Accumulation Mechanism and Risk Assessment of Artemisia selengensis Seedling In Vitro with the Hydroponic Culture under Cadmium Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture In Vitro and Cd Treatment
2.2. Analysis of Plant Biomass and Cd Content
2.3. Determination of Malondialdehyde (MDA) Content in Plants
2.4. Extraction of Subcellular Components of Root
2.5. Analysis of Transmission Electron Microscopy (TEM)
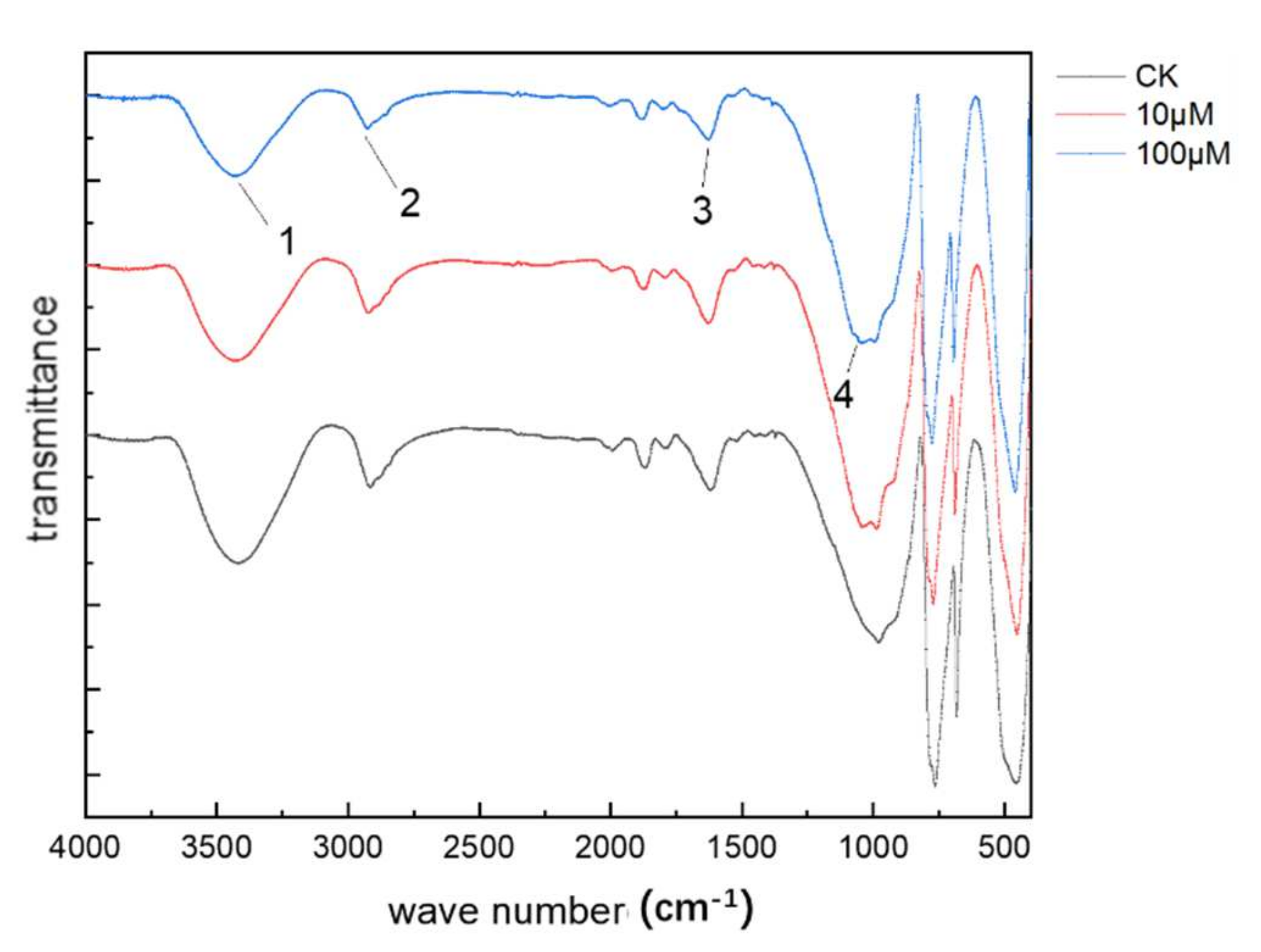
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.7. Statistical Analysis
3. Results
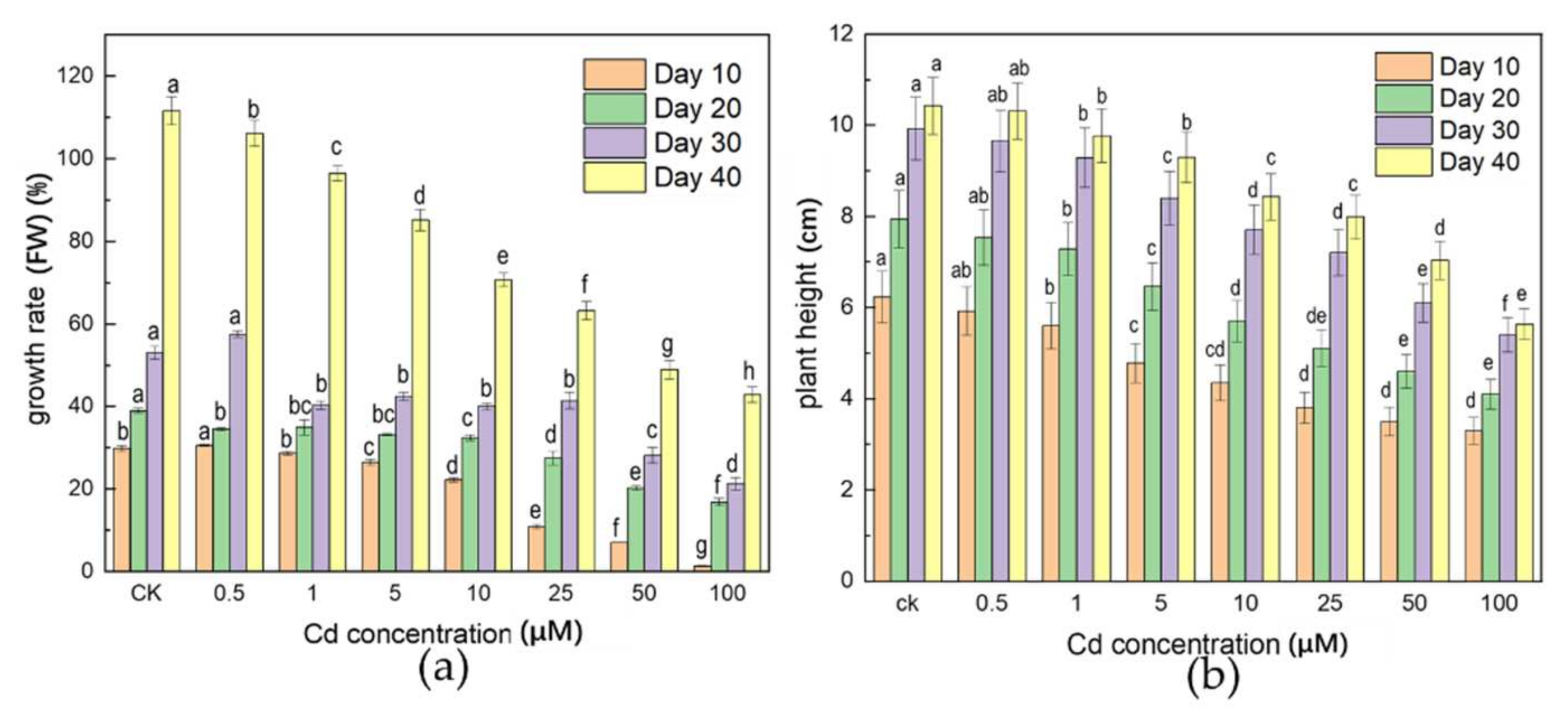
3.1. Effects of Cd Stress on Plant Biomass
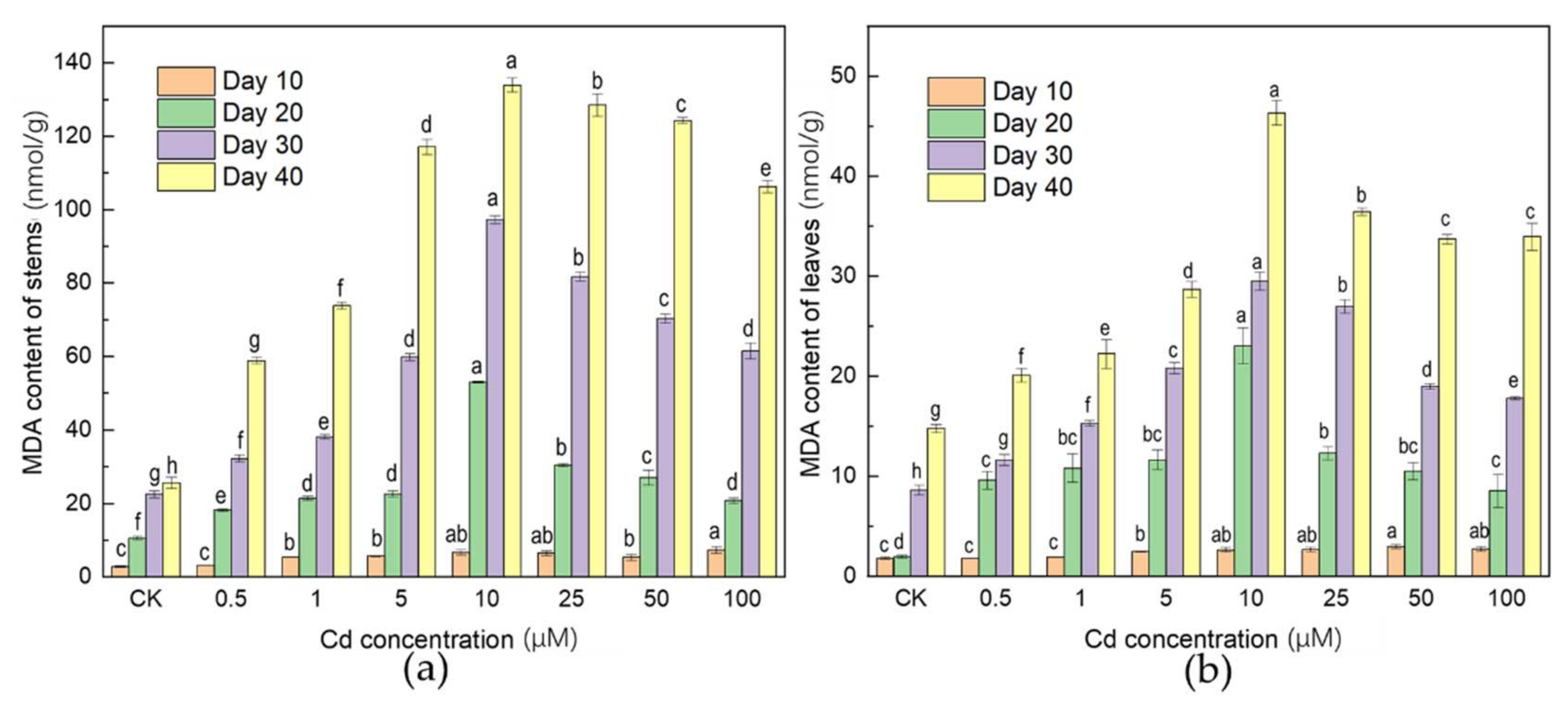
3.2. Effects of Cd Stress on MDA Concentration
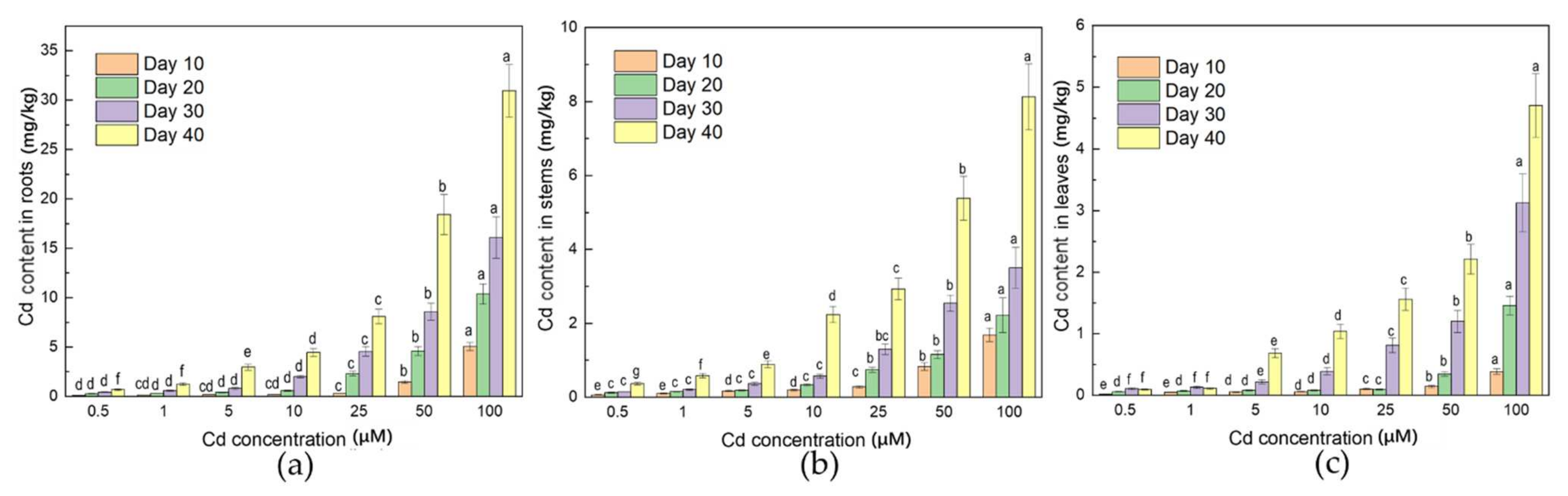
3.3. Distribution of Cd in Plant
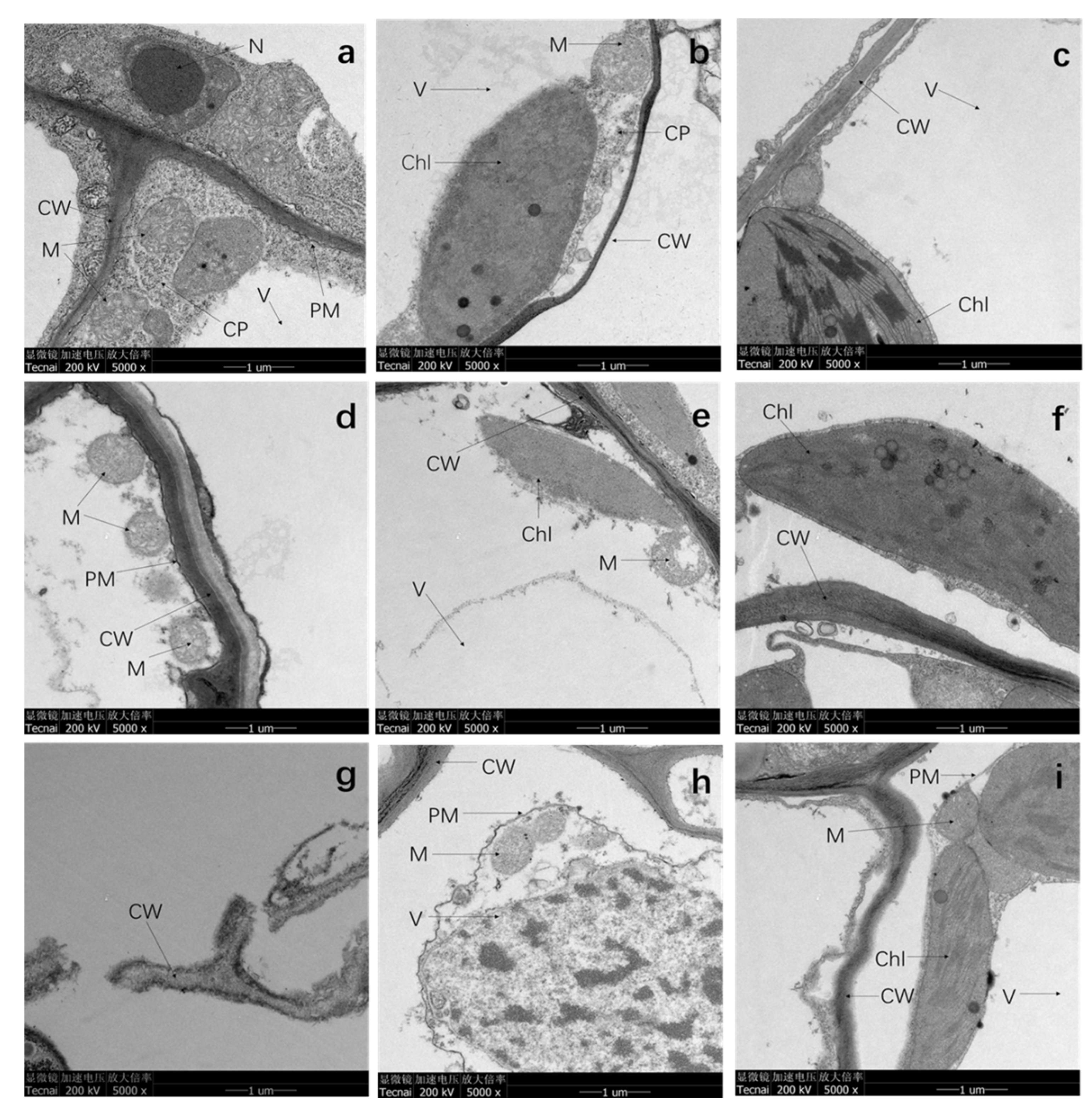
3.4. TEM Analysis
3.5. Subcellular Distribution of Cd in Plant Roots
3.6. FTIR Analysis
3.7. Health Risk Assessments
4. Discussion
4.1. Effects of Cd Stress on Plant Growth
4.2. Oxidative Stress Response of Plant to Cd Stress
4.3. Roots Could Be the Main Part of Cd Enrichment
4.4. Cd Stress Deformed the Subcellular Structure of Plants
4.5. Root Cell Wall Might Play a Key Role in the Cd Accumulation
4.6. The Health Risk Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Li, K.; Yu, H.; Li, T.; Chen, G.; Huang, F. Cadmium accumulation characteristics of low-cadmium rice (Oryza sativa L.) line and F1 hybrids grown in cadmium-contaminated soils. Environ. Sci. Pollut. Res. Int. 2017, 24, 17566–17576. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, H.; He, L.; Wang, Q.; Sheng, X. Cd immobilization and reduced tissue Cd accumulation of rice (Oryza sativa wuyun-23) in the presence of heavy metal-resistant bacteria. Ecotoxicol. Environ. Saf. 2017, 138, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, R.; Yan, X.; Liang, X.; Xu, Y. Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2020, 194, 110369–110378. [Google Scholar] [CrossRef]
- Li, L.Z.; Tu, C.; Wu, L.H.; Peijnenburg, W.J.G.M.; Ebbs, S.; Luo, Y.M. Pathways of root uptake and membrane transport of Cd2+ in the zinc/cadmium hyperaccumulating plant Sedum plumbizincicola. Environ. Toxicol. Chem. 2017, 36, 1038–1046. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhang, S.L.; Wu, K.J.; Li, R.; He, X.R.; He, C.H.; Wei, H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. under Cd stress. Ecotoxicol. Environ. Saf. 2019, 187, 109790–109799. [Google Scholar] [CrossRef]
- Zeng, L.; Zhu, T.; Gao, Y.; Wang, Y.; Ning, C.; Bjorn, L.O. Effects of Ca addition on the uptake, translocation, and distribution of Cd in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2017, 139, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Zare, A.A.; Khoshgoftarmanesh, A.H.; Malakouti, M.J.; Bahrami, H.A.; Chaney, R.L. Root uptake and shoot accumulation of cadmium by lettuce at various Cd: Zn ratios in nutrient solution. Ecotoxicol. Environ. Saf. 2018, 148, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef] [Green Version]
- Alves, L.R.; Andre, G.; Priscila. Heavy metals in agricultural soils: From plants to our daily life. Cientifica 2016, 44, 346–361. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Chen, H.S.; Huang, Q.Y.; Liu, L.N.; Cai, P.; Liang, W.; Ming, L.I. Poultry Manure Compost Alleviates the Phytotoxicity of Soil Cadmium: Influence on Growth of Pakchoi (Brassica chinensis L.). Pedosphere 2010, 1, 63–70. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Mu, Y.X.; He, C.T.; Fu, H.L.; Wang, X.S.; Gong, F.Y.; Yang, Z.Y. Cadmium and lead accumulations and agronomic quality of a newly bred pollution-safe cultivar (psc) of water spinach. Environ. Sci. Pollut. Res. Int. 2018, 25, 11152–11162. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Q.; Zhang, Z.; Xu, J.; Yang, J.; Wong, M.H. Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J. Sci. Food Agric. 2005, 85, 147. [Google Scholar] [CrossRef]
- Duan, G.; Shao, G.; Zhong, T.; Chen, H.; Zhao, F.J. Genotypic and environmental variations in grain cadmium and arsenic concentrations among a panel of high yielding rice cultivars. Rice 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Huang, X.; Sun, L.; Li, S.; Chen, Y.; Cao, X.; Wang, W.; Dai, J.; Rinnan, R. Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 2020, 241, 125065. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nan, Y.; Mu, G.; Shen, Z.; Shinwari, K.I. Screening for cd-safe cultivars of chinese cabbage and a preliminary study on the mechanisms of Cd accumulation. Int. J. Environ. Res. Public Health 2017, 14, 395. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lu, H.D.; Muhammad, U.; Han, J.Z.; Wei, Z.H.; Lu, Z.X. Ultrasound-assisted extraction of polysaccharides from Artemisia selengensis turcz and its antioxidant and anticancer activities. J. Food Sci. Technol. 2016, 53, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Wang, Y.; Zhu, H.; Chen, Q. Fingerprint profile of active components for Artemisia selengensis Turcz by HPLC-PAD combined with chemometrics. Food Chem. 2011, 125, 1064–1071. [Google Scholar] [CrossRef]
- Xu, C.; Sun, X.L.; Hu, P.J.; Cheng, Y.; Lou, Y.M.; Wu, L.H.; Peter, C. Concentrations of heavy metals in suburban horticultural soils and their uptake by Artemisia selengensis. Pedosphere 2015, 25, 82–91. [Google Scholar] [CrossRef]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, C.; Zhang, Q.; Li, Y.; Chen, Z.; Li, M. Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control 2010, 21, 1757–1763. [Google Scholar] [CrossRef]
- Haider, F.U.; Cai, L.; Coulter, J.A.; Cheema, S.A.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887–111908. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, Y.; Zeng, H.; Zhou, X.; Peng, L.; Zeng, Q. Phytoextraction of cadmium-contaminated soil and potential of regenerated tobacco biomass for recovery of cadmium. Sci. Rep. 2017, 7, 7210–7219. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Seth, C.S.; Misra, V.; Chauhan, L.K.S.; Singh, R.R. Genotoxicity of cadmium on root meristem cells of Allium cepa: Cytogenetic and Comet assay approach. Ecotoxicol. Environ. Saf. 2008, 71, 711–716. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, Q.; Li, S.; Zhu, M.; Yu, W. Toxicological effects of single and joint sulfamethazine and cadmium stress in soil on pakchoi (Brassica chinensis L.). Chemosphere 2021, 263, 128296–128305. [Google Scholar] [CrossRef] [PubMed]
- Ulusu, Y.; Öztürk, L.; Elmasta, S.M. Antioxidant capacity and cadmium accumulation in parsley seedlings exposed to cadmium stress. Russ. J. Plant Physiol. 2017, 64, 883–888. [Google Scholar] [CrossRef]
- Dai, H.; Yang, Z. Variation in Cd accumulation among radish cultivars and identification of low-Cd cultivars. Environ. Sci. Pollut. Res. 2017, 24, 15116–15124. [Google Scholar] [CrossRef]
- Haisel, D.; Cyrusová, T.; Vaněk, T.; Podlipná, R. The effect of nanoparticles on the photosynthetic pigments in cadmium-zinc interactions. Environ. Sci. Pollut. Res. 2019, 26, 4147–4151. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qajjam, M.F.; Abbass, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar] [CrossRef]
- Delavari, P.; Baghizadeh, A.; Enteshari, S.; Kalantari, K.M.; Yazdanpanah, A.; Mousavi, E. The effects of salicylic acid on some of biochemical and morphological characteristic of Ocimum basilicucm under salinity stress. Aust. J. Basic Appl. Sci. 2010, 4, 4832–4845. [Google Scholar]
- Gutsch, A.; Sergeant, K.; Keunen, E.; Prinsen, E.; Cuypers, A. Does long-term cadmium exposure influence the composition of pectic polysaccharides in the cell wall of Medicago sativa stems? BMC Plant Biol. 2019, 19, 271–287. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Zhang, L.; Wang, L.; Zhou, C.; Shangguan., Y.; Yang, Y. Antioxidative enzymes activity and thiol metabolism in three leafy vegetables under cd stress. Ecotoxicol. Environ. Saf. 2019, 173, 214–224. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.W.; Li, W.S.; Tu, S.X.; Ding, Y.Z.; Feng, R.W. Differences in cadmium absorption by 71 leaf vegetable varieties from different families and genera and their health risk assessment. Ecotoxicol. Environ. Saf. 2019, 184, 109593–109600. [Google Scholar] [CrossRef]
- Guo, J.J.; Tan, X.; Fu, H.L.; Chen, J.X.; Lin, X.X.; Ma, Y.; Yang, Z.Y. Selection for Cd pollution-safe cultivars of Chinese kale (Brassica alboglabra L. H. Bailey) and biochemical mechanisms of the cultivar-dependent Cd accumulation involving in Cd subcellular distribution. J. Agric. Food Chem. 2018, 66, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Stolt, P.; Asp, H.; Hultin, S. Genetic variation in wheat cadmium accumulation on soils with different cadmium concentrations. J. Agron. Crop Sci. 2006, 192, 201–208. [Google Scholar] [CrossRef]
- Xin, J.L.; Huang, B.F.; Yang, Z.Y.; Yuan, J.G.; Zhang, Y.D. Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil 2013, 372, 431–444. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Y.; Sun, Y.; Liang, X.; Lin, D. Identification of pakchoi cultivars with low cadmium accumulation and soil factors that affect their cadmium uptake and translocation. Front. Environ. Sci. Eng. 2014, 8, 877–887. [Google Scholar] [CrossRef]
- Xue, M.; Zhou, Y.; Yang, Z.; Lin, B.; Yuan, J.; Shanshan, W.U. Comparisons in subcellular and biochemical behaviors of cadmium between low-Cd and high-Cd accumulation cultivars of pakchoi (Brassica chinensis L.). Front. Environ. Sci. Eng. 2014, 8, 226–238. [Google Scholar] [CrossRef]
- Xiao, L.; Guan, D.; Peart, M.R.; Chen, Y.; Li, Q.; Dai, J. The influence of bioavailable heavy metals and microbial parameters of soil on the metal accumulation in rice grain. Chemosphere 2017, 185, 868–878. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, F.; Yang, S.; Yan, C. Subcellular distribution and tolerance of cadmium in canna indica L. Ecotoxicol. Environ. Saf. 2019, 185, 109692–109699. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Kochian, L.V. Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol. Plant. 2010, 116, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Rafiq, M.T.; Aziz, R.; Yang, X.; Xiao, W.; Stoffella, P.J.; Saghir, A.; Azam, M.; Li, T. Phytoavailability of cadmium (Cd) to Pak choi (Brassica chinensis L.) grown in Chinese soils: A model to evaluate the impact of soil Cd pollution on potential dietary toxicity. PLoS ONE 2014, 9, e111461. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Farsaraei, S.; Moghaddam, M. Biochar application modified growth and physiological parameters of Ocimum ciliatum L. and reduced human risk assessment under cadmium stress. J. Hazard. Mater. 2020, 409, 124954–124965. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Lin, S.Z.; Lai, Z.X. Cadmium impairs iron homeostasis in Arabidopsis thaliana by increasing the polysaccharide contents and the iron-binding capacity of root cell walls. Plant Soil 2015, 392, 71–85. [Google Scholar] [CrossRef]
- Muschitz, A.; Riou, C.; Mollet, J.C.; Gloaguen, V.; Faugeron, C. Modifications of cell wall pectin in tomato cell suspension in response to cadmium and zinc. Acta Physiol. Plant. 2015, 37, 245–255. [Google Scholar] [CrossRef]
- Jia, W.; Lv, S.; Feng, J.; Li, J.; Li, Y.; Li, S. Morphophysiological characteristic analysis demonstrated the potential of sweet sorghum (Sorghum bicolor (L.) Moench) in the phytoremediation of cadmium-contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 18823–18831. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, B.; Wan, H.; Fang, X.; Yang, C. The differences of cell wall in roots between two contrasting soybean cultivars exposed to cadmium at young seedlings. Environ. Sci. Pollut. Res. 2018, 25, 29705–29714. [Google Scholar] [CrossRef]
- Ali, M.B.; Singh, N.; Shohael, A.M.; Hahn, E.J.; Paek, K.Y. Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci. 2006, 171, 147–154. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [Green Version]
- Konno, H.; Nakashima, S.; Katoh, K. Metal-tolerant moss Scopelophila cataractae accumulates copper in the cell wall pectin of the protonema. J. Plant Physiol. 2010, 167, 358–364. [Google Scholar] [CrossRef]
- Chen, G.C.; Liu, Y.Q.; Wang, R.M. Cadmium adsorption by willow root: The role of cell walls and their subfractions. Environ. Sci. Pollut. Res. 2013, 20, 5665–5672. [Google Scholar] [CrossRef]
- Yang, X.; Lin, R.; Zhang, W.; Xu, Y.; Wei, X.; Zhuo, C.; Qin, J.; Li, H. Comparison of Cd subcellular distribution and Cd detoxification between low/high Cd-accumulative rice cultivars and sea rice. Ecotoxicol. Environ. Saf. 2019, 185, 109698–109705. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yang, X.; Geng, G.; Wan, X.; Ma, R.; Zhang, Q.; Liang, Y. Absorption and subcellular distribution of cadmium in tea plant (Camellia sinensis cv. “Shuchazao”). Environ. Sci. Pollut. Res. Int. 2018, 25, 15357–15367. [Google Scholar] [CrossRef]
- Fu, X.; Dou, C.; Chen, Y.; Chen, X.; Shi, J.; Yu, M.; Xu, J. Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J. Hazard. Mater. 2011, 186, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Zavoi, S.; Fetea, F.; Ranga, F.; Pop, R.M.; Baciu, A.; Socaciu, C. Comparative fingerprint and extraction yield of medicinal herb phenolics with hepatoprotective potential, as determined by UV-Vis and FT-MIR spectroscopy. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.H.; Sheng, L.; Zhang, C.Y.; Deng, H.P. Physiological response of Arundo donax to cadmium stress by fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 88–91. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Ahmad, A.; Hadi, F.; Ali, N. Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L.) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int. J. Phytoremedia. 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuan, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef] [PubMed]

| Cd | Day 10 | Day 20 | Day 30 | Day 40 | ||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μM) | Leaf | Stem | Leaf | Stem | Leaf | Stem | Leaf | Stem |
| 0.5 | 0.07 | 0.31 | 0.30 | 0.68 | 0.40 | 0.80 | 0.55 | 2.00 |
| 1 | 0.17 | 0.50 | 0.37 | 0.81 | 0.73 | 1.07 | 0.64 | 3.17 |
| 5 | 0.30 | 0.96 | 0.45 | 1.06 | 1.21 | 2.25 | 3.77 | 4.90 |
| 10 | 0.32 | 1.12 | 0.44 | 1.86 | 2.13 | 3.04 | 5.65 | 12.15 |
| 25 | 0.57 | 1.55 | 0.90 | 4.78 | 4.46 | 7.64 | 8.62 | 16.00 |
| 50 | 0.79 | 4.71 | 1.89 | 6.32 | 5.47 | 10.10 | 12.47 | 29.38 |
| 100 | 2.23 | 9.04 | 8.01 | 14.72 | 17.26 | 26.71 | 25.67 | 44.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Kang, W.; Shen, M.; Chen, L.; Zhao, X.; Wang, Y.; Xu, S.; Ming, A.; Feng, T.; Deng, H.; et al. Accumulation Mechanism and Risk Assessment of Artemisia selengensis Seedling In Vitro with the Hydroponic Culture under Cadmium Pressure. Int. J. Environ. Res. Public Health 2022, 19, 1183. https://doi.org/10.3390/ijerph19031183
Tang T, Kang W, Shen M, Chen L, Zhao X, Wang Y, Xu S, Ming A, Feng T, Deng H, et al. Accumulation Mechanism and Risk Assessment of Artemisia selengensis Seedling In Vitro with the Hydroponic Culture under Cadmium Pressure. International Journal of Environmental Research and Public Health. 2022; 19(3):1183. https://doi.org/10.3390/ijerph19031183
Chicago/Turabian StyleTang, Tao, Wei Kang, Mi Shen, Lin Chen, Xude Zhao, Yongkui Wang, Shunwen Xu, Anhuai Ming, Tao Feng, Haiyan Deng, and et al. 2022. "Accumulation Mechanism and Risk Assessment of Artemisia selengensis Seedling In Vitro with the Hydroponic Culture under Cadmium Pressure" International Journal of Environmental Research and Public Health 19, no. 3: 1183. https://doi.org/10.3390/ijerph19031183
APA StyleTang, T., Kang, W., Shen, M., Chen, L., Zhao, X., Wang, Y., Xu, S., Ming, A., Feng, T., Deng, H., & Zheng, S. (2022). Accumulation Mechanism and Risk Assessment of Artemisia selengensis Seedling In Vitro with the Hydroponic Culture under Cadmium Pressure. International Journal of Environmental Research and Public Health, 19(3), 1183. https://doi.org/10.3390/ijerph19031183





