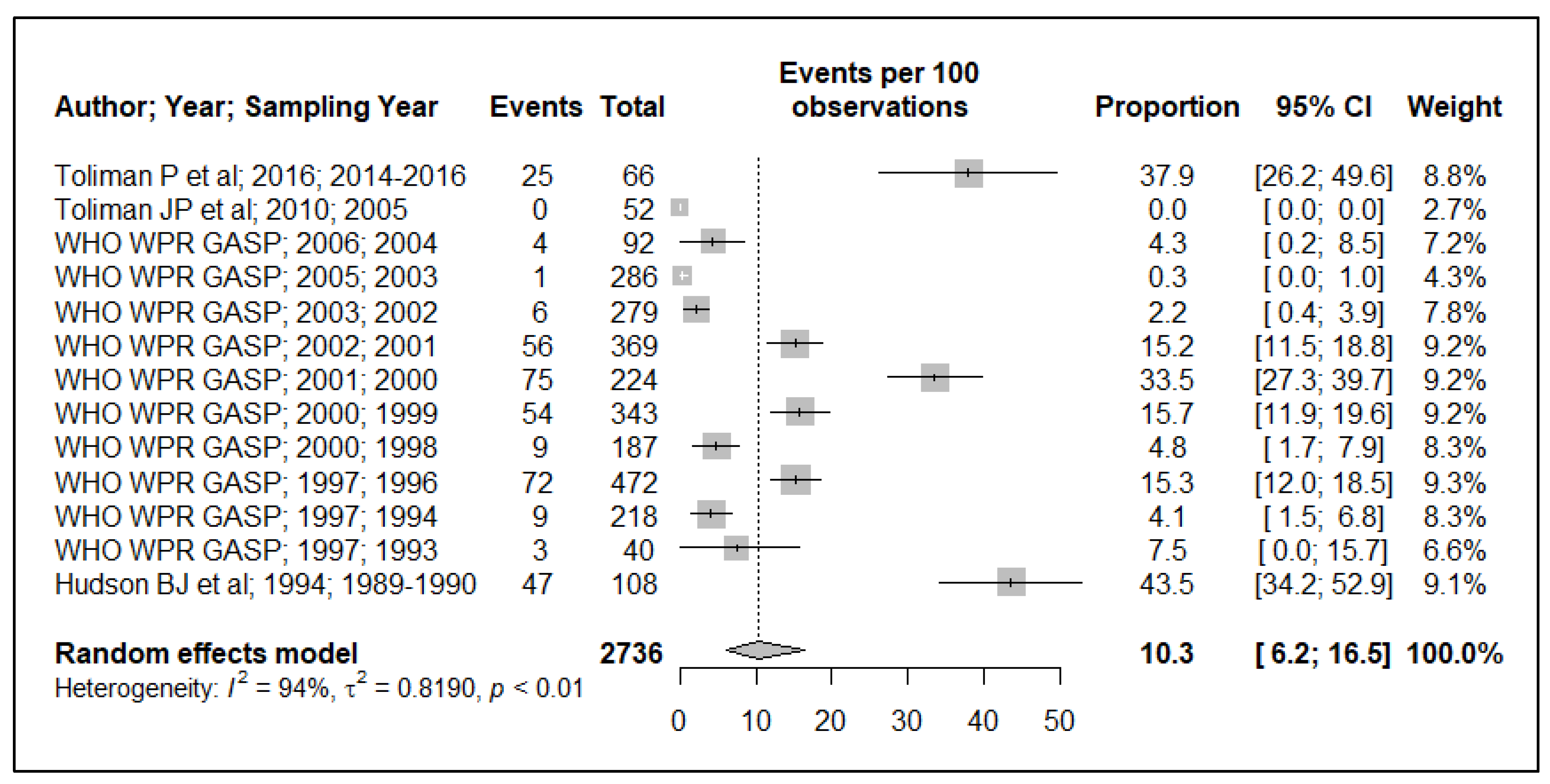
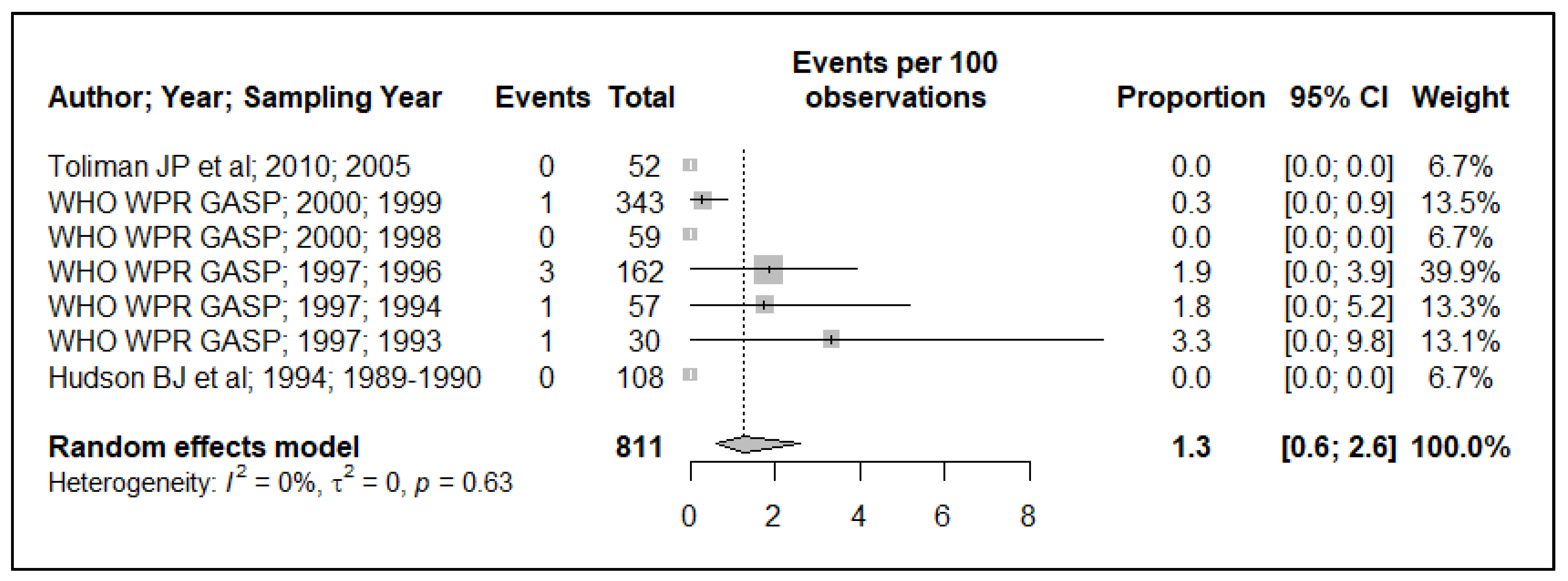
3.2. Tetracycline Resistance
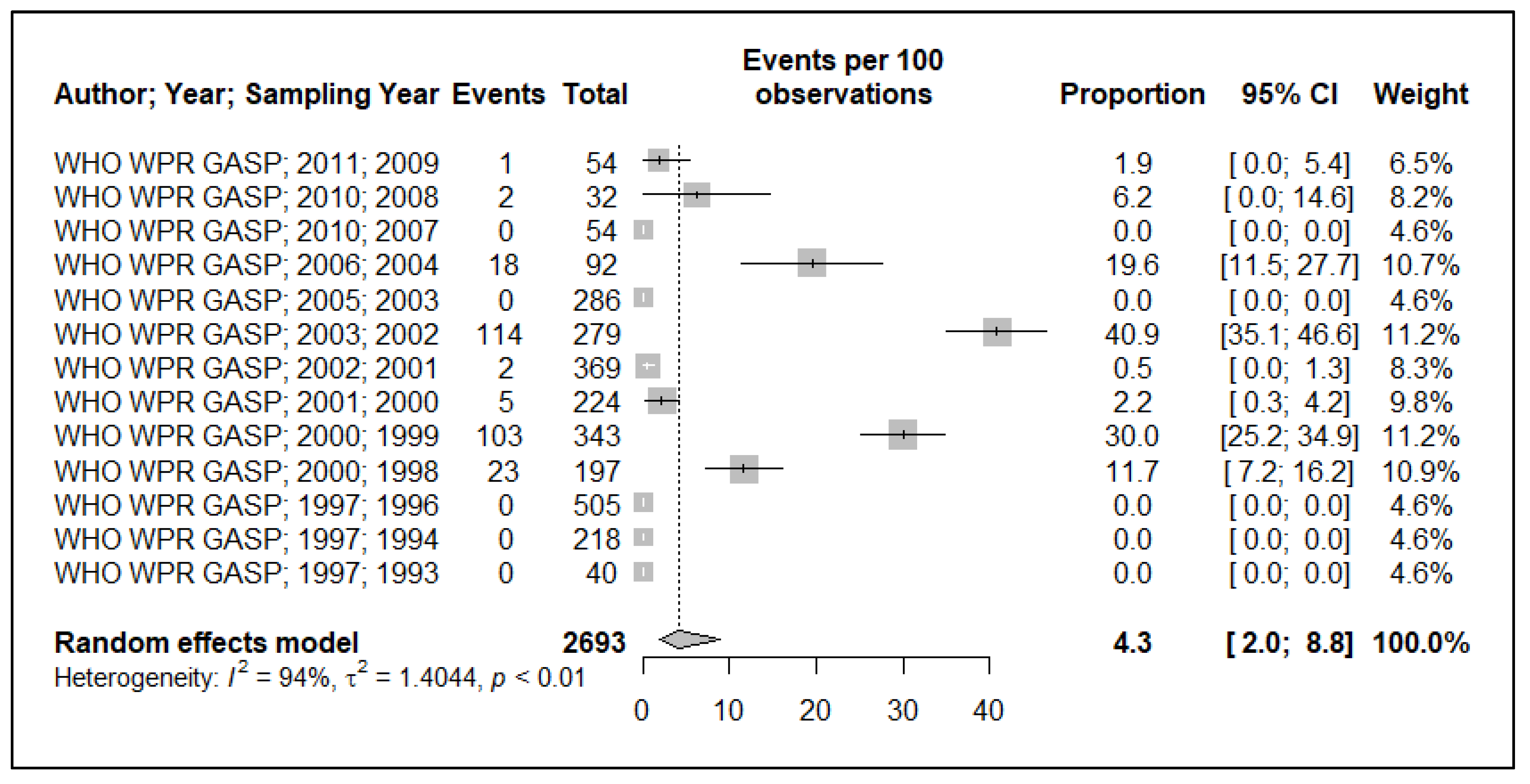
3.3. Quinolone Resistance
3.4. Aminoglycoside Resistance
3.5. Cephalosporin Resistance
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whiley, M.D.; Goire, N.; Lahra, M.M.; Donovan, B.; Limnios, E.A.; Nissen, D.M.; Sloots, P.T. The ticking time bomb: Escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J. Antimicrob. Chemother. 2012, 67, 2059–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemo, M.; Shafer, M.W. Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Młynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Młynarczyk, G.; Majewski, S. Multiresistant Neisseria gonorrhoeae: A new threat in second decade of the XXI century. Med. Microbiol. Immunol. 2020, 209, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Whiley, D.M.; Lahra, M.M.; Unemo, M. Prospects of untreatable gonorrhea and ways forward. Future Microbiol. 2015, 10, 313–316. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae. 2012. Available online: https://apps.who.int/iris/handle/10665/44863 (accessed on 14 December 2020).
- WHO. Global Action Plan on Antimicrobial Resistance. 2015; Cited 2020. Available online: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 14 December 2020).
- Tapsal, J. Antimicrobial Resistance in Neisseria Gonorrhoeae; WHO: Geneva, Switzerland, 2001.
- Unemo, M.; Ballard, R.; Ison, C.; Lewis, L.; Ndowa, F.; Peeling, R. Laboratory Diagnosis of Sexually Transmitted Infections, Including Human Immunodeficiency Virus; WHO, Ed.; World Health Organization: Geneva, Switzerland, 2013.
- WHO. Guidelines for the Treatment of Neisseria gonorrhoeae. 2016; Cited 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/246114/9789241549691-eng.pdf;jsessionid=CDF3A406C819BC7FBA92DFB2F48DB6D9?sequence=1 (accessed on 14 December 2020).
- Whiley, D.M.; Trembizki, E.; Buckley, C.; Freeman, K.; Baird, R.W.; Beaman, M.; Chen, M.; Donovan, B.; Kundu, R.L.; Fairley, C.K.; et al. Molecular Antimicrobial Resistance Surveillance for Neisseria gonorrhoeae, Northern Territory, Australia. Emerg. Infect. Dis. 2017, 23, 1478–1485. [Google Scholar] [CrossRef] [Green Version]
- WHO. Review of National Treatment Guidelines for Sexually Transmitted Infections in the Western Pacific Region; WHO: Geneva, Switzerland, 2018.
- Unemo, M.; Lahra, M.M.; Cole, M.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.R.; Ramon-Pardo, P.; Bolan, G.; Wi, T. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex. Health 2019, 16, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, T.; Nand, D.; David, N.; Bauri, M.; Carney, R.; Htin, K.C.W.; Shwe, Y.Y.; Gurung, A.; Mahiane, G.; Ishikawa, N.; et al. Prevalence of syphilis, gonorrhoea and chlamydia in women in Fiji, the Federated States of Micronesia, Papua New Guinea and Samoa, 1995–2017: Spectrum-STI model estimates. West. Pac. Surveill. Response J. 2020, 11, 29–40. [Google Scholar] [CrossRef]
- Kelly-Hanku, A.; Willie, B.; Weikum, D.; Boli Neo, R.; Kupul, M.; Coy, K.; Hou, P.; Aeno, H.; Ase, S.; Gabuzzi, J.; et al. Kauntim mi tu: Multi-Site Summary Report from the Key Population Integrated Bio-Behavioural Survey, Papua New Guinea; Papua New Guinea Institute of Medical Research and Kirby Institute; UNSW Sydney: Goroka, Papua New Guinea, 2018. [Google Scholar]
- Vallely, A.; Page, A.; Shannon Dias, S.; Siba, P.; Lupiwa, T.; Law, G.; Millan, J.; Wilson, P.D.; Murray, M.J.; Toole, M.; et al. The Prevalence of Sexually Transmitted Infections in Papua New Guinea: A Systematic Review and Meta-Analysis. PLoS ONE 2010, 5, e15586. [Google Scholar] [CrossRef] [Green Version]
- Toliman, J.P.; Lupiwa, T.; Law, J.G.; Reeder, C.J.; Siba, M.P. Neisseria gonorrhoeae isolates from four centres in Papua New Guinea remain susceptible to amoxycillin-clavulanate therapy. PNG Med. J. 2010, 53, 15–20. [Google Scholar]
- NDoH. Standard Treatment Guidelines For Common Illness of Adults in Papua New Guinea. A Manual for Nurses, Health Extension Officers and Doctors, National Department of Health; P.N.G. National Department of Health, Ed.; National Deaprtment of Health: Port Moresby, Papua New Guinea, 2012.
- NDoH. Papua New Guinea Standard Management of Sexually Transmitted Infections and Genital Conditions; NDoH: Port Moresby, Papua New Guinea, 2019.
- PRISMA. Transparent Reporting of Systematic Reviews and Meta-Analyses. Cited 2020. Available online: http://www.prisma-statement.org (accessed on 14 December 2020).
- National Library of Medicine. National Library of Medicine. National Center for Biotechnology Information. 2020; Cited 2020. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 14 December 2020).
- EMBASE. Cited. 2020. Available online: https://www.embase.com/#search (accessed on 14 December 2020).
- The EndNote Team. EndNote; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, 964–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Western Pacific Region Gonococcal Antimicrobial Surveillance Programme. Surveillance of Antibiotic Susceptibility of Neisseria gonorrhoeae in the WHO Western Pacific region 1992-4. Genitourin. Med. 1997, 73, 355–361. [Google Scholar] [CrossRef] [Green Version]
- The WHO Western Pacific Region Gonococcal Antimicrobial Surveillance Programme. Antimicrobial resistance in gonococci, WHO Western Pacific Region, 1996. Commun. Dis. Intell. 1997, 21, 349–353. [Google Scholar]
- The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 1998. Commun. Dis. Intell. 2000, 24, 1–4. [Google Scholar]
- The WHO Western Pacific Region Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 1999. Commun. Dis. Intell. 2000, 24, 269–271. [Google Scholar]
- The WHO Western Pacific Gonococcal antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2000. Commun. Dis. Intell. 2001, 35, 274–277. [Google Scholar]
- The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2001. Commun. Dis. Intell. 2002, 26, 541–545. [Google Scholar]
- WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2002. Commun. Dis. Intell. 2003, 27, 488–491. [Google Scholar]
- The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the World Health Organization Western Pacific Region, 2003. Commun. Dis. Intell. 2005, 29, 62–64. [Google Scholar]
- The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2004. Commun. Dis. Intell. 2006, 30, 129–132. [Google Scholar]
- The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of Antibiotic Resistance in Neisseria gonorrhoeae in The Who Western Pacific Region, 2006. Commun. Dis. Intell. 2008, 32, 48–51. [Google Scholar]
- The WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programmes. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2007–2008. Commun. Dis. Intell. 2010, 34, 1–7. [Google Scholar]
- The WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programmes. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2009. Commun. Dis. Intell. 2011, 35, 2–7. [Google Scholar]
- Hudson, B.J.; van der Meijden, W.I.; Lupiwa, T.; Howard, P.; Tabua, T.; Tapsall, J.W.; Phillips, E.A.; Lennox, V.A.; Backhouse, J.L.; Pyakalyia, T. A survey of sexually transmitted diseases in five STD clinics in Papua New Guinea. Papua New Guin. Med. J. 1994, 37, 152–160. [Google Scholar]
- Toliman, P.; Yoannes, M.; Toto, B.; Koata, A. Gonococcal Antimicrobial Susceptibility Survey Goroka, Eastern Highlands Province; Papua New Guinea Institute of Medical Research: Goroka, Papua New Guinea, 2016. [Google Scholar]
- The WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programmes. Surveillance of Antibiotic Resistance in Neisseria Gonorrhoeae in The Who Western Pacific And South East Asian Regions, 2010. Commun. Dis. Intell. 2012, 36, 95–100. [Google Scholar]
- Lahra, M.M.; Enriquez, R.; Robert George, C.R.R. Australian Gonococcal Surveillance Programme Annual Report, 2017. Commun. Dis. Intell. 2018, 15, 43. [Google Scholar] [CrossRef]
- Arya, R.; Antonisamy, B.; Kumar, S. Sample size estimation in prevalence studies. Indian J. Pediatrics 2012, 79, 1482–1488. [Google Scholar] [CrossRef]
- WHO. Gonococcal Antimicrobial Resistance in the Western Pacific Region. 2017. Available online: https://apps.who.int/iris/rest/bitstreams/1148004/retrieve (accessed on 14 December 2020).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willie, B.; Sweeney, E.L.; Badman, S.G.; Chatfield, M.; Vallely, A.J.; Kelly-Hanku, A.; Whiley, D.M. The Prevalence of Antimicrobial Resistant Neisseria gonorrhoeae in Papua New Guinea: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1520. https://doi.org/10.3390/ijerph19031520
Willie B, Sweeney EL, Badman SG, Chatfield M, Vallely AJ, Kelly-Hanku A, Whiley DM. The Prevalence of Antimicrobial Resistant Neisseria gonorrhoeae in Papua New Guinea: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1520. https://doi.org/10.3390/ijerph19031520
Chicago/Turabian StyleWillie, Barne, Emma L. Sweeney, Steven G. Badman, Mark Chatfield, Andrew J. Vallely, Angela Kelly-Hanku, and David M. Whiley. 2022. "The Prevalence of Antimicrobial Resistant Neisseria gonorrhoeae in Papua New Guinea: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1520. https://doi.org/10.3390/ijerph19031520
APA StyleWillie, B., Sweeney, E. L., Badman, S. G., Chatfield, M., Vallely, A. J., Kelly-Hanku, A., & Whiley, D. M. (2022). The Prevalence of Antimicrobial Resistant Neisseria gonorrhoeae in Papua New Guinea: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(3), 1520. https://doi.org/10.3390/ijerph19031520




