Shorter Incubation Period among Unvaccinated Delta Variant Coronavirus Disease 2019 Patients in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Unvaccinated COVID-19 Cases
2.3. Participant COVID-19 Cases with Delta Variant and Non-Delta Strains
2.4. Statistical Analysis
2.5. Ethical Approval
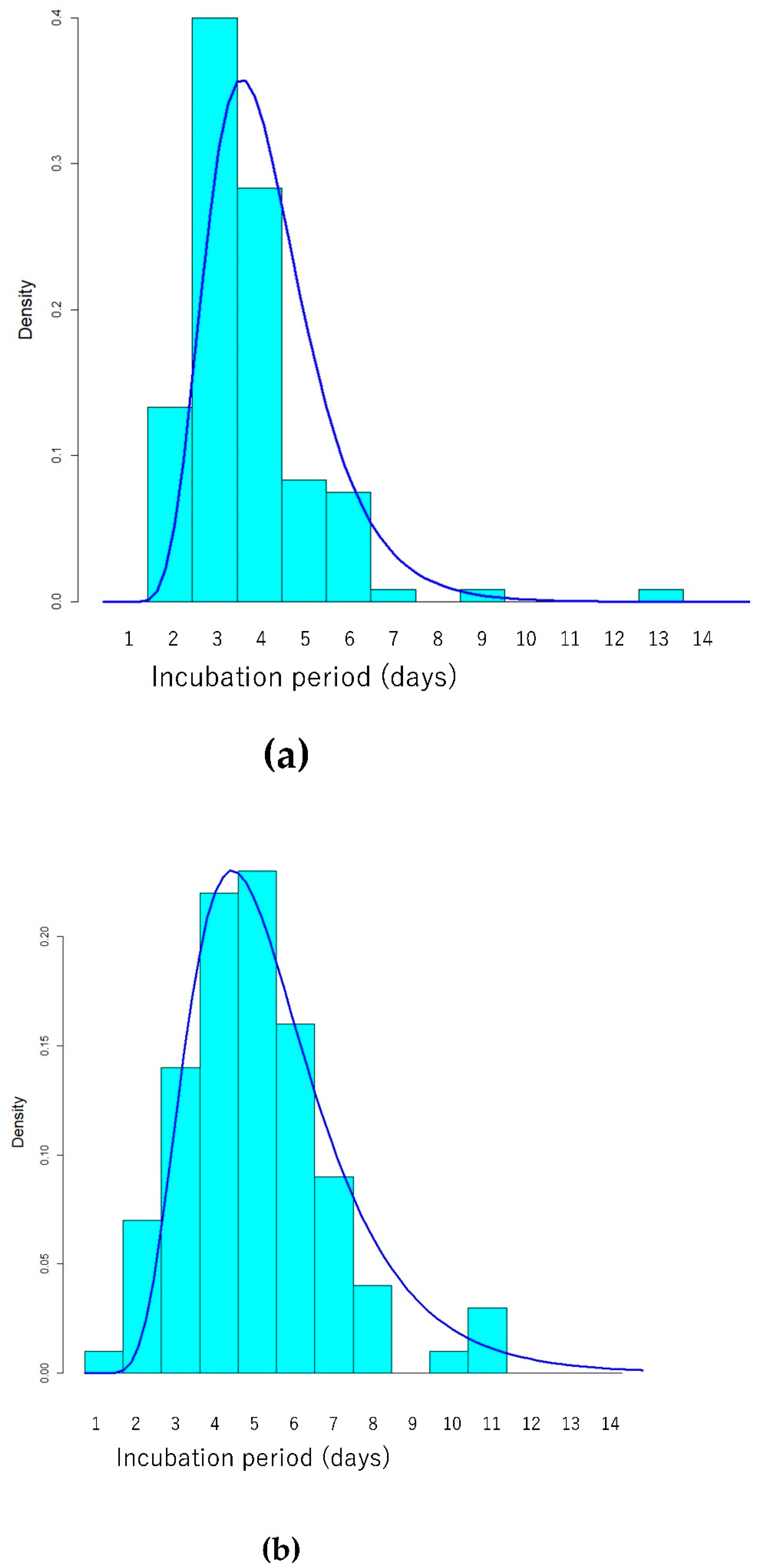
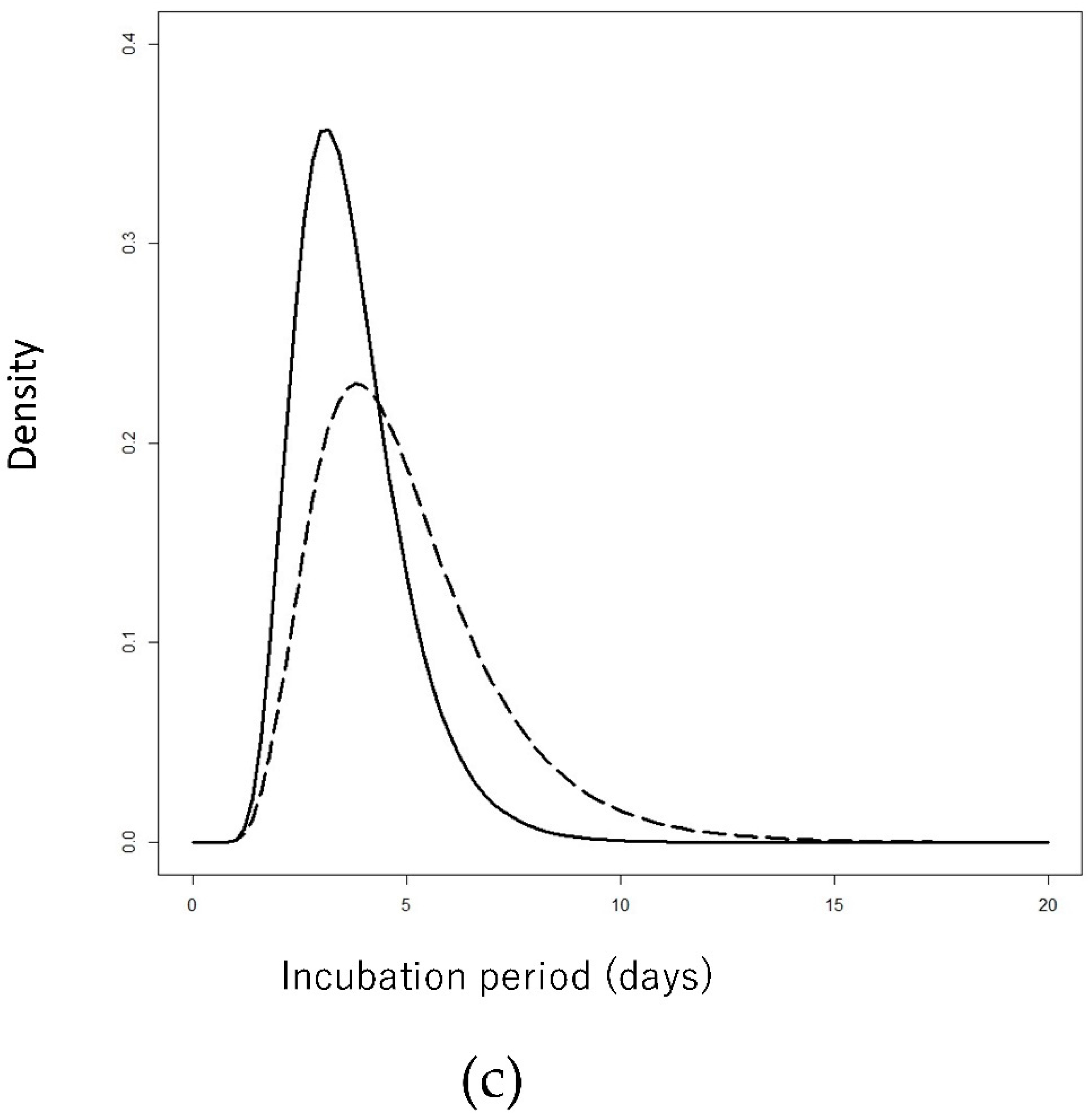
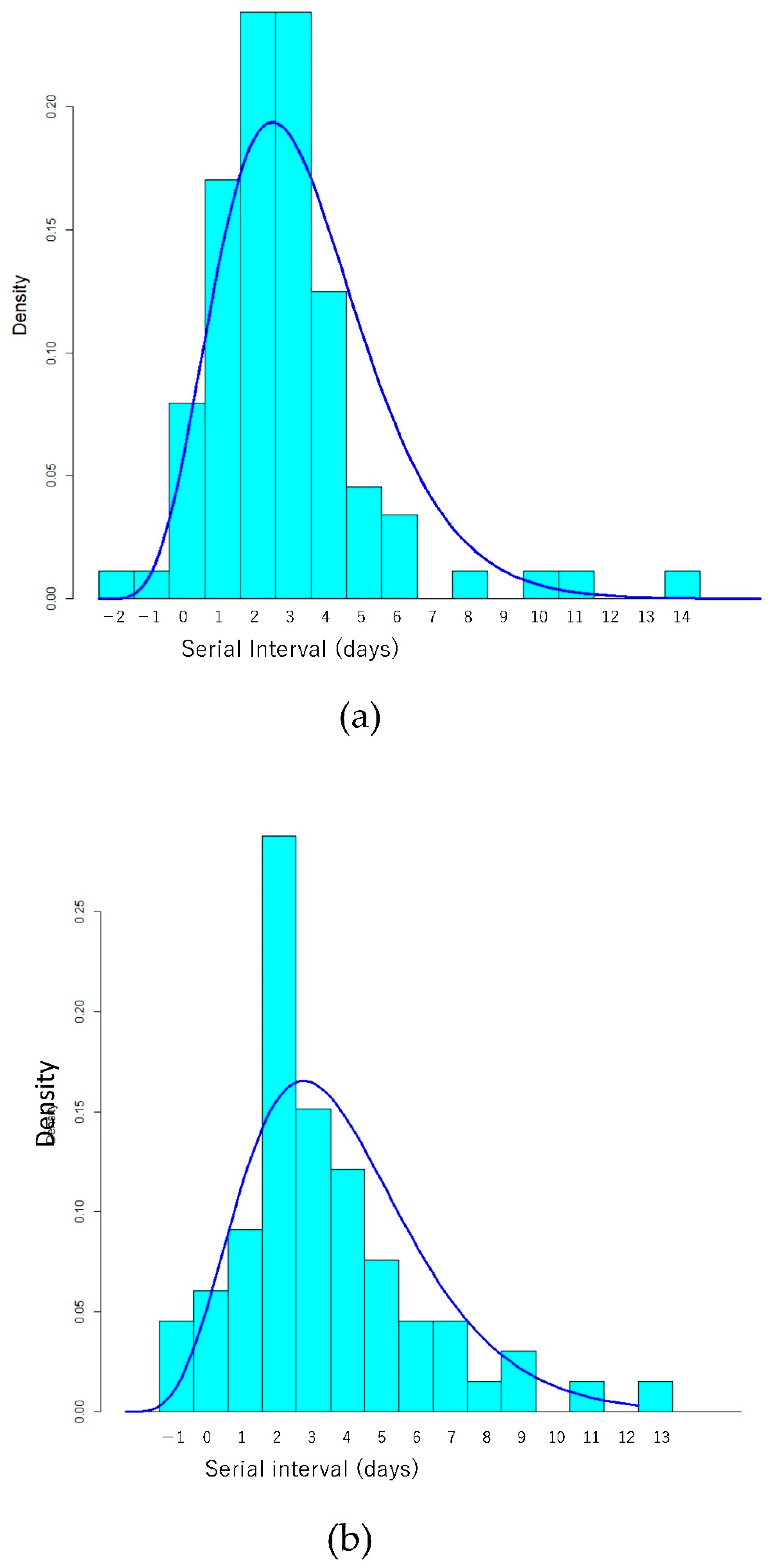
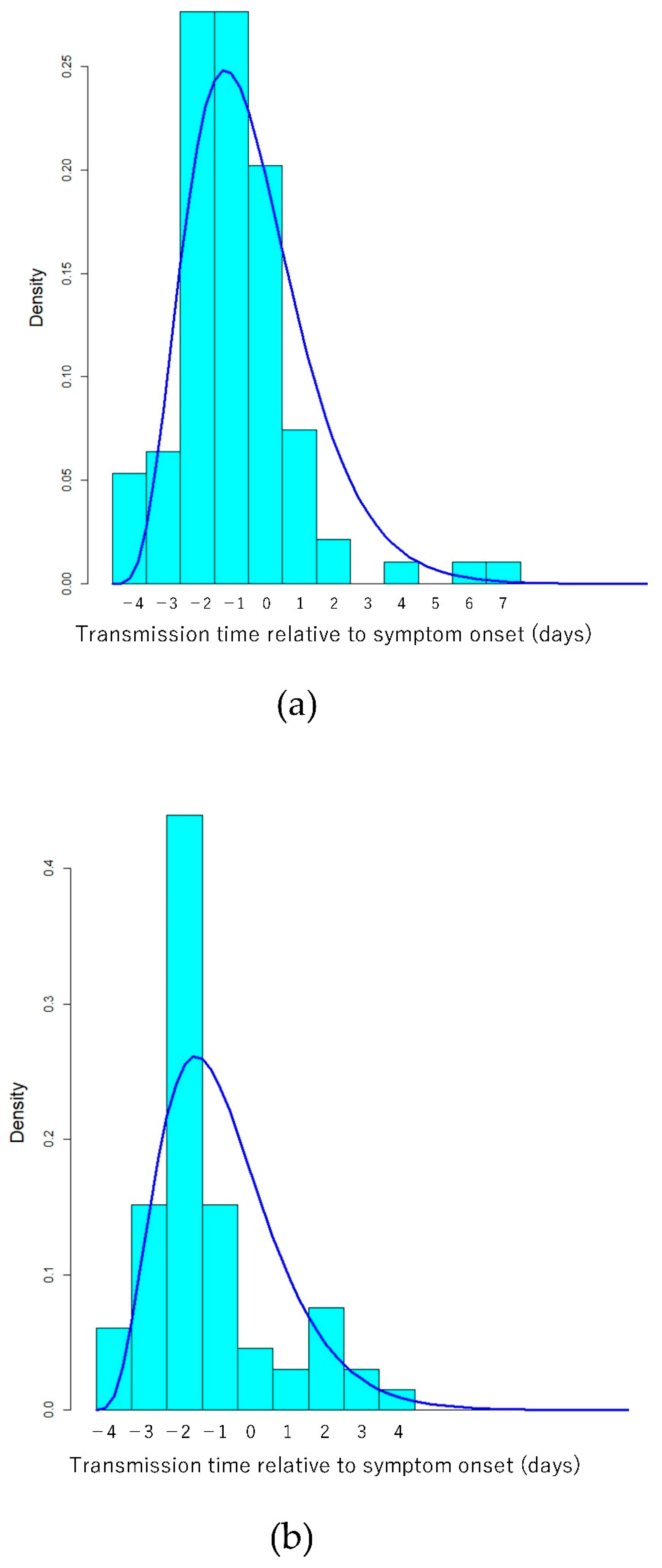
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 1 December 2021).
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef]
- Deng, X.; Garcia-Knight, M.A.; Khalid, M.M.; Servellita, V.; Wang, C.; Morris, M.K.; Sotomayor-González, A.; Glasner, D.R.; Reyes, K.R.; Gliwa, A.S.; et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell 2021, 184, 3426–3437.e8. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Update on COVID-19–13 October 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---13-october-2021 (accessed on 1 December 2020).
- Wang, Y.; Chen, R.; Hu, F.; Lan, Y.; Yang, Z.; Zhan, C.; Shi, J.; Deng, X.; Jiang, M.; Zhong, S.; et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine 2021, 40, 101129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, J.; Deng, A.; Zhang, Y.; Zhuang, Y.; Hu, T.; Li, J.; Tu, H.; Li, B.; Zhou, Y.; et al. Transmission Dynamics of an Outbreak of the COVID-19 Delta Variant B.1.617.2—Guangdong Province, China, May–June 2021. China CDC Wkly. 2021, 3, 584–586. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Infectious Diseases, Infectious Disease Epidemiology Center. Manual Conducting for Active Epidemiological Surveillance of Patients with Novel Coronavirus Infection (Provisional Version on May 29). January 2021 (in Japanese). Available online: https://www.niid.go.jp/niid/images/epi/corona/COVID19-02-210108.pdf (accessed on 15 December 2021).
- Centers for Disease Control and Prevention (CDC). Interim Guidance on Developing a COVID-19 Case Investigation & Contact Tracing Plan: Overview: Appendices. Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/appendix.html#contact (accessed on 1 December 2021).
- Centers for Disease Control and Prevention (CDC). National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Quarantine and Isolation (Updated 19 October 2021). Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html (accessed on 1 December 2021).
- World Health Organization. Contact Tracing in the Context of COVID-19. 2021. Available online: https://www.who.int/publications/i/item/contact-tracing-in-the-context-of-covid-19 (accessed on 1 December 2021).
- Tu, H.; Hu, K.; Zhang, M.; Zhuang, Y.; Song, T. Effectiveness of 14 day quarantine strategy: Chinese experience of prevention and control. BMJ 2021, 375, e066121. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- McAloon, C.; Collins, Á.; Hunt, K.; Barber, A.; Byrne, A.W.; Butler, F.; Casey, M.; Griffin, J.; Lane, E.; McEvoy, D.; et al. Incubation period of COVID-19: A rapid systematic review and meta-analysis of observational research. BMJ Open 2020, 10, e039652. [Google Scholar] [CrossRef]
- Xin, H.; Wong, J.Y.; Murphy, C.; Yeung, A.; Ali, S.T.; Wu, P.; Cowling, B.J. The incubation period distribution of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin. Infect. Dis. 2021, 73, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.E.; Li, Z.; Chiew, C.J.; Yong, S.E.; Toh, M.P.; Lee, V.J. Presymptomatic Transmission of SARS-CoV-2—Singapore, 23 January–16 March 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owusu, D.; Pomeroy, M.A.; Lewis, N.M.; Wadhwa, A.; Yousaf, A.R.; Whitaker, B.; Dietrich, E.; Hall, A.J.; Chu, V.; Thornburg, N.; et al. Persistent SARS-CoV-2 RNA Shedding Without Evidence of Infectiousness: A Cohort Study of Individuals With COVID-19. J. Infect. Dis. 2021, 224, 1362–1371. [Google Scholar] [CrossRef]
- Casey-Bryars, M.; Griffin, J.; McAloon, C.; Byrne, A.; Madden, J.; Mc Evoy, D.; Collins, Á.; Hunt, K.; Barber, A.; Butler, F.; et al. Presymptomatic transmission of SARS-CoV-2 infection: A secondary analysis using published data. BMJ Open 2021, 11, e041240. [Google Scholar] [CrossRef]
- National Institute of Infectious Diseases. Current Situation of Infection, 26 May 2021. Available online: https://www.niid.go.jp/niid/en/2019-ncov-e/10415-covid19-ab36th-en.html (accessed on 1 December 2021).
- COVID-19 Advisory Board of the Ministry of Health, Labor and Welfare. Current Situation of Infection and Others (in Japanese). Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00294.html (accessed on 1 December 2021).
- Ministry of Health, Labor and Welfare. Visualizing the Data: Information on COVID-19 Infections. Available online: https://covid19.mhlw.go.jp/en/ (accessed on 15 December 2021).
- Tindale, L.C.; Stockdale, J.E.; Coombe, M.; Garlock, E.S.; Lau, W.Y.V.; Saraswat, M.; Zhang, L.; Chen, D.; Wallinga, J.; Colijn, C. Evidence for transmission of COVID-19 prior to symptom onset. Elife 2020, 9, e57149. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowling, B.J.; Muller, M.P.; Wong, I.O.; Ho, L.M.; Louie, M.; McGeer, A.; Leung, G.M. Alternative methods of estimating an incubation distribution: Examples from severe acute respiratory syndrome. Epidemiology 2007, 18, 253–259. [Google Scholar] [CrossRef]
- Institute of Health of Ibaraki Prefectural Government. Situation of Tests on Variant Virus of SARS-CoV-2 (in Japanese). Available online: https://www.pref.ibaraki.jp/hokenfukushi/eiken/kikaku/covid-19_ibarakieiken_kensa.html (accessed on 1 December 2021).
- Government of Osaka Prefecture. Proof of Positive Cases in Mutation PCR Test (Screening Test), 8 June 2021 (in Japanese). Available online: https://www.pref.osaka.lg.jp/attach/23711/00400840/0608heni.pdf (accessed on 1 December 2021).
- Du, Z.; Xu, X.; Wu, Y.; Wang, L.; Cowling, B.J.; Meyers, L.A. Serial Interval of COVID-19 among Publicly Reported Confirmed Cases. Emerg Infect. Dis. 2020, 26, 1341–1343. [Google Scholar] [CrossRef]
- Li, L.; Han, Z.G.; Qin, P.Z.; Liu, W.H.; Yang, Z.; Chen, Z.Q.; Li, K.; Xie, C.J.; Ma, Y.; Wang, H.; et al. Transmission and containment of the SARS-CoV-2 Delta variant of concern in Guangzhou, China: A population-based study. PLoS Negl. Trop. Dis. 2022, 16, e0010048. [Google Scholar] [CrossRef] [PubMed]
- Pung, R.; Mak, T.M.; Kucharski, A.J.; Lee, V.J. Serial intervals in SARS-CoV-2 B.1.617.2 variant cases. Lancet 2021, 398, 837–838. [Google Scholar] [CrossRef]
- Hwang, H.; Lim, J.S.; Song, S.A.; Achangwa, C.; Sim, W.; Kim, G.; Ryu, S. Transmission dynamics of the Delta variant of SARS-CoV-2 infections in South Korea. J. Infect. Dis. 2021, jiab586, online ahead of print. [Google Scholar] [CrossRef]
- Nishiura, H.; Linton, N.M.; Akhmetzhanov, A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020, 93, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.K.; Liu, X.F.; Wu, Y.; Ali, S.T.; Du, Z.; Bosetti, P.; Lau, E.H.Y.; Cowling, B.J.; Wang, L. Reconstruction of Transmission Pairs for Novel Coronavirus Disease 2019 (COVID-19) in Mainland China: Estimation of Superspreading Events, Serial Interval, and Hazard of Infection. Clin. Infect. Dis. 2020, 71, 3163–3167. [Google Scholar] [CrossRef]
- Ali, S.T.; Wang, L.; Lau, E.H.Y.; Xu, X.K.; Du, Z.; Wu, Y.; Leung, G.M.; Cowling, B.J. Serial interval of SARS-CoV-2 was shortened over time by nonpharmaceutical interventions. Science 2020, 369, 1106–1109. [Google Scholar] [CrossRef]
- Siedner, M.J.; Boucau, J.; Gilbert, R.F.; Uddin, R.; Luu, J.; Haneuse, S.; Vyas, T.; Reynolds, Z.; Iyer, S.; Chamberlin, G.C.; et al. Duration of viral shedding and culture positivity with post-vaccination SARS-CoV-2 delta variant infections. JCI Insight 2021, e155483. [Google Scholar] [CrossRef]
- Hellewell, J.; Abbott, S.; Gimma, A.; Bosse, N.I.; Jarvis, C.I.; Russell, T.W.; Munday, J.D.; Kucharski, A.J.; Edmunds, W.J.; Funk, S.; et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health 2020, 8, e488–e496. [Google Scholar] [CrossRef] [Green Version]
- Fontal, A.; Bouma, M.J.; San-José, A.; López, L.; Pascual, M.; Rodó, X. Climatic signatures in the different COVID-19 pandemic waves across both hemispheres. Nat. Comput. Sci. 2021, 1, 655–665. [Google Scholar] [CrossRef]
- Qin, J.; You, C.; Lin, Q.; Hu, T.; Yu, S.; Zhou, X.H. Estimation of incubation period distribution of COVID-19 using disease onset forward time: A novel cross-sectional and forward follow-up study. Sci. Adv. 2020, 6, eabc1202. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, M.M.; Buchholz, U.; Corman, V.M.; Hoch, M.; Katz, K.; Marosevic, D.V.; Böhm, S.; Woudenberg, T.; Ackermann, N.; Konrad, R.; et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: A case series. Lancet Infect. Dis. 2020, 20, 920–928. [Google Scholar] [CrossRef]
- Allen, H.; Vusirikala, A.; Flannagan, J.; Twohig, K.A.; Zaidi, A.; Chudasama, D.; Lamagni, T.; Groves, N.; Turner, C.; Rawlinson, C.; et al. Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): National case-control study. Lancet Reg. Health Eur. 2022, 12, 100252. [Google Scholar] [CrossRef]

| Variables | N |
|---|---|
| Total | 224 |
| Sex | |
| Male | 156 |
| Female | 68 |
| Age | |
| ≤29 | 119 |
| ≥30 | 105 |
| Delta mutation | |
| Delta | 121 |
| Non-Delta | 103 |
| Parameters | Delta | Non-Delta | Mann–Whitney U Test | ||
|---|---|---|---|---|---|
| N | Mean | N | Mean | p-Value | |
| (Days) | (Days) | ||||
| Incubation period | 120 | 3.7 | 100 | 4.9 | 0.000 |
| Serial interval | 88 | 2.8 | 66 | 3.3 | 0.227 |
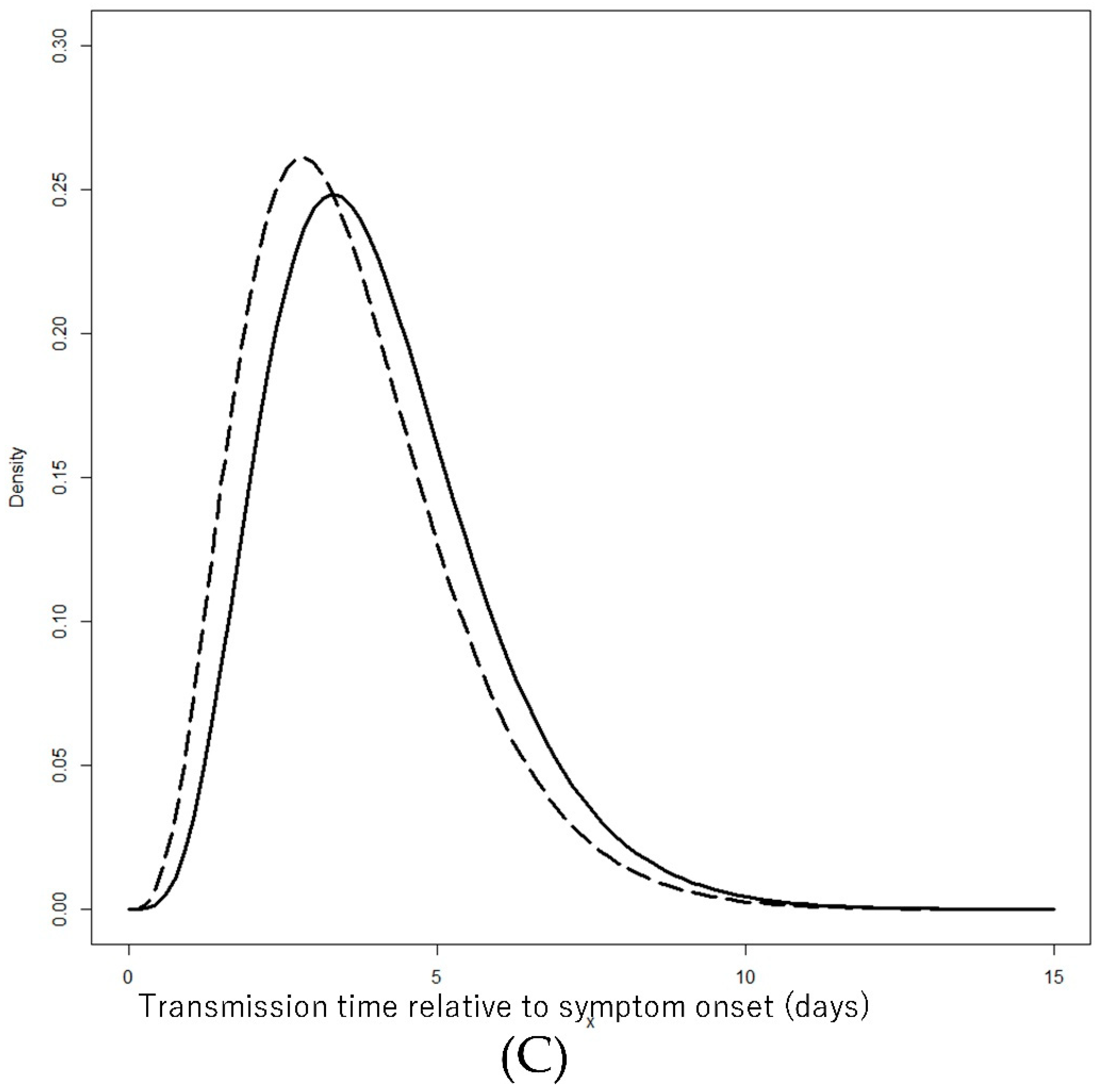
| Transmission time relative to onset | 94 | −0.94 | 66 | −1.39 | 0.012 |
| Parameters | Fitted Distribution | Delta | Non-Delta | ||
|---|---|---|---|---|---|
| Meanlog/Shape | Sdlog/Rate | Meanlog/Shape | Sdlog/Rate | ||
| Days (95% CI) | Days (95% CI) | Days (95% CI) | Days (95% CI) | ||
| Incubation period | Lognormal (Meanlog/Sdlog) | 1.25 (1.19–1.31) | 0.34 (0.30–0.39) | 1.52 (1.43–1.60) | 0.42(0.37–0.48) |
| Serial interval (added 3 days) | Gamma (Shape/Rate) | 7.1 (5.1–9.5) | 1.24 (0.88–1.67) | 6.1 (4.2–8.2) | 0.97 (2.6–4.0) |
| Transmission time relative to onset (added 5 days) | Gamma (Shape/Rate) | 5.5 (4.0–7.3) | 1.37 (0.98–1.81) | 4.7 (3.3–6.3) | 1.32(0.90–1.80) |
| Indicators | Delta | Non-Delta |
|---|---|---|
| Days (95% CI) | Days (95% CI) | |
| Mean | 3.7 (3.4–4.0) | 5.0 (4.5–5.6) |
| Median | 3.5 (3.3–3.7) | 4.6 (4.2–5.0) |
| 2.5th percentile | 1.8 (1.5–2.1) | 2.0 (1.6–2.4) |
| 97.5th percentile | 6.9 (5.9–8.0) | 10.4 (8.6–12.7) |
| Variables | Mean | Mono-Variable Mean Estimate (95% CI) | |
|---|---|---|---|
| N | (Days) | (Days) | |
| Total | 120 | 3.7 | 3.7 (3.4–4.0) |
| Sex | |||
| Male | 82 | 3.6 | 3.7 (3.4–4.0) |
| Female | 38 | 3.8 | 3.9 (3.3–4.6) |
| Age | |||
| ≤29 | 77 | 3.6 | 3.6 (3.3–4.0) |
| ≥30 | 43 | 3.9 | 3.9 (3.4–4.5) |
| Eating of infector and/or patient at exposure | |||
| Yes | 58 | 3.9 | 3.8 (3.4–4.4) |
| No or unknown | 62 | 3.6 | 3.6 (3.3–3.9) |
| Setting | |||
| Restaurant | 33 | 3.9 | 3.9 (3.2–4.8) |
| School | 29 | 3.8 | 3.8 (3.2–4.6) |
| House | 22 | 3.8 | 3.8 (3.3–4.6) |
| Others | 36 | 3.4 | 3.4 (3.1–3.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, T.; Tanaka, H.; Irie, F.; Hirayama, A.; Takahashi, Y. Shorter Incubation Period among Unvaccinated Delta Variant Coronavirus Disease 2019 Patients in Japan. Int. J. Environ. Res. Public Health 2022, 19, 1127. https://doi.org/10.3390/ijerph19031127
Ogata T, Tanaka H, Irie F, Hirayama A, Takahashi Y. Shorter Incubation Period among Unvaccinated Delta Variant Coronavirus Disease 2019 Patients in Japan. International Journal of Environmental Research and Public Health. 2022; 19(3):1127. https://doi.org/10.3390/ijerph19031127
Chicago/Turabian StyleOgata, Tsuyoshi, Hideo Tanaka, Fujiko Irie, Atsushi Hirayama, and Yuki Takahashi. 2022. "Shorter Incubation Period among Unvaccinated Delta Variant Coronavirus Disease 2019 Patients in Japan" International Journal of Environmental Research and Public Health 19, no. 3: 1127. https://doi.org/10.3390/ijerph19031127
APA StyleOgata, T., Tanaka, H., Irie, F., Hirayama, A., & Takahashi, Y. (2022). Shorter Incubation Period among Unvaccinated Delta Variant Coronavirus Disease 2019 Patients in Japan. International Journal of Environmental Research and Public Health, 19(3), 1127. https://doi.org/10.3390/ijerph19031127





