Can We Identify Patients in Danger of Complications in Retrograde Intrarenal Surgery?—A Retrospective Risk Factors Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somani, B.K.; Aboumarzouk, O.; Srivastava, A.; Traxer, O. Flexible ureterorenoscopy: Tips and tricks. Urol. Ann. 2013, 5, 1–6. [Google Scholar] [CrossRef]
- Rodríguez-Monsalve Herrero, M.; Doizi, S.; Keller, E.X.; De Coninck, V.; Traxer, O. Retrograde intrarenal surgery: An expanding role in treatment of urolithiasis. Asian J. Urol. 2018, 5, 264–273. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Rukin, N.J.; Siddiqui, Z.A.; Chedgy, E.; Somani, B.K. Trends in Upper Tract Stone Disease in England: Evidence from the Hospital Episodes Statistics Database. Urol. Int. 2017, 98, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Türk, C.; Petřík, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur. Urol. 2016, 69, 475–482. [Google Scholar] [CrossRef]
- Kim, C.H.; Chung, D.Y.; Rha, K.H.; Lee, J.Y.; Lee, S.H. Effectiveness of Percutaneous Nephrolithotomy, Retrograde Intrarenal Surgery, and Extracorporeal Shock Wave Lithotripsy for Treatment of Renal Stones: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 26. [Google Scholar] [CrossRef] [PubMed]
- Miernik, A.; Wilhelm, K.; Ardelt, P.U.; Adams, F.; Kuehhas, F.E.; Schoenthaler, M. Standardized flexible ureteroscopic technique to improve stone-free rates. Urology 2012, 80, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Traxer, O.; Thomas, A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J. Urol. 2013, 189, 580–584. [Google Scholar] [CrossRef]
- Miernik, A.; Schoenthaler, M.; Wilhelm, K.; Wetterauer, U.; Zyczkowski, M.; Paradysz, A.; Bryniarski, P. Combined semirigid and flexible ureterorenoscopy via a large ureteral access sheath for kidney stones >2 cm: A bicentric prospective assessment. World J. Urol. 2014, 32, 697–702. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Jones, P.; Whitehurst, L.; Somani, B.K. Role of ‘dusting and pop-dusting’ using a high-powered (100 W) laser machine in the treatment of large stones (≥ 15 mm): Prospective outcomes over 16 months. Urolithiasis 2019, 47, 391–394. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, V.; Keller, E.X.; Somani, B.; Giusti, G.; Proietti, S.; Rodriguez-Socarras, M.; Rodriguez-Monsalve, M.; Doizi, S.; Ventimiglia, E.; Traxer, O. Complications of ureteroscopy: A complete overview. World J. Urol. 2020, 38, 2147–2166. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, E.; Traxer, O. What Is Moses Effect: A Historical Perspective. J. Endourol. 2019, 33, 353–357. [Google Scholar] [CrossRef]
- Elhilali, M.M.; Badaan, S.; Ibrahim, A.; Andonian, S. Use of the Moses Technology to Improve Holmium Laser Lithotripsy Outcomes: A Preclinical Study. J. Endourol. 2017, 31, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Traxer, O.; Keller, E.X. Thulium fiber laser: The new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J. Urol. 2020, 38, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, V.; Vinarov, A.; Yaroslavsky, I.; Kovalenko, A.; Vybornov, A.; Rapoport, L.; Enikeev, D.; Sorokin, N.; Dymov, A.; Tsarichenko, D.; et al. Preclinical comparison of superpulse thulium fiber laser and a holmium:YAG laser for lithotripsy. World J. Urol. 2020, 38, 497–503. [Google Scholar] [CrossRef]
- Corrales, M.; Traxer, O. Initial clinical experience with the new thulium fiber laser: First 50 cases. World J. Urol. 2021, 39, 3945–3950. [Google Scholar] [CrossRef]
- Enikeev, D.; Taratkin, M.; Klimov, R.; Inoyatov, J.; Azilgareeva, C.; Ali, S.; Korolev, D.; Corrales, M.; Traxer, O.; Glybochko, P. Superpulsed Thulium Fiber Laser for Stone Dusting: In Search of a Perfect Ablation Regimen-A Prospective Single-Center Study. J. Endourol. 2020, 34, 1175–1179. [Google Scholar] [CrossRef]
- De Coninck, V.M.J.; Keller, E.X.; Kovalenko, A.; Vinnichenko, V. Dusting efficiency comparison between Moses technology of Ho: YAG laser and superpulse thulium fiber laser. Eur. Urol. Suppl. 2019, 18, e1757–e1758. [Google Scholar] [CrossRef]
- Traxer, O.; Rapoport, L.; Tsarichenko, D.; Dymov, A.; Enikeev, D.; Sorokin, N.; Akopyan, S.; Korolev, A.; Proskura, V.; Klimov, R. First clinical study on superpulse thulium fiber laser for lithotripsy. J. Urol. 2018, 199, e321–e322. [Google Scholar] [CrossRef]
- Petzold, R.; Suarez-Ibarrola, R.; Miernik, A. Temperature assessment of a novel pulsed Thulium solid-state laser compared to a Holmium:YAG laser. J. Endourol. 2021, 35, 853–859. [Google Scholar] [CrossRef]
- Petzold, R.; Miernik, A.; Suarez-Ibarrola, R. In Vitro Dusting Performance of a New Solid State Thulium Laser Compared to Holmium Laser Lithotripsy. J. Endourol. 2021, 35, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Petzold, R.; Miernik, A.; Suarez-Ibarrola, R. Retropulsion force in laser lithotripsy—An in vitro study comparing a Holmium device to a novel pulsed solid-state Thulium laser. World J. Urol. 2021, 39, 3651–3656. [Google Scholar] [CrossRef] [PubMed]
- Guliciuc, M.; Maier, A.C.; Maier, I.M.; Kraft, A.; Cucuruzac, R.R.; Marinescu, M.; Şerban, C.; Rebegea, L.; Constantin, G.B.; Firescu, D. The Urosepsis—A Literature Review. Medicina 2021, 57, 872. [Google Scholar] [CrossRef]
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock-Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 2020, 104, 573–585. [Google Scholar] [CrossRef]
- Stoller, J.; Halpin, L.; Weis, M.; Aplin, B.; Qu, W.; Georgescu, C.; Nazzal, M. Epidemiology of severe sepsis: 2008–2012. J. Crit. Care 2016, 31, 58–62. [Google Scholar] [CrossRef]
- Khwannimit, B.; Bhurayanontachai, R. The direct costs of intensive care management and risk factors for financial burden of patients with severe sepsis and septic shock. J. Crit. Care 2015, 30, 929–934. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Tandogdu, Z.; Bartoletti, R.; Cai, T.; Cek, M.; Kulchavenya, E.; Köves, B.; Naber, K.; Perepanova, T.; Tenke, P.; et al. The Global Prevalence of Infections in Urology Study: A Long-Term, Worldwide Surveillance Study on Urological Infections. Pathogens 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Cindolo, L.; Castellan, P.; Scoffone, C.M.; Cracco, C.M.; Celia, A.; Paccaduscio, A.; Schips, L.; Proietti, S.; Breda, A.; Giusti, G. Mortality and flexible ureteroscopy: Analysis of six cases. World J. Urol. 2016, 34, 305–310. [Google Scholar] [CrossRef]
- Cindolo, L.; Castellan, P.; Primiceri, G.; Hoznek, A.; Cracco, C.M.; Scoffone, C.M.; Galfano, A.; Petralia, G.; De Angelis, M.; Annino, F.; et al. Life-threatening complications after ureteroscopy for urinary stones: Survey and systematic literature review. Minerva Urol. Nefrol. 2017, 69, 421–431. [Google Scholar] [CrossRef]
- Tanimoto, R.; Cleary, R.C.; Bagley, D.H.; Hubosky, S.G. Ureteral avulsion associated with ureteroscopy: Insights from the MAUDE database. J. Endourol. 2016, 30, 257–261. [Google Scholar] [CrossRef]
- Taie, K.; Jasemi, M.; Khazaeli, D.; Fatholahi, A. Prevalence and management of complications of ureteroscopy: A seven-year experience with introduction of a new maneuver to prevent ureteral avulsion. Urol. J. 2012, 9, 356–360. [Google Scholar]
- Schoenthaler, M.; Buchholz, N.; Farin, E.; Ather, H.; Bach, C.; Bach, T.; Denstedt, J.D.; Fritsche, H.M.; Grasso, M.; Hakenberg, O.W.; et al. The Post-Ureteroscopic Lesion Scale (PULS): A multicenter video-based evaluation of inter-rater reliability. World J. Urol. 2014, 32, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ambani, S.N.; Faerber, G.J.; Roberts, W.W.; Hollingsworth, J.M.; Wolf, J.S., Jr. Ureteral stents for impassable ureteroscopy. J. Endourol. 2013, 27, 549–553. [Google Scholar] [CrossRef]
- Lildal, S.K.; Andreassen, K.H.; Jung, H.; Pedersen, M.R.; Osther, P.J.S. Evaluation of ureteral lesions in ureterorenoscopy: Impact of access sheath use. Scand. J. Urol. 2018, 52, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chotikawanich, E.; Korman, E.; Monga, M. Complications of stone baskets: 14-year review of the manufacturer and user facility device experience database. J. Urol. 2011, 185, 179–183. [Google Scholar] [CrossRef]
- Bonkat, G.; Pilatz, A.; Wagenlehner, F. Time to Adapt Our Practice? The European Commission Has Restricted the Use of Fluoroquinolones since March 2019. Eur. Urol. 2019, 76, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Somani, B.K.; Giusti, G.; Sun, Y.; Osther, P.J.; Frank, M.; De Sio, M.; Turna, B.; de la Rosette, J. Complications associated with ureterorenoscopy (URS) related to treatment of urolithiasis: The Clinical Research Office of Endourological Society URS Global study. World J. Urol. 2017, 35, 675–681. [Google Scholar] [CrossRef]
- Giusti, G.; Proietti, S.; Villa, L.; Cloutier, J.; Rosso, M.; Gadda, G.M.; Doizi, S.; Suardi, N.; Montorsi, F.; Gaboardi, F.; et al. Current Standard Technique for Modern Flexible Ureteroscopy: Tips and Tricks. Eur. Urol. 2016, 70, 188–194. [Google Scholar] [CrossRef]
- Berardinelli, F.; Proietti, S.; Cindolo, L.; Pellegrini, F.; Peschechera, R.; Derek, H.; Dalpiaz, O.; Schips, L.; Giusti, G. A prospective multicenter European study on flexible ureterorenoscopy for the management of renal stone. Int. Braz. J. Urol. 2016, 42, 479–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wagenius, M.; Rydberg, M.; Popiolek, M.; Forsvall, A.; Stranne, J.; Linder, A. Ureteroscopy: A population based study of clinical complications and possible risk factors for stone surgery. Cent. Eur. J. Urol. 2019, 72, 285–295. [Google Scholar] [CrossRef]
- Chugh, S.; Pietropaolo, A.; Montanari, E.; Sarica, K.; Somani, B.K. Predictors of Urinary Infections and Urosepsis After Ureteroscopy for Stone Disease: A Systematic Review from EAU Section of Urolithiasis (EULIS). Curr. Urol. Rep. 2020, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, X.-Z.; Lai, D.-H.; Li, X.; He, Y.-Z. Fever and systemic inflammatory response syndrome after retrograde intrarenal surgery: Risk factors and predictive model. Kaohsiung J. Med. Sci. 2018, 201834, 400–408. [Google Scholar] [CrossRef]
- Uchida, Y.; Takazawa, R.; Kitayama, S.; Tsujii, T. Predictive risk factors for systemic inflammatory response syndrome following ureteroscopic laser lithotripsy. Urolithiasis 2018, 46, 375–381. [Google Scholar] [CrossRef]
- Hu, M.; Zhong, X.; Cui, X.; Xu, X.; Zhang, Z.; Guan, L.; Feng, Q.; Huang, Y.; Hu, W. Development and validation of a risk-prediction nomogram for patients with ureteral calculi associated with urosepsis: A retrospective analysis. PLoS ONE 2018, 13, e0201515. [Google Scholar] [CrossRef]
- Czajkowski, K.; Broś-Konopielko, M.; Teliga-Czajkowska, J. Urinary tract infection in women. Menopause Rev. 2021, 20, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sammon, J.D.; Ghani, K.R.; Karakiewicz, P.I.; Bhojani, N.; Ravi, P.; Sun, M.; Sukumar, S.; Trinh, V.Q.; Kowalczyk, K.J.; Kim, S.P.; et al. Temporal trends, practice patterns, and treatment outcomes for infected upper urinary tract stones in the United States. Eur. Urol. 2013, 64, 85–92. [Google Scholar] [CrossRef]
- Southern, J.B.; Higgins, A.M.; Young, A.J.; Kost, K.A.; Schreiter, B.R.; Clifton, M.; Fulmer, B.R.; Garg, T. Risk factors for postoperative fever and systemic inflammatory response syndrome after ureteroscopy for stone disease. J. Endourol. 2019, 33, 516–522. [Google Scholar] [CrossRef]
- Baboudjian, M.; Gondran-Tellier, B.; Abdallah, R.; Sichez, P.C.; Akiki, A.; Gaillet, S.; Delaporte, V.; Karsenty, G.; Lechevallier, E.; Boissier, R. Predictive risk factors of urinary tract infection following flexible ureteroscopy despite preoperative precautions to avoid infectious complications. World J. Urol. 2019, 38, 1253–1259. [Google Scholar] [CrossRef]
- Senocak, C.; Ozcan, C.; Sahin, T.; Yilmaz, G.; Ozyuvali, E.; Sarikaya, S.; Resorlu, B.; Oguz, U.; Bozkurt, O.F.; Unsal, A.; et al. Risk Factors of Infectious Complications after Flexible Uretero-renoscopy with Laser Lithotripsy. Urol. J. 2018, 15, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Berardinelli, F.; De Francesco, P.; Marchioni, M.; Cera, N.; Proietti, S.; Hennessey, D.; Dalpiaz, O.; Cracco, C.; Scoffone, C.; Schips, L.; et al. Infective complications after retrograde intrarenal surgery: A new standardized classification system. Int. Urol. Nephrol. 2016, 48, 1757–1762. [Google Scholar] [CrossRef]
- Blackmur, J.P.; Maitra, N.U.; Marri, R.R.; Housami, F.; Malki, M.; Mcilhenny, C. Analysis of factors’ association with risk of postoperative urosepsis in patients undergoing ureteroscopy for treatment of stone disease. J. Endourol. 2016, 30, 963–969. [Google Scholar] [CrossRef]
- Youssef, R.F.; Neisius, A.; Goldsmith, Z.G.; Ghaffar, M.; Tsivian, M.; Shin, R.H.; Cabrera, F.; Ferrandino, M.N.; Scales, C.D.; Preminger, G.M.; et al. Clinical outcomes after ureteroscopic lithotripsy in patients who initially presented with urosepsis: Matched pair comparison with elective ureteroscopy. J. Endourol. 2014, 28, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, A.; Hendry, J.; Kyriakides, R.; Geraghty, R.; Jones, P.; Aboumarzouk, O.; Somani, B.K. Outcomes of Elective Ureteroscopy for Ureteric Stones in Patients with Prior Urosepsis and Emergency Drainage: Prospective Study over 5 yr from a Tertiary Endourology Centre. Eur. Urol. Focus. 2020, 6, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Matsuda, A.; Sakamoto, H.; Higashi, Y.; Yamada, H. Safety and efficacy of ureteroscopy after obstructive pyelonephritis treatment. Int. J. Urol. 2013, 20, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Nevo, A.; Mano, R.; Baniel, J.; Lifshitz, D.A. Ureteric stent dwelling time: A risk factor for post-ureteroscopy sepsis. BJU Int. 2017, 120, 117–122. [Google Scholar] [CrossRef]
- Tokas, T.; Herrmann, T.R.W.; Skolarikos, A.; Nagele, U. Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Pressure matters: Intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J. Urol. 2019, 37, 125–131. [Google Scholar] [CrossRef]
- Tokas, T.; Skolarikos, A.; Herrmann, T.R.W.; Nagele, U. Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Pressure matters 2: Intrarenal pressure ranges during upper-tract endourological procedures. World J. Urol. 2019, 37, 133–142. [Google Scholar] [CrossRef]
- Geraghty, R.M.; Ishii, H.; Somani, B.K. Outcomes of flexible ureteroscopy and laser fragmentation for treatment of large renal stones with and without the use of ureteral access sheaths: Results from a university hospital with a review of literature. Scand. J. Urol. 2016, 50, 216–219. [Google Scholar] [CrossRef]
- Skolarikos, A.; Gross, A.J.; Krebs, A.; Unal, D.; Bercowsky, E.; Eltahawy, E.; Somani, B.K.; de la Rosette, J. Outcomes of Flexible Ureterorenoscopy for Solitary Renal Stones in the CROES URS Global Study. J. Urol. 2015, 194, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Prattley, S.; Voss, J.; Cheung, S.; Geraghty, R.; Jones, P.; Somani, B.K. Ureteroscopy and stone treatment in the elderly (≥70 years): Prospective outcomes over 5- years with a review of literature. Int. Braz. J. Urol. 2018, 44, 750–757. [Google Scholar] [CrossRef]
- Emiliani, E.; Piccirilli, A.; Cepeda-Delgado, M.; Kanashiro, A.K.; Mantilla, D.; Amaya, C.A.; Sanchez-Martin, F.M.; Millan-Rodriguez, F.; Territo, A.; Amón-Sesmero, J.H.; et al. Flexible ureteroscopy in extreme elderly patients (80 years of age and older) is feasible and safe. World J. Urol. 2020, 39, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Cocci, A.; Coccarelli, F.; Ruggera, L.; Lanzafame, P.; Caciagli, P.; Malossini, G.; Crisci, A.; Trinchieri, A.; Perletti, G.; et al. Infectious Complications After Laser Vaporization of Urinary Stones During Retrograde Intrarenal Surgery Are Not Associated with Spreading of Bacteria into Irrigation Fluid but with Previous Use of Fluoroquinolones. Eur. Urol. Focus. 2021, 7, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.Y.; Ma, Y.C.; Liu, R.; Li, H.; Wang, K. Preoperative positive urine nitrite and albumin-globulin ratio are independent risk factors for predicting postoperative fever after retrograde Intrarenal surgery based on a retrospective cohort. BMC Urol. 2020, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Aboumarzouk, O.M.; Somani, B.K.; Monga, M. Flexible ureteroscopy and holmium:YAG laser lithotripsy for stone disease in patients with bleeding diathesis: A systematic review of the literature. Int. Braz. J. Urol. 2012, 38, 298–306. [Google Scholar] [CrossRef]
- Sharaf, A.; Amer, T.; Somani, B.K.; Aboumarzouk, O.M. Ureteroscopy in Patients with Bleeding Diatheses, Anticoagulated, and on Anti-Platelet Agents: A Systematic Review and Meta-Analysis of the Literature. J. Endourol. 2017, 31, 1217–1225. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Reeves, T.; Aboumarzouk, O.; Kallidonis, P.; Ozsoy, M.; Skolarikos, A.; Tailly, T.; Liatsikos, E.; Traxer, O.; Somani, B.K. Endourologic Management (PCNL, URS, SWL) of Stones in Solitary Kidney: A Systematic Review from European Association of Urologists Young Academic Urologists and Uro-Technology Groups. J. Endourol. 2020, 34, 7–17. [Google Scholar] [CrossRef]
- Grosso, A.A.; Sessa, F.; Campi, R.; Viola, L.; Polverino, P.; Crisci, A.; Salvi, M.; Liatsikos, E.; Feu, O.A.; Di Maida, F.; et al. Intraoperative and postoperative surgical complications after ureteroscopy, retrograde intrarenal surgery, and percutaneous nephrolithotomy: A systematic review. Minerva Urol. Nephrol. 2021, 73, 309–332. [Google Scholar] [CrossRef]
- Krambeck, A.; Wijnstok, N.; Olbert, P.; Mitroi, G.; Bariol, S.; Shah, H.N.; El-Abd, A.S.; Onal, B.; de la Rosette, J. The Influence of Body Mass Index on Outcomes in Ureteroscopy: Results from the Clinical Research Office of Endourological Society URS Global Study. J. Endourol. 2017, 31, 20–26. [Google Scholar] [CrossRef]
- Ishii, H.; Couzins, M.; Aboumarzouk, O.; Biyani, C.S.; Somani, B.K. Outcomes of Systematic Review of Ureteroscopy for Stone Disease in the Obese and Morbidly Obese Population. J. Endourol. 2016, 30, 135–145. [Google Scholar] [CrossRef]
- Aboumarzouk, O.M.; Somani, B.K.; Monga, M. Safety and efficacy of ureteroscopic lithotripsy for stone disease in obese patients: A systematic review of the literature. BJU Int. 2012, 110, E374–E380. [Google Scholar] [CrossRef] [PubMed]
- Komori, M.; Izaki, H.; Daizumoto, K.; Tsuda, M.; Kusuhara, Y.; Mori, H.; Kagawa, J.; Yamaguchi, K.; Yamamoto, Y.; Fukumori, T.; et al. Complications of Flexible Ureteroscopic Treatment for Renal and Ureteral Calculi during the Learning Curve. Urol. Int. 2015, 95, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bres-Niewada, E.; Dybowski, B.; Zapała, P.; Poletajew, S.; Miązek-Zapała, N.; Michałek, I.; Radziszewski, P. A stone pushed back to the collecting system—Long therapeutic path in centers with limited access to flexible instruments. Cent. Eur. J. Urol. 2018, 71, 186–189. [Google Scholar] [CrossRef]
- Talso, M.; Goumas, I.K.; Kamphuis, G.M.; Dragos, L.; Tefik, T.; Traxer, O.; Somani, B.K. Reusable flexible ureterorenoscopes are more cost-effective than single-use scopes: Results of a systematic review from PETRA Uro-group. Transl. Androl. Urol. 2019, 8, S418–S425. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, T.; Schneider, M.H.; Hupe, M.C.; Wiessmeyer, J.R.; Cordes, J.; Chlosta, P.L.; Merseburger, A.S.; Kramer, M.W. Retrospective Cost Analysis of a Single-Center Reusable Flexible Ureterorenoscopy Program: A Comparative Cost Simulation of Disposable fURS as an Alternative. J. Endourol. 2017, 31, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
| Parameters | % or SD Value | |
|---|---|---|
| Age (years) | 58 (13.7) | |
| Sex | Women 53.4% | |
| Men 46.6% | ||
| BMI (kg/m2) | 27.8 (5.5) | |
| Operation side | Left 50.7% | |
| Right 49.3% | ||
| Operation time (min) | 45 (15) | |
| Stone size (mm) | Occurrence | Size (SD) |
| Largest stone | 100% | 10 (3.9) |
| 2nd | 37.20% | 6 (2.8) |
| 3rd | 12.60% | 5 (1.9) |
| Largest stone location: | ||
| Upper calyx | 11.80% | |
| Medium calyx | 21.10% | |
| Lower calyx | 45.60% | |
| Kidney pelvis | 21.60% | |
| 2nd Largest stone location: | ||
| Upper calyx | 10.40% | |
| Medium calyx | 40.30% | |
| Lower calyx | 37.70% | |
| Kidney pelvis | 11.70% | |
| Comorbidities: | ||
| Ischemic heart disease | 8.6% (n = 18) | |
| Diabetes | 15.9% (n = 33) | |
| Recurrent/chronic UTI | 20.3% (n = 42) | |
| Chronic kidney disease | 4.3% (n = 9) | |
| Hypertension | 23.2% (n = 48) | |
| Hypothyroidism | 8.2% (n = 17) | |
| Gout | 4.8% (n = 10) | |
| Chronic obstructive pulmonary disease | 2.4% (n = 5) | |
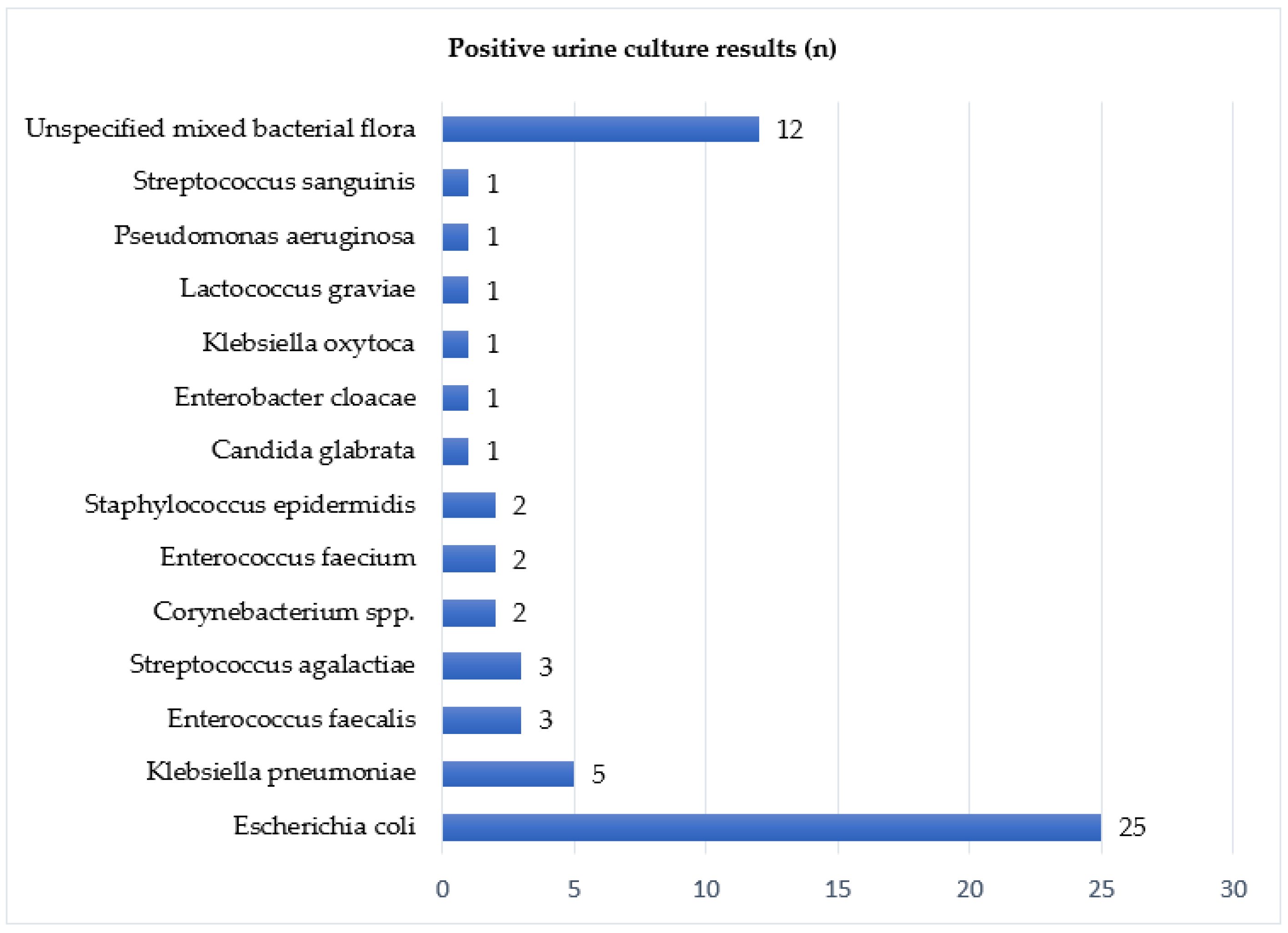
| Mid-Stream Urine Culture: | % (n) |
|---|---|
| Total Available | 88.4% (183) |
| Negative/sterile | 67.2% (123) |
| Positive | 32.8% (60) |
| Not available | 11.6% (24) |
| Grade | Description | n | % |
|---|---|---|---|
| I | Any deviation from the normal post-operative course without the need for pharmacological treatment or surgical, endoscopic and radiological interventions. Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics and electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside. | 23 | 11.1 |
| II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included. | 12 | 5.8 |
| III | Requiring surgical, endoscopic or radiological intervention. | null | null |
| IV | Life-threatening complications (including CNS complications) requiring IC/ICU-management. | 5 | 2.4 |
| V | Death of a patient. | null | null |
| Level of Effect | Odds Ratio | Lower CI 95.0% | Upper CI 95.0% | p | |
|---|---|---|---|---|---|
| Largest stone diameter (mm) | 0.878796 | 0.760866 | 1.01500 | 0.078850 | |
| Normal weight | BMI 18.5–25 | 2.749022 | 0.579156 | 13.04850 | 0.142517 |
| Underwieght | BMI < 18.5 | 0.216355 | 0.013141 | 3.56211 | 0.119581 |
| Overweight | BMI > 25 | 2.526093 | 0.630934 | 10.11381 | 0.148003 |
| UTI | 4.852125 | 1.308780 | 17.98860 | 0.018153 | |
| Diabetes | 2.977971 | 0.635067 | 13.96436 | 0.166329 | |
| Urine culture | Non-sterile | 0.139426 | 0.041352 | 0.47010 | 0.001487 |
| Intercept | Largest Stone Diameter (mm) | BMI 18.5–25 | BMI < 18.5 | BMI > 25 | History of Infection | Diabetes | Positive Urine Culture | |
|---|---|---|---|---|---|---|---|---|
| Intercept | 0.980160 | −0.062800 | −0.001948 | 0.316145 | −0.118121 | 0.045744 | −0.024435 | −0.008929 |
| Largest stone diameter (mm) | −0.062800 | 0.005405 | −0.004559 | −0.000998 | 0.001139 | −0.008407 | −0.006558 | 0.000237 |
| BMI 18.5–25 | −0.001948 | −0.004559 | 0.384616 | −0.394674 | −0.003049 | 0.021113 | −0.015871 | −0.022897 |
| BMI < 18.5 | 0.316145 | −0.000998 | −0.394674 | 1.100130 | −0.370720 | −0.043936 | −0.018200 | 0.030884 |
| BMI > 25 | −0.118121 | 0.001139 | −0.003049 | −0.370720 | 0.325158 | 0.012591 | 0.016305 | −0.021175 |
| History of infection | 0.045744 | −0.008407 | 0.021113 | −0.043936 | 0.012591 | 0.111738 | 0.043180 | −0.010176 |
| Diabetes | −0.024435 | −0.006558 | −0.015871 | −0.018200 | 0.016305 | 0.043180 | 0.155400 | −0.017252 |
| Positive urine culture | −0.008929 | 0.000237 | −0.022897 | 0.030884 | −0.021175 | −0.010176 | −0.017252 | 0.096138 |
| Intercept | Largest Stone Diameter (mm) | BMI 18.5–25 | BMI < 18.5 | BMI > 25 | History of Infection | Diabetes | Positive Urine Culture | |
|---|---|---|---|---|---|---|---|---|
| Intercept | 1.000000 | −0.862795 | −0.003173 | 0.304449 | −0.209234 | 0.138224 | −0.062608 | −0.029089 |
| Largest stone diameter (mm) | −0.862795 | 1.000000 | −0.099989 | −0.012945 | 0.027170 | −0.342084 | −0.226265 | 0.010375 |
| BMI 18.5–25 | −0.003173 | −0.099989 | 1.000000 | −0.606740 | −0.008621 | 0.101843 | −0.064918 | −0.119077 |
| BMI < 18.5 | 0.304449 | −0.012945 | −0.606740 | 1.000000 | −0.619836 | −0.125313 | −0.044017 | 0.094964 |
| BMI > 25 | −0.209234 | 0.027170 | −0.008621 | −0.619836 | 1.000000 | 0.066057 | 0.072534 | −0.119763 |
| History of infection | 0.138224 | −0.342084 | 0.101843 | −0.125313 | 0.066057 | 1.000000 | 0.327683 | −0.098180 |
| Diabetes | −0.062608 | −0.226265 | −0.064918 | −0.044017 | 0.072534 | 0.327683 | 1.000000 | −0.141146 |
| Positive urine culture | −0.029089 | 0.010375 | −0.119077 | 0.094964 | −0.119763 | −0.098180 | −0.141146 | 1.000000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, J.M.; Hladun, T.; Krenz, B.; Bromber, K.; Salagierski, M.; Marczak, M. Can We Identify Patients in Danger of Complications in Retrograde Intrarenal Surgery?—A Retrospective Risk Factors Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1114. https://doi.org/10.3390/ijerph19031114
Ratajczak JM, Hladun T, Krenz B, Bromber K, Salagierski M, Marczak M. Can We Identify Patients in Danger of Complications in Retrograde Intrarenal Surgery?—A Retrospective Risk Factors Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1114. https://doi.org/10.3390/ijerph19031114
Chicago/Turabian StyleRatajczak, Jakub Marek, Taras Hladun, Bartosz Krenz, Krzysztof Bromber, Maciej Salagierski, and Michał Marczak. 2022. "Can We Identify Patients in Danger of Complications in Retrograde Intrarenal Surgery?—A Retrospective Risk Factors Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1114. https://doi.org/10.3390/ijerph19031114
APA StyleRatajczak, J. M., Hladun, T., Krenz, B., Bromber, K., Salagierski, M., & Marczak, M. (2022). Can We Identify Patients in Danger of Complications in Retrograde Intrarenal Surgery?—A Retrospective Risk Factors Analysis. International Journal of Environmental Research and Public Health, 19(3), 1114. https://doi.org/10.3390/ijerph19031114






