HAPPY MAMA Project (Part 2)—Maternal Distress and Self-Efficacy: A Pilot Randomized Controlled Field Trial
Abstract
:1. Introduction
2. Methods
2.1. Design of the Study
2.1.1. Ethical Approval and Registration of the Protocol
2.1.2. Eligibility Criteria for Participants
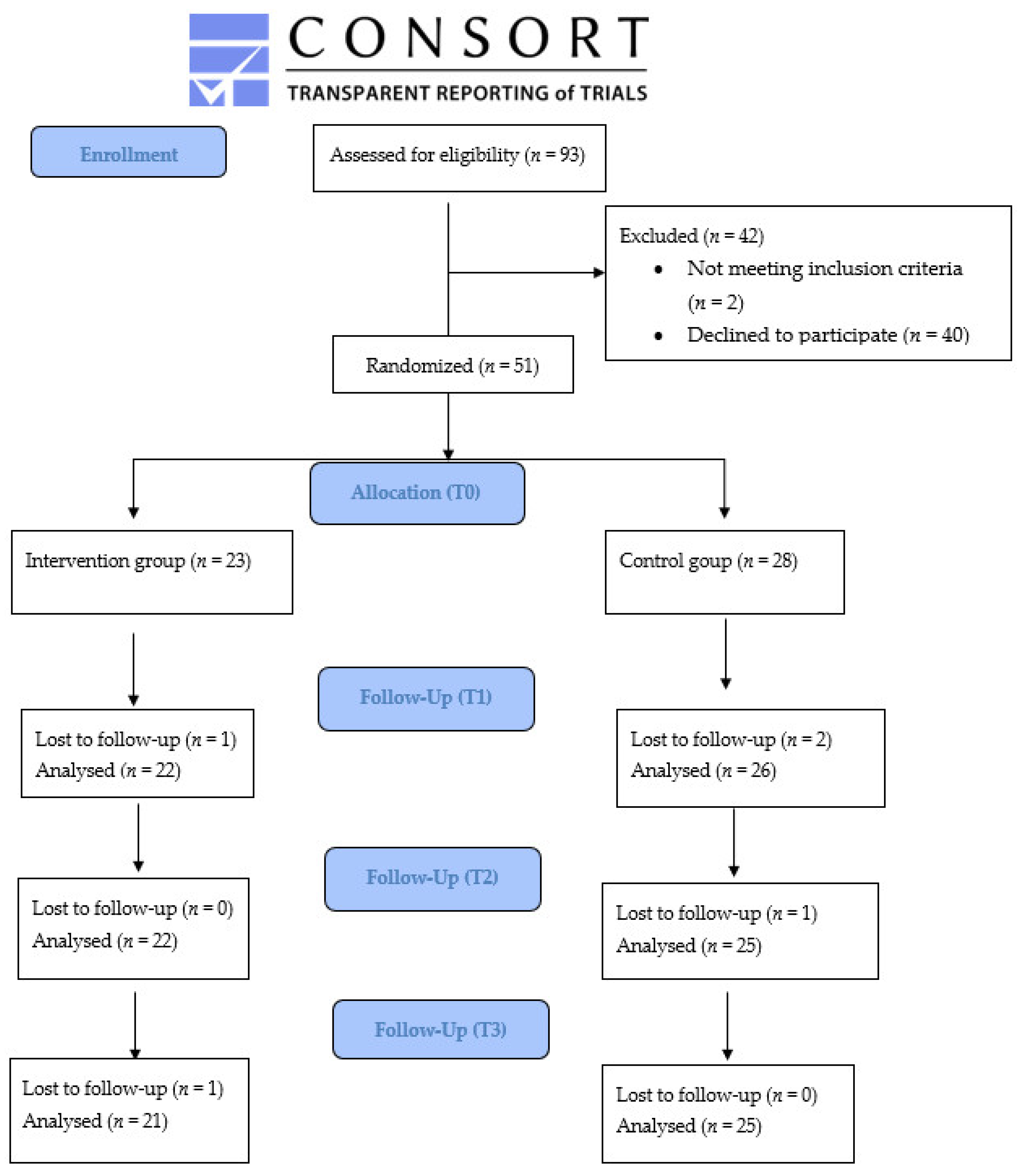
2.1.3. Randomization and Blinding
- age (>34 years, 34 is the mean age of Italian women at childbirth) [38];
- vaginal delivery (Yes/No).
2.2. Data Collection
- At T0: about one week after the hospital delivery;
- At T1: about one months after the delivery and after the home intervention;
- At T2: three months after the delivery;
- At T3: six months after the delivery.
2.3. Questionnaire
- The Karitane Parenting Confidence Scale (KPCS) [40] was used. More precisely, the Italian version (KPCS-IT) validated by Mannocci et al. was included [41]. The KPCS scale measures perceived parental self-efficacy (PPSE), which is defined as ‘‘beliefs or judgments a parent holds of their capabilities to organize and execute a set of tasks related to parenting a child’’ [42]. The 15-item scale, based on self-efficacy theory [15], was developed to assess the PPSE of parents with infants aged 0–12 months. The factor analysis has revealed a three-factor structure: efficacy, support, and child development. This 15-item questionnaire was scored on a five-point Likert scale, where 0 = No, hardly ever; 1 = No, not very often; 2 = Yes, some of the time; 3 = Yes, most of the time). The internal consistency of the questionnaires KPCS-IT was estimated as 0.801 [41].
- The Parental Stress Scale (PSS) [42] was used; more precisely, the Italian version (PSS-IT) validated by Mannocci et al. [41]. The PSS scale consisted of 18 items rated on a 5-point Likert scale (1 = low agree/5 = strong agree) The total score was obtained by summing up the value for each item. A higher score indicates a higher level of parental stress. The internal consistency of the PSS-IT studied by Mannocci et al. reported a Cronbach’s alpha = 0.862 [41].
- The Italian version of the Edinburgh Postnatal Depression Scale (EPDS) [43,44,45]. The EPDS version published by Benvenuti et al. [43] is used to measure maternal depressive symptoms. The EPDS is a self-report screening measure used to detect symptoms of postpartum depression. Scores >12 on the EPDS are correlated with a diagnosis of major depressive disorder (MDD) [46].
2.4. HAPPY MAMA Intervention
2.4.1. Personnel Involved in the Intervention
2.4.2. Intervention
- Listening and establishing relationship phases
- 2.
- Analysis of the problems
- 3.
- Assessment
- 4.
- Definition of the problem and the goal of the intervention
2.4.3. Sample Size
- average depression score measured with EPDS after childbirth is equal to mean = 5.1 and SD = 2.96 [43];
- hypothesis: SD is similar in the “IG” and the standardized difference (effect size) of EPDS will be 0.1 ≤ d ≤0.3 (small effect) [49], namely that the EPDS mean in the IG was lower 4.2 ≤ mean EPDS ≤ 4.8. The hypothesis of a small effect size was chosen because it is the first time that the HAPPY MAMA intervention was carried out and the effects are unknown. The small effect observed in the literature for other similar interventions was also considered [27,51];
- the level of significance and power of the study are 95% and 80%, respectively.
2.5. Statistical Methods
3. Results
3.1. KPCS Score
3.2. PSS Score
3.3. EPDS Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, C.A. The trauma of birth. Health Care Women Int. 2017, 38, 999–1010. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wickrama, K.A.S. Maternal life stress and health during the first 3 years postpartum. Women Health 2017, 58, 565–582. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Li, Q. Integrative Review of Research on General Health Status and Prevalence of Common Physical Health Conditions of Women After Childbirth. Women’s Health Issues 2008, 18, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Begley, C.; Clarke, M. Determinants of breastfeeding initiation in Ireland. Ir. J. Med. Sci. 2015, 185, 663–668. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, M.W.; McCabe, J.E. Postpartum Depression: Current Status and Future Directions. Annu. Rev. Clin. Psychol. 2013, 9, 379–407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Fowles, E.R.; Walker, L.O. Continuing education module: Postpartum maternal health care in the United States: A critical review. J. Périnat. Educ. 2006, 15, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, R.; de Visser, R.; Ayers, S. Not identifying with postnatal depression: A qualitative study of women’s postnatal symptoms of distress and need for support. J. Psychosom. Obstet. Gynecol. 2015, 36, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potharst, E.S.; Aktar, E.; Rexwinkel, M.; Rigterink, M.; Bögels, S.M. Mindful with Your Baby: Feasibility, Acceptability, and Effects of a Mindful Parenting Group Training for Mothers and Their Babies in a Mental Health Context. Mindfulness 2017, 8, 1236–1250. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Folkman, S. Cognitive Theories of Stress and the Issue of Circularity. In Dynamics of Stress: Physiological, Psychological and Social Perspectives; Appley, M.H., Trumbull, R., Eds.; Springer: Berlin, Germany, 1986; pp. 63–80. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Bennett, A.E.; Kearney, J.M. Factors Associated with Maternal Wellbeing at Four Months Post-Partum in Ireland. Nutrients 2018, 10, 609. [Google Scholar] [CrossRef] [Green Version]
- Soysa, C.K.; Wilcomb, C.J. Mindfulness, Self-compassion, Self-efficacy, and Gender as Predictors of Depression, Anxiety, Stress, and Well-being. Mindfulness 2015, 6, 217–226. [Google Scholar] [CrossRef]
- Firth, A.M.; Cavallini, I.; Sütterlin, S.; Lugo, R.G. Mindfulness and self-efficacy in pain perception, stress and academic performance. The influence of mindfulness on cognitive processes. Psychol. Res. Behav. Manag. 2019, 12, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Adv. Behav. Res. Ther. 1978, 1, 139–161. [Google Scholar] [CrossRef]
- Bandura, A. Regulation of cognitive processes through perceived self-efficacy. Dev. Psychol. 1989, 25, 729–735. [Google Scholar] [CrossRef]
- Bandura, A.; Freeman, W.H.; Lightsey, R. Self-Efficacy: The Exercise of Control. J. Cogn. Psychother. 1999, 13, 158–166. [Google Scholar] [CrossRef]
- Leahy-Warren, P.; McCarthy, G. Maternal parental self-efficacy in the postpartum period. Midwifery 2011, 27, 802–810. [Google Scholar] [CrossRef]
- Coleman, P.K.; Karraker, K.H. Maternal self-efficacy beliefs, competence in parenting, and toddlers’ behavior and developmental status. Infant Ment. Health J. 2003, 24, 126–148. [Google Scholar] [CrossRef]
- Troutman, B.; Moran, T.E.; Arndt, S.; Johnson, R.F.; Chmielewski, M. Development of parenting self-efficacy in mothers of infants with high negative emotionality. Infant Ment. Health J. 2012, 33, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Leahy-Warren, P. First-time mothers: Social support and confidence in infant care. J. Adv. Nurs. 2005, 50, 479–488. [Google Scholar] [CrossRef]
- Sanders, M.R.; Wooley, M.L. The relationship between maternal self-efficacy and parenting practices: Implications for parent training. Child Care Health Dev. 2005, 31, 65–73. [Google Scholar] [CrossRef]
- Ngai, F.-W.; Chan, S.W.-C.; Ip, W.-Y. Predictors and Correlates of Maternal Role Competence and Satisfaction. Nurs. Res. 2010, 59, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.G.; Teasdale, J.D.; Segal, Z.V.; Kabat-Zinn, J. The Mindful way Through Depression: Freeing Yourself from Chronic Unhappiness; Guilford Press: New York, NY, USA, 2007. [Google Scholar]
- Hughes, A.; Williams, M.; Bardacke, N.; Duncan, L.G.; Dimidjian, S.; Goodman, S.H. Mindfulness approaches to childbirth and parenting. Br. J. Midwifery 2009, 17, 630–635. [Google Scholar] [CrossRef]
- Dhillon, A.; Sparkes, E.; Duarte, R.V. Mindfulness-Based Interventions During Pregnancy: A Systematic Review and Meta-analysis. Mindfulness 2017, 8, 1421–1437. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Macbeth, A. The Effectiveness of Mindfulness-Based Interventions on Maternal Perinatal Mental Health Outcomes: A Systematic Review. Mindfulness 2017, 8, 823–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Blasco, J.; Viguer, P.; Rodrigo, M.F. Effects of a mindfulness-based intervention on psychological distress, well-being, and maternal self-efficacy in breast-feeding mothers: Results of a pilot study. Arch. Women’s Ment. Health 2013, 16, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Coates, R.; Ayers, S.; De Visser, R. Women’s experiences of postnatal distress: A qualitative study. BMC Pregnancy Childbirth 2014, 14, 359. [Google Scholar] [CrossRef] [Green Version]
- Tarkka, M.; Paunonen, M.; Laippala, P. Factors related to successful breast feeding by first-time mothers when the child is 3 months old. J. Adv. Nurs. 1999, 29, 113–118. [Google Scholar] [CrossRef]
- Jones, T.L.; Prinz, R.J. Potential roles of parental self-efficacy in parent and child adjustment: A review. Clin. Psychol. Rev. 2005, 25, 341–363. [Google Scholar] [CrossRef]
- Coleman, P.K.; Karraker, K.H. Self-Efficacy and Parenting Quality: Findings and Future Applications. Dev. Rev. 1998, 18, 47–85. [Google Scholar] [CrossRef]
- Montigny, F.; Lacharité, C. Perceived parental efficacy: Concept analysis. J. Adv. Nurs. 2005, 49, 387–396. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, F.C.; Lubchenco, L.O. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967, 71, 159–163. [Google Scholar] [CrossRef]
- Apgar, V. A proposal for a new method of evaluation of the newborn infant. Curr. Res. Anesth. Analg. 1953, 32, 260–267. [Google Scholar] [CrossRef]
- Brown, S.; Lumley, J. Physical health problems after childbirth and maternal depression at six to seven months postpartum. BJOG: Int. J. Obstet. Gynaecol. 2000, 107, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-H.; Lai, J.C.-Y.; Hwang, S.-J.; Huang, N.; Chou, Y.-J.; Chien, L.-Y. Understanding the relationship between cesarean birth and stress, anxiety, and depression after childbirth: A nationwide cohort study. Birth 2017, 44, 369–376. [Google Scholar] [CrossRef]
- Crncec, R.; Barnett, B.; Matthey, S. Development of an instrument to assess perceived self-efficacy in the parents of infants. Res. Nurs. Health 2008, 31, 442–453. [Google Scholar] [CrossRef]
- Mannocci, A.; Massimi, A.; Scaglietta, F.; Ciavardini, S.; Scollo, M.; Scaglione, C.; La Torre, G. HAPPY MAMA Project (PART 1). Assessing the Reliability of the Italian Karitane Parenting Confidence Scale (KPCS-IT) and Parental Stress Scale (PSS-IT): A Cross-Sectional Study among Mothers Who Gave Birth in the Last 12 Months. Int. J. Environ. Res. Public Health 2021, 18, 4066. [Google Scholar] [CrossRef]
- Berry, J.O.; Jones, W.H. The Parental Stress Scale: Initial Psychometric Evidence. J. Soc. Pers. Relatsh. 1995, 12, 463–472. [Google Scholar] [CrossRef]
- Benvenuti, P.; Ferrara, M.; Niccolai, C.; Valoriani, V.; Cox, J.L. The Edinburgh Postnatal Depression Scale: Validation for an Italian sample. J. Affect. Disord. 1999, 53, 137–141. [Google Scholar] [CrossRef]
- Carpiniello, B.; Pariante, C.M.; Serri, F.; Costa, G.; Carta, M.G. Validation of the Edinburgh Postnatal Depression Scale in Italy. J. Psychosom. Obstet. Gynecol. 1997, 18, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry J. Ment. Sci. 1987, 150, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Jardri, R.; Pelta, J.; Maron, M.; Thomas, P.; Delion, P.; Codaccioni, X.; Goudemand, M. Predictive validation study of the Edinburgh Postnatal Depression Scale in the first week after delivery and risk analysis for postnatal depression. J. Affect. Disord. 2006, 93, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nezu, A.M.; Nezu, C.M.; D’Zurilla, T.J. Problem-Solving Therapy: A Treatment Manual; Springer Publishing Company: New York, NY, USA, 2013. [Google Scholar]
- Goldfried, M.R.; Davison, G.C. Clinical Behavior Therapy; Holt, Rinehart & Winston: New York, NY, USA, 1976. [Google Scholar]
- Bell, M.L.; Whitehead, A.L.; A Julious, S. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin. Epidemiol. 2018, 10, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Missler, M.; Van Straten, A.; Denissen, J.; Donker, T.; Beijers, R. Effectiveness of a psycho-educational intervention for expecting parents to prevent postpartum parenting stress, depression and anxiety: A randomized controlled trial. BMC Pregnancy Childbirth 2020, 20, 658. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministery of Health, Evento nascita, Il Rapporto Con l’analisi Dei Dati CeDAP. 2016. Available online: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=3882 (accessed on 13 March 2021).
- Italian Ministry of Health. Puerperio. Retrieved. Available online: http://www.salute.gov.it/portale/donna/dettaglioContenutiDonna.jsp?lingua=italiano&id=4481&area=Salute%20donna&menu=nascita (accessed on 13 March 2021).
- Kristensen, I.H.; Simonsen, M.; Trillingsgaard, T.; Pontoppidan, M.; Kronborg, H. First-time mothers’ confidence mood and stress in the first months postpartum. A cohort study. Sex. Reprod. Health 2018, 17, 43–49. [Google Scholar] [CrossRef]
- Albanese, A.M.; Russo, G.R.; Geller, P.A. The role of parental self-efficacy in parent and child well-being: A systematic review of associated outcomes. Child Care Health Dev. 2019, 45, 333–363. [Google Scholar] [CrossRef]
- Masarik, A.S.; Conger, R.D. Stress and child development: A review of the Family Stress Model. Curr. Opin. Psychol. 2017, 13, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Abidin, R. Parenting Stress Index: Manual; Pediatric Psychology Press: Charlottesville, VA, USA, 1983. [Google Scholar]
- Shorey, S.; Chan, S.W.C.; Chong, Y.S.; He, H.-G. A randomized controlled trial of the effectiveness of a postnatal psychoeducation programme on self-efficacy, social support and postnatal depression among primiparas. J. Adv. Nurs. 2015, 71, 1260–1273. [Google Scholar] [CrossRef]
- Barnes, C.R.; Adamson-Macedo, E. Perceived Maternal Parenting Self Efficacy (PMP S-E) tool: Development and validation with mothers of hospitalized preterm neonates. J. Adv. Nurs. 2007, 60, 550–560. [Google Scholar] [CrossRef]
- Mohammadi, F.; Kohan, S.; Farzi, S.; Khosravi1, M.; Heidari, Z. The effect of pregnancy training classes based on bandura self-efficacy theory on postpartum depression and anxiety and type of delivery. J. Educ. Health Promot. 2021, 10, 273. [Google Scholar] [CrossRef]
- ISTAT. Livelli Di Istruzione E Ritorni Occupazionali. Anno 21019. 2020. Available online: https://www.istat.it/it/files//2020/07/Livelli-di-istruzione-e-ritorni-occupazionali.pdf (accessed on 8 November 2021).
- Wittkowski, A.; Garrett, C.; Calam, R.; Weisberg, D. Self-Report Measures of Parental Self-Efficacy: A Systematic Review of the Current Literature. J. Child Fam. Stud. 2017, 26, 2960–2978. [Google Scholar] [CrossRef]
- Milani, H.S.; Amiri, P.; Mohseny, M.; Abadi, A.; Vaziri, S.M.; Vejdani, M. Postpartum home care and its effects on mothers’ health: A clinical trial. J. Res. Med. Sci. 2017, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.B.; Rossi, D.M.; Sander, T.M. Women’s successful transition to motherhood during the early postnatal period: A qualitative systematic review of postnatal and midwifery home care literature. Midwifery 2019, 79, 102552. [Google Scholar] [CrossRef] [PubMed]
- Lambermon, F.; Vandenbussche, F.; Dedding, C.; van Duijnhoven, N. Maternal self-care in the early postpartum period: An integrative review. Midwifery 2020, 90, 102799. [Google Scholar] [CrossRef] [PubMed]
- Spelke, B.; Werner, E. The Fourth Trimester of Pregnancy: Committing to Maternal Health and Well-Being Postpartum. Rhode Isl. Med. J. 2018, 101, 30–33. [Google Scholar]
- Tully, K.P.; Stuebe, A.M.; Verbiest, S.B. The fourth trimester: A critical transition period with unmet maternal health needs. Am. J. Obstet. Gynecol. 2017, 217, 37–41. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Aune, I.; Dahlberg, U.; Haugan, G. Health-promoting influences among Norwegian women following early postnatal home visit by a midwife. Nord. J. Nurs. Res. 2018, 38, 177–186. [Google Scholar] [CrossRef]
- Ministry of Health. Azioni Regionali Su Accordo Percorso Nascita. Available online: http://www.salute.gov.it/portale/donna/dettaglioContenutiDonna.jsp?lingua=italiano&id=4483&area=Salute%20donna&menu=nascita (accessed on 18 April 2018).
| Variables | Total (N = 51) | CG (N = 28) | IG (N = 23) | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Educational level | Middle school | 3 (5.9) | 1 (3.6) | 2 (8.7) |
| High school | 7 (13.7) | 3 (10.7) | 4 (17.4) | |
| University | 41 (80.4) | 24 (85.7) | 17 (73.9) | |
| Employment | Worker | 42 (82.3) | 26 (92.9) | 16 (69.6) |
| Housewife | 2 (3.9) | 0 (0.0) | 2 (8.7) | |
| Student | 1 (2.0) | 0 (0.0) | 1 (4.3) | |
| No worker | 6 (11.8) | 2 (7.1) | 4 (17.4) | |
| Number of children | 1 | 34 (66.7) | 18 (64.3) | 16 (69.6) |
| 2 | 13 (25.5) | 9 (32.1) | 4 (17.4) | |
| >2 | 4 (7.8) | 1 (3.6) | 3 (13.0) | |
| Type of birth | Vaginal birth | 37 (72.5) | 22 (78.6) | 15 (65.2) |
| Caesarean section | 14 (27.5) | 6 (21.4) | 8 (34.8) | |
| Support received during pregnancy | No | 10 (19.6) | 6 (21.4) | 4 (17.4) |
| Yes (Hospital/ASL) | 37 (72.6) | 19 (67.9) | 18 (78.3) | |
| Yes (Private) | 4 (7.8) | 3 (10.7) | 1 (4.3) | |
| Visits/counselling post-partum | No | 33 (64.7) | 19 (67.9) | 14 (60.9) |
| Midwife/childcare | 13 (25.5) | 7 (25.0) | 6 (26.1) | |
| Clinician | 5 (9.8) | 2 (7.1) | 3 (13.0) | |
| Kind of breastfeeding | Exclusive | 11 (21.6) | 6 (21.4) | 5 (21.7) |
| Partial | 39 (76.4) | 22 (78.6) | 17 (74.0) | |
| No (bottle) | 1 (2.0) | 0 (0.0) | 1 (4.3) | |
| Number of feedings | 4–5/day | 2 (3.9) | 1 (3.6) | 1 (4.3) |
| 6–8/day | 31 (60.8) | 18 (64.3) | 13 (56.6) | |
| 9–10/day | 13 (25.5) | 6 (21.4) | 7 (30.4) | |
| >10/day | 5 (9.8) | 3 (10.7) | 2 (8.7) | |
| Variables | (Follow-Up) | CG | IG | p * |
|---|---|---|---|---|
| Mean ± SD Median (Min-Max) | Mean ± SD Median (Min-Max) | |||
| KPCS | (T0) | 35.8 ± 6.0 36.5 (15.0–45.0) | 35.0 ± 5.8 35.0 (24.0–44.0) | 0.544 |
| (T1) | 37.0 ± 4.9 37.5 (27.0–44.0) | 39.7 ± 4.2 41.0 (32.0–45.0) | 0.039 | |
| (T2) | 39.3 ± 3.6 40.0 (32.0–44.0) | 39.0 ± 5.6 41.0 (22.0–45.0) | 0.614 | |
| (T3) | 39.7 ± 4.2 41.0 (28.0–45.0) | 40.5 ± 3.8 41.0 (32.0–45.0) | 0.458 | |
| PSS | (T0) | 31.1 ± 6.2 30.0 (21.0–45.0) | 34.3 ± 7.5 33.0 (24.0–50.0) | 0.105 |
| (T1) | 32.9 ± 9.1 29.5 (22.0–55.0) | 27.7 ± 5.6 26.0 (20.0–39.0) | 0.024 | |
| (T2) | 31.1 ± 7.7 30.5 (20.0–51.0) | 31.3 ± 9.7 30.0 (18.0–52.0) | 0.864 | |
| (T3) | 30.1 ± 8.9 28.5 (19.0–54.0) | 30.0 ± 8.8 28.0 (18.0–48.0) | 0.894 | |
| EPDS | (T0) | 8.4 ± 4.1 8.0 (0.0–19.0) | 8.0 ± 3.2 8.0 (1.0–16.0) | 0.924 |
| (T1) | 7.5 ± 4.1 8.0 (0.0–15.0) | 6.3 ± 3.5 7.0 (0.0–12.0) | 0.246 | |
| (T2) | 6.9 ± 3.5 7.0 (1.0–14.0) | 6.2 ± 4.2 6.0 (0.0–13.0) | 0.575 | |
| (T3) | 6.6 ± 5.0 7.0 (0.0–15.0) | 6.0 ± 4.8 7.0 (0.0–16.0) | 0.575 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannocci, A.; Ciavardini, S.; Mattioli, F.; Massimi, A.; D’Egidio, V.; Lia, L.; Scaglietta, F.; Giannini, A.; Antico, R.; Dorelli, B.; et al. HAPPY MAMA Project (Part 2)—Maternal Distress and Self-Efficacy: A Pilot Randomized Controlled Field Trial. Int. J. Environ. Res. Public Health 2022, 19, 1461. https://doi.org/10.3390/ijerph19031461
Mannocci A, Ciavardini S, Mattioli F, Massimi A, D’Egidio V, Lia L, Scaglietta F, Giannini A, Antico R, Dorelli B, et al. HAPPY MAMA Project (Part 2)—Maternal Distress and Self-Efficacy: A Pilot Randomized Controlled Field Trial. International Journal of Environmental Research and Public Health. 2022; 19(3):1461. https://doi.org/10.3390/ijerph19031461
Chicago/Turabian StyleMannocci, Alice, Sara Ciavardini, Federica Mattioli, Azzurra Massimi, Valeria D’Egidio, Lorenza Lia, Franca Scaglietta, Andrea Giannini, Roberta Antico, Barbara Dorelli, and et al. 2022. "HAPPY MAMA Project (Part 2)—Maternal Distress and Self-Efficacy: A Pilot Randomized Controlled Field Trial" International Journal of Environmental Research and Public Health 19, no. 3: 1461. https://doi.org/10.3390/ijerph19031461
APA StyleMannocci, A., Ciavardini, S., Mattioli, F., Massimi, A., D’Egidio, V., Lia, L., Scaglietta, F., Giannini, A., Antico, R., Dorelli, B., Svelato, A., Orfeo, L., Benedetti Panici, P., Ragusa, A., La Torre, G., & Group, H. M. (2022). HAPPY MAMA Project (Part 2)—Maternal Distress and Self-Efficacy: A Pilot Randomized Controlled Field Trial. International Journal of Environmental Research and Public Health, 19(3), 1461. https://doi.org/10.3390/ijerph19031461









