Using a Syndemics Framework to Understand How Substance Use Contributes to Morbidity and Mortality among People Living with HIV in Africa: A Call to Action
Abstract
1. Substance Use Varies by African Regions
2. Substance Use and HIV Prevalence among Populations in Uganda
3. Substance Use and HIV Prevalence among South African Populations
4. Substance Use and HIV Prevalence among Populations in Nigeria
5. Health Implications and Outcomes of Substance Use among People Living with HIV
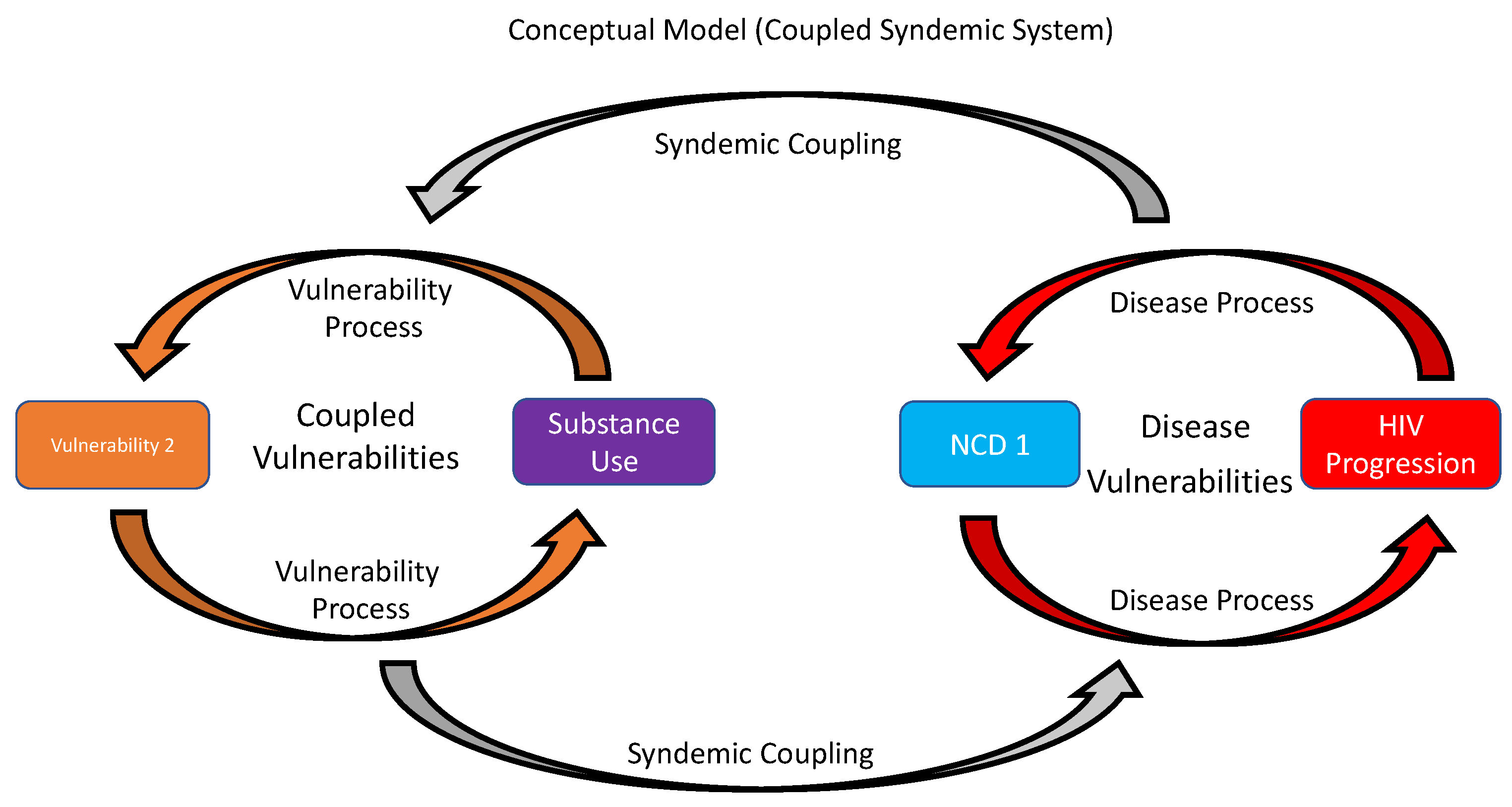
6. Using a Holistic, Equity-Based Syndemics Framework for PWH with SU
7. Conclusions
| Central | Eastern | Southern | Western | |
|---|---|---|---|---|
| Alcohol | 1413.3 | 1611.0 | 1515.0 | 1168.1 |
| Amphetamine | 6.7 | 6.2 | 27.3 | 6.1 |
| Cocaine | 14.6 | 13.8 | 20.0 | 14.4 |
| Opioids | 240.1 | 212.1 | 376.8 | 276.4 |
| Tobacco | 179.1 | 230.9 | 189.0 | 116.8 |
| Other substances | 33.6 | 34.0 | 40.2 | 36.2 |
| Alcohol DALYs (%) | 2733.4 (2.9) | 2010.6 (2.7) | 3178.8 (5.1) | 1166.0 (1.7) |
| Nigeria | South Africa | Uganda | |
|---|---|---|---|
| Alcohol DALYs | 1527.0 (922.6, 2257.0) | 3012.6 (2409.0, 3610.2) | 3694.5 (1945.1, 5565.9) |
| Drug DALYs | 577.9 (467.8, 725.8) | 572.0 (470.8, 705.0) | 147.8 (113.5, 187.4) |
| Deaths attributable to alcohol | 46.7 (23.0, 79.8) | 88.5 (64.8, 110.1) | 122.2 (56.6, 198.4) |
| Deaths attributable to substances | 9.7 (7.6, 12.3) | 376.7 (305.7, 502.1) | 1.2 (0.9, 1.4) |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degenhardt, L.; Charlson, F.; Ferrari, A.; Santomauro, D.; Erskine, H.; Mantilla-Herrara, A.; Whiteford, H.; Leung, J.; Naghavi, M.; Griswold, M.; et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar] [CrossRef]
- Paschen-Wolff, M.M.; Campbell, A.N.; Tross, S.; Choo, T.-H.; Pavlicova, M.; Jarlais, D.D. DSM-5 substance use disorder symptom clusters and HIV antiretroviral therapy (ART) adherence. AIDS Care 2020, 32, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Remmert, J.E.; Psaros, C.; Pinkston, M.; Safren, S.A. Learning to address multiple syndemics for people living with HIV through client perspectives on CBT. Psychother. Res. 2017, 29, 492–502. [Google Scholar] [CrossRef]
- Evangeli, M. Mental health and substance use in HIV-infected adolescents. Curr. Opin. HIV AIDS 2018, 13, 204–211. [Google Scholar] [CrossRef]
- Haas, A.D.; Technau, K.; Pahad, S.; Braithwaite, K.; Madzivhandila, M.; Sorour, G.; Sawry, S.; Maxwell, N.; Von Groote, P.; Tlali, M.; et al. Mental health, substance use and viral suppression in adolescents receiving ART at a paediatric HIV clinic in South Africa. J. Int. AIDS Soc. 2020, 23, e25644. [Google Scholar] [CrossRef] [PubMed]
- Kharsany, A.B.; Karim, Q.A. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016, 10, 34–48. [Google Scholar] [CrossRef]
- Sorsdahl, K.; Naledi, T.; Lund, C.; Levitt, N.S.; Joska, J.A.; Stein, D.J.; Myers, B. Integration of mental health counselling into chronic disease services at the primary health care level: Formative research on dedicated versus designated strategies in the Western Cape, South Africa. J. Health Serv. Res. Policy 2021, 26, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Sibeko, G.; Cummings, B.; Myers, B.; Sorsdahl, K.; Stein, D.J.; Kuo, C.; Becker, S.J. Training the addiction treatment workforce in HIV endemic regions: An overview of the South Africa HIV Addiction Technology Transfer Center initiative. Train. Educ. Prof. Psychol. 2020, 14, 78–85. [Google Scholar] [CrossRef]
- Lancaster, K.E.; Hetrick, A.; Jaquet, A.; Adedimeji, A.; Atwoli, L.; Colby, D.J.; Mayor, A.M.; Parcesepe, A.; Syvertsen, J. Substance use and universal access to HIV testing and treatment in sub-Saharan Africa: Implications and research priorities. J. Virus Erad. 2018, 4, 26–32. [Google Scholar] [CrossRef]
- Charlson, F.J.; Diminic, S.; Lund, C.; Degenhardt, L.; Whiteford, H. Mental and Substance Use Disorders in Sub-Saharan Africa: Predictions of Epidemiological Changes and Mental Health Workforce Requirements for the Next 40 Years. PLoS ONE 2014, 9, e110208. [Google Scholar] [CrossRef] [PubMed]
- Wechsberg, W.M.; Myers, B.; Kline, T.L.; Carney, T.; Browne, F.A.; Novak, S.P. The Relationship of Alcohol and Other Drug Use Typologies to Sex Risk Behaviors among Vulnerable Women in Cape Town, South Africa. J. AIDS Clin. Res. 2012, S1, 15. [Google Scholar] [CrossRef]
- Mbwambo, J.; McCurdy, S.A.; Myers, B.; Lambdin, B.; Kilonzo, G.P.; Kaduri, P. Drug trafficking, use, and HIV risk: The need for comprehensive interventions. SAHARA-J J. Soc. Asp. HIV/AIDS 2012, 9, 154–159. [Google Scholar] [CrossRef][Green Version]
- Bukenya, D.; Mayanja, B.N.; Nakamanya, S.; Muhumuza, R.; Seeley, J. What causes non-adherence among some individuals on long term antiretroviral therapy? Experiences of individuals with poor viral suppression in Uganda. AIDS Res. Ther. 2019, 16, 2. [Google Scholar] [CrossRef]
- Sileo, K.M.; Wanyenze, R.K.; Kizito, W.; Reed, E.; Brodine, S.K.; Chemusto, H.; Kiene, S.M. Multi-level Determinants of Clinic Attendance and Antiretroviral Treatment Ad-herence Among Fishermen Living with HIV/AIDS in Communities on Lake Victoria, Uganda. AIDS Behav. 2019, 23, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, T.G.M.; Knox, J.R.; Alcala, C.; El-Bassel, N.; Kuo, I.; Smith, L.R. Substance Use and HIV Risk Among Men Who Have Sex with Men in Africa: A Systematic Review. J. Acquir. Immune. Defic. Syndr. 2017, 76, e34–e46. [Google Scholar] [CrossRef]
- Magidson, J.F.; Iyer, H.S.; Regenauer, K.S.; Grelotti, D.J.; Dietrich, J.J.; Courtney, I.; Tshabalala, G.; Orrell, C.; Gray, G.E.; Bangsberg, D.R.; et al. Recreational ART use among individuals living with HIV/AIDS in South Africa: Examining longitudinal ART initiation and viral suppression. Drug Alcohol Depend. 2019, 198, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wandera, B.; Tumwesigye, N.M.; Nankabirwa, J.I.; Kambugu, A.D.; Parkes-Ratanshi, R.; Mafigiri, D.; Kapiga, S.; Sethi, A.K. Alcohol Consumption among HIV-Infected Persons in a Large Urban HIV Clinic in Kampala Uganda: A Constellation of Harmful Behaviors. PLoS ONE 2015, 10, e0126236. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.A.; Vandepitte, J.; Bukenya, J.N.; Mayanja, Y.; Nakubulwa, S.; Kamali, A.; Seeley, J.; Grosskurth, H. High Levels of Persistent Problem Drinking in Women at High Risk for HIV in Kampala, Uganda: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2016, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Woolf-King, S.E.; Fatch, R.; Cheng, D.M.; Muyindike, W.; Ngabirano, C.; Kekibiina, A.; Emenyonu, N.; Hahn, J.A. Alcohol Use and Unprotected Sex Among HIV-Infected Ugandan Adults: Findings from an Event-Level Study. Arch. Sex. Behav. 2018, 47, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, K.; Ramlagan, S.; Johnson, B.D.; Phaswana-Mafuya, N. Illicit Drug Use and Treatment in South Africa: A Review. Subst. Use Misuse 2010, 45, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Rosselló, J.; Rawson, R.A.; Zarza, M.J.; Bellows, A.; Busse, A.; Sáenz, E.; Freese, T.; Shawkey, M.; Carise, D.; Ali, R.; et al. United Nations Office on Drugs and Crime International Network of Drug Dependence Treatment and Rehabilitation Resource Centres: Treatnet. Subst. Abus. 2010, 31, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Haysom, S.; Gastrow, P.; Shaw, M. Tackling heroin trafficking on the East African coast. ENACT Policy Brief Issue 2018. [Google Scholar]
- Emerson, S.; Solomon, H. Trafficking in drugs and small arms. In African Security in the Twenty-First Century; Manchester University Press: Manchester, UK, 2018. [Google Scholar]
- Wyler, L.S.; Cook, N. Illegal Drug Trade in Africa: Trends and U.S. Policy; Congressional Research Service: Washington, DC, USA, 2009. [Google Scholar]
- Whiteford, H.A.; Ferrari, A.J.; Degenhardt, L.; Feigin, V.; Vos, T. The global burden of mental, neurological and substance use disorders: An analysis from the Global Burden of Disease Study 2010. PLoS ONE 2015, 10, e0116820. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 DALYs and HALE Collaborators; Murray, C.J.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological tran-sition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- UNOODC. World Drug Report 2021. CrimRxiv 2021. [Google Scholar] [CrossRef]
- Uganda Ministry of Health. Uganda Population-Based HIV Impact Assessment (UPHIA) 2016–2017; United States Department of Health and Human Services: New York, NY, USA, 2019.
- WHO. Atlas on Substance Use (2010): Resources for the Prevention and Treatment of Substance Use Disorders; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Kabwama, S.N.; Ndyanabangi, S.; Mutungi, G.; Wesonga, R.; Bahendeka, S.K.; Guwatudde, D. Tobacco use and associated factors among Adults in Uganda: Findings from a nationwide survey. Tob. Induc. Dis. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Plange-Rhule, J.; Iwelunmor, J.; Lee, D.; Blackstone, S.R.; Mitchell, A.; Ntim, M.; Apusiga, K.; Tayo, B.; Yeboah-Awudzi, K.; et al. Training nurses in task-shifting strategies for the management and control of hypertension in Ghana: A mixed-methods study. BMC Health Serv. Res. 2017, 17, 104. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Baluku, M.; Wamala, T.; Muhangi, D. HIV- and hepatitis C-related risk behaviors among people who inject drugs in Uganda: Implications for policy and programming. Harm Reduct. J. 2019, 16, 56. [Google Scholar] [CrossRef]
- Statistics South Africa. Mid-Year Population Estimates: Statistical Release P0302. 2021. Available online: http://www.statssa.gov.za/publications/P0302/P03022021.pdf (accessed on 14 December 2021).
- Pasche, S.; Myers, B. Substance misuse trends in South Africa. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 338–341. [Google Scholar] [CrossRef]
- Harker Burnhams, N.; Bharat, C.; Williams, D.R.; Stein, D.J.; Myers, B. Transitions between lifetime alcohol use, regular use and remission: Results from the 2004 South African Stress and Health Survey. S. Afr. Med. J. 2018, 109, 40–46. [Google Scholar] [CrossRef]
- Harker, N.; Lucas, W.C.; Laubscher, R.; Dada, S.; Myers, B.; Parry, C.D. Is South Africa being spared the global opioid crisis? A review of trends in drug treatment demand for heroin, nyaope and codeine-related medicines in South Africa (2012–2017). Int. J. Drug Policy 2020, 83, 102839. [Google Scholar] [CrossRef] [PubMed]
- Meade, C.S.; Towe, S.L.; Watt, M.H.; Hobkirk, A.L.; Skinner, D.; Myers, B.; Kimani, S.M.; Pieterse, D. HIV Testing Behaviors and Attitudes Among Community Recruited Methamphetamine Users in a South African Township. AIDS Behav. 2014, 19, 186–191. [Google Scholar] [CrossRef][Green Version]
- Dada, S.; Burnhams, N.H.; Laubscher, R.; Parry, C.; Myers, B. Alcohol and other drug use among women seeking substance abuse treatment in the Western Cape, South Africa. S. Afr. J. Sci. 2018, 114, 1–7. [Google Scholar] [CrossRef]
- Trangenstein, P.J.; Morojele, N.K.; Lombard, C.; Jernigan, D.H.; Parry, C.D.H. Heavy drinking and contextual risk factors among adults in South Africa: Findings from the International Alcohol Control study. Subst. Abus. Treat. Prev. Policy 2018, 13, 43. [Google Scholar] [CrossRef]
- Kader, R.; Seedat, S.; Govender, R.; Koch, J.R.; Parry, C.D. Hazardous and Harmful use of Alcohol and/or Other Drugs and Health Status Among South African Patients Attending HIV Clinics. AIDS Behav. 2013, 18, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.D.; Londani, M.; Shuper, P.A.; Myers, B.; Kekwaletswe, C.T.; Nkosi, S.; Morojele, N.K. Characteristics and drinking behaviour of patients on antiretroviral therapy who drink and attend HIV clinics in Tshwane, South Africa: Implications for intervention. S. Afr. Med. J. 2019, 109, 784–791. [Google Scholar] [CrossRef]
- Morojele, N.K.; Nkosi, S.; Kekwaletswe, C.T.; Shuper, P.A.; Manda, S.O.; Myers, B.; Parry, C.D.H. Utility of Brief Versions of the Alcohol Use Disorders Identification Test (AUDIT) to Identify Excessive Drinking Among Patients in HIV Care in South Africa. J. Stud. Alcohol Drugs 2017, 78, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Lombard, C.; Joska, J.A.; Abdullah, F.; Naledi, T.; Lund, C.; Williams, P.P.; Stein, D.J.; Sorsdahl, K.R. Associations Between Patterns of Alcohol Use and Viral Load Suppression Amongst Women Living with HIV in South Africa. AIDS Behav. 2021, 25, 3758–3769. [Google Scholar] [CrossRef]
- Veld, D.H.I.T.; Pengpid, S.; Colebunders, R.; Skaal, L.; Peltzer, K. High-risk alcohol use and associated socio-demographic, health and psychosocial factors in patients with HIV infection in three primary health care clinics in South Africa. Int. J. STD AIDS 2017, 28, 651–659. [Google Scholar] [CrossRef]
- Cichowitz, C.; Maraba, N.; Hamilton, R.; Charalambous, S.; Hoffmann, C.J. Depression and alcohol use disorder at antiretroviral therapy initiation led to disengagement from care in South Africa. PLoS ONE 2017, 12, e0189820. [Google Scholar] [CrossRef]
- Reddy, P.; Zuma, K.; Shisana, O.; Kim, J.; Sewpaul, R. Prevalence of tobacco use among adults in South Africa: Results from the first South African National Health and Nutrition Examination Survey. S. Afr. Med. J. 2015, 105, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Agaku, I.; Egbe, C.; Ayo-Yusuf, O. Utilisation of smoking cessation aids among South African adult smokers: Findings from a national survey of 18 208 South African adults. Fam. Med. Community Health 2021, 9, e000637. [Google Scholar] [CrossRef]
- Euromonitor International. Passport. Cigarettes in South Africa. 2016. Available online: https://www.euromonitor.com/cigarettes-in-south-africa/report (accessed on 15 April 2021).
- Mutemwa, M.; Peer, N.; De Villiers, A.; Faber, M.; Kengne, A.-P. Tobacco smoking and associated factors in human immunodeficiency virus-infected adults attending human immunodeficiency virus clinics in the Western Cape province, South Africa. S. Afr. J. HIV Med. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Egbe, C.O.; Bialous, S.A.; Glantz, S. Framework Convention on Tobacco Control Implementation in Nigeria: Lessons for Low- and Middle-Income Countries. Nicotine Tob. Res. 2018, 21, 1122–1130. [Google Scholar] [CrossRef]
- Reddy, S.P.; James, S.; Sewpaul, R.; Sifunda, S.; Ellahebokus, A.; Kambaran, N.S.; Omardien, R.G. Umthente Uhlaba Usamila: The 3rd South African National Youth Risk Behaviour Survey 2011; South African Medical Research Council: Cape Town, South Africa, 2013. [Google Scholar]
- Peltzer, K.; Phaswana-Mafuya, N. Drug use among youth and adults in a population-based survey in South Africa. S. Afr. J. Psychiatry 2018, 24, 1139. [Google Scholar] [CrossRef] [PubMed]
- Pengpid, S.; Peltzer, K.; Ramlagan, S. Prevalence and correlates of hazardous, harmful or dependent alcohol use and drug use amongst persons 15 years and older in South Africa: Results of a national survey in 2017. Afr. J. Prim. Health Care Fam. Med. 2021, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Regenauer, K.S.; Myers, B.; Batchelder, A.W.; Magidson, J.F. “That person stopped being human”: Intersecting HIV and substance use stigma among patients and providers in South Africa. Drug Alcohol Depend. 2020, 216, 108322. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Carney, T.; Wechsberg, W.M. “Not on the agenda”: A qualitative study of influences on health services use among poor young women who use drugs in Cape Town, South Africa. Int. J. Drug Policy 2016, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Jatau, A.I.; Sha’Aban, A.; Gulma, K.A.; Shitu, Z.; Khalid, G.M.; Isa, A.; Wada, A.S.; Mustapha, M. The Burden of Drug Abuse in Nigeria: A Scoping Review of Epidemiological Studies and Drug Laws. Public Health Rev. 2021, 42. [Google Scholar] [CrossRef]
- Lindblad, R.; Hu, L.; Oden, N.; Wakim, P.; Rosa, C.; VanVeldhuisen, P. Mortality Rates Among Substance Use Disorder Participants in Clinical Trials: Pooled Analysis of Twenty-Two Clinical Trials Within the National Drug Abuse Treatment Clinical Trials Network. J. Subst. Abus. Treat. 2016, 70, 73–80. [Google Scholar] [CrossRef]
- Meyer, J.P.; Althoff, A.L.; Altice, F. Optimizing Care for HIV-Infected People Who Use Drugs: Evidence-Based Approaches to Overcoming Healthcare Disparities. Clin. Infect. Dis. 2013, 57, 1309–1317. [Google Scholar] [CrossRef]
- Amin, P.; Douaihy, A. Substance Use Disorders in People Living with Human Immunodeficiency Virus/AIDS. Nurs. Clin. N. Am. 2018, 53, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, R.; Miller, T. Substance Abuse Treatment in Persons with HIV/AIDS: Challenges in Managing Triple Diagnosis. Behav. Med. 2014, 40, 43–52. [Google Scholar] [CrossRef]
- Kader, R.; Govender, R.; Seedat, S.; Koch, J.R.; Parry, C. Understanding the Impact of Hazardous and Harmful Use of Alcohol and/or Other Drugs on ARV Adherence and Disease Progression. PLoS ONE 2015, 10, e0125088. [Google Scholar] [CrossRef]
- Kader, R.; Seedat, S.; Koch, J.; Parry, C. A preliminary investigation of the AUDIT and DUDIT in comparison to biomarkers for alcohol and drug use among HIV-infected clinic attendees in Cape Town, South Africa. Afr. J. Psychiatry 2012, 15, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; D’Sa, C.; Kimmerling, A.; Siedlarz, K.M.; Tuit, K.L.; Stowe, R.; Sinha, R. Immune system inflammation in cocaine dependent individuals: Implications for med-ications development. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 156–166. [Google Scholar] [CrossRef]
- Loftis, J.M.; Huckans, M. Substance use disorders: Psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol. Ther. 2013, 139, 289–300. [Google Scholar] [CrossRef][Green Version]
- Asanuma, M.; Miyazaki, I.; Higashi, Y.; Tsuji, T.; Ogawa, N. Specific Gene Expression and Possible Involvement of Inflammation in Methamphetamine-Induced Neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1025, 69–75. [Google Scholar] [CrossRef] [PubMed]
- So-Armah, K.A.; Tate, J.P.; Chang, C.-C.H.; Butt, A.A.; Gerschenson, M.; Gibert, C.L.; Leaf, D.; Rimland, D.; Rodriguez-Barradas, M.C.; Budoff, M.J.; et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? JAIDS J. Acquir. Immune Defic. Syndr. 2016, 72, 206–213. [Google Scholar] [CrossRef]
- Monnig, M.A.; Kahler, C.W.; Cioe, P.A.; Tucker, L.; Monti, P.M.; Mayer, K.H.; Ramratnam, B. Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV. AIDS Care 2016, 28, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Kline, T.L.; Doherty, I.A.; Carney, T.; Wechsberg, W.M. Perceived need for substance use treatment among young women from disadvantaged communities in Cape Town, South Africa. BMC Psychiatry 2014, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Williams, P.P.; Govender, R.; Manderscheid, R.; Koch, J.R. Substance abuse treatment engagement, completion and short-term outcomes in the Western Cape province, South Africa: Findings from the Service Quality Measures Initiative. Drug Alcohol Depend. 2018, 185, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Farhoudian, A.; Radfar, S.R.; Mohaddes Ardabili, H.; Rafei, P.; Ebrahimi, M.; Khojasteh Zonoozi, A.; De Jong, G.A.J.; Vahidi, M.; Yunesian, M.; Kouimtsidis, C.; et al. A Global Survey on Changes in the Supply, Price, and Use of Illicit Drugs and Alcohol, and Related Complications During the 2020 COVID-19 Pandemic. Front. Psychiatry 2021, 12, 646206. [Google Scholar] [CrossRef]
- Radfar, S.R.; De Jong, C.A.; Farhoudian, A.; Ebrahimi, M.; Rafei, P.; Vahidi, M.; Yunesian, M.; Kouimtsidis, C.; Arunogiri, S.; Massah, O.; et al. Reorganization of Substance Use Treatment and Harm Reduction Services During the COVID-19 Pandemic: A Global Survey. Front. Psychiatry 2021, 12, 639393. [Google Scholar] [CrossRef]
- Cribbs, S.K.; Crothers, K.; Morris, A. Pathogenesis of HIV-Related Lung Disease: Immunity, Infection, and Inflammation. Physiol. Rev. 2020, 100, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; George, M.P.; Crothers, K.; Huang, L.; Lucht, L.; Kessinger, C.; Kleerup, E.C. HIV and Chronic Obstructive Pulmonary Disease: Is It Worse and Why? Proc. Am. Thorac. Soc. 2011, 8, 320–325. [Google Scholar] [CrossRef]
- Plymoth, M.; Sanders, E.J.; Van Der Elst, E.M.; Medstrand, P.; Tesfaye, F.; Winqvist, N.; Balcha, T.; Björkman, P. Socio-economic condition and lack of virological suppression among adults and adolescents receiving antiretroviral therapy in Ethiopia. PLoS ONE 2020, 15, e0244066. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Hatcher, A.M.; Weiser, S.D.; Johnson, M.O.; Rice, W.; Turan, J.M. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. Am. J. Public Health 2017, 107, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Turan, J.M.; Elafros, M.A.; Logie, C.H.; Banik, S.; Turan, B.; Crockett, K.B.; Pescosolido, B.; Murray, S.M. Challenges and opportunities in examining and addressing intersectional stigma and health. BMC Med. 2019, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.T.; Shin, L.M.; Mehta, N.N.; Pitman, R.K.; Fayad, Z.A.; Tawakol, A. Disentangling the Links Between Psychosocial Stress and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010931. [Google Scholar] [CrossRef]
- Dar, T.; Radfar, A.; Abohashem, S.; Pitman, R.K.; Tawakol, A.; Osborne, M.T. Psychosocial Stress and Cardiovascular Disease. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 23. [Google Scholar] [CrossRef]
- Backé, E.M.; Seidler, A.; Latza, U.; Rossnagel, K.; Schumann, B. The role of psychosocial stress at work for the development of car-diovascular diseases: A systematic review. Int. Arch. Occup. Environ. Health 2012, 85, 67–79. [Google Scholar] [CrossRef]
- Anema, A.; Vogenthaler, N.; Frongillo, E.A.; Kadiyala, S.; Weiser, S.D. Food insecurity and HIV/AIDS: Current knowledge, gaps, and research priorities. Curr. HIV/AIDS Rep. 2009, 6, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Peprah, E.; Armstrong-Hough, M.; Cook, S.; Mukasa, B.; Taylor, J.; Xu, H.; Chang, L.; Gyamfi, J.; Ryan, N.; Ojo, T.; et al. An Emerging Syndemic of Smoking and Cardiopulmonary Diseases in People Living with HIV in Africa. Int. J. Environ. Res. Public Health 2021, 18, 3111. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Providing Sustainable Mental and Neurological Health Care in Ghana and Kenya: Workshop Summary; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Altevogt, B.M.; Wizemann, T.M.; Norris, S.M.P.; Pankevich, D.E. Improving Access to Essential Medicines for Mental, Neurological, and Substance Use Disorders in Sub-Saharan Africa: Workshop Summary; National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Patel, V.; Chisholm, D.; Parikh, R.; Charlson, F.J.; Degenhardt, L.; Dua, T.; Ferrari, A.J.; Hyman, S.; Laxminarayan, R.; Levin, C.; et al. Global Priorities for Addressing the Burden of Mental, Neurological, and Substance Use Disorders. In Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 4. [Google Scholar]
- Cuff, P.; Ssali, Z.N.; Hanson, S.L.; Altevogt, B.M. Mental, Neurological, and Substance Use Disorders in Sub-Saharan Africa: Reducing the Treatment Gap, Improving Quality of Care: Summary of a Joint Workshop by the Institute of Medicine and the Uganda National Academy of Sciences; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Turner, H.A.; Turner, R.J. Gender, Social Status, and Emotional Reliance. J. Health Soc. Behav. 1999, 40, 360–373. [Google Scholar] [CrossRef]
- Earnshaw, V.A.; Eaton, L.A.; Collier, Z.K.; Watson, R.J.; Maksut, J.L.; Rucinski, K.; Kelly, J.F.; Kalichman, S.C. HIV Stigma, Depressive Symptoms, and Substance Use. AIDS Patient Care STDs 2020, 34, 275–280. [Google Scholar] [CrossRef]
- Esber, A.; Dear, N.; Reed, D.; Bahemana, E.; Owouth, J.; Maswai, J.; Kibuuka, H.; Iroezindu, M.; Crowell, T.A.; Polyak, C.S.; et al. Temporal trends in self-reported HIV stigma and association with adherence and viral sup-pression in the African Cohort Study. AIDS Care 2021, 1–8. [Google Scholar]
- Ingram, R.E. Origins of Cognitive Vulnerability to Depression. Cogn. Ther. Res. 2003, 27, 77–88. [Google Scholar] [CrossRef]
- Peacock, W.G.; Ragsdale, A.K. Social systems, ecological networks and disasters: Toward a socio-political ecology of disasters. Hurric. Ethn. Gend. Sociol. Disasters 1997, 20–35. [Google Scholar]
- Kuran, C.H.A.; Morsut, C.; Kruke, B.I.; Krüger, M.; Segnestam, L.; Orru, K.; Nævestad, T.O.; Airola, M.; Keränen, J.; Gabel, F.; et al. Vulnerability and vulnerable groups from an intersectionality perspective. Int. J. Disaster Risk Reduct. 2020, 50, 101826. [Google Scholar] [CrossRef]
- Schmutter, K.; Nash, M.; Dovey, L. Ocean acidification: Assessing the vulnerability of socioeconomic systems in Small Island Developing States. Reg. Environ. Chang. 2017, 17, 973–987. [Google Scholar] [CrossRef]
- Tsai, A.C.; Venkataramani, A.S. Syndemics and Health Disparities: A Methodological Note. AIDS Behav. 2016, 20, 423–430. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Auta, A.; Fawibe, A.; Gadanya, M.; Ezeigwe, N.; Mpazanje, R.G.; Dewan, M.T.; Omoyele, C.; Alemu, W.; Harhay, M.O.; et al. Current prevalence pattern of tobacco smoking in Nigeria: A systematic review and me-ta-analysis. BMC Public Health 2019, 19, 1719. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peprah, E.; Myers, B.; Kengne, A.-P.; Peer, N.; El-Shahawy, O.; Ojo, T.; Mukasa, B.; Ezechi, O.; Iwelunmor, J.; Ryan, N.; et al. Using a Syndemics Framework to Understand How Substance Use Contributes to Morbidity and Mortality among People Living with HIV in Africa: A Call to Action. Int. J. Environ. Res. Public Health 2022, 19, 1097. https://doi.org/10.3390/ijerph19031097
Peprah E, Myers B, Kengne A-P, Peer N, El-Shahawy O, Ojo T, Mukasa B, Ezechi O, Iwelunmor J, Ryan N, et al. Using a Syndemics Framework to Understand How Substance Use Contributes to Morbidity and Mortality among People Living with HIV in Africa: A Call to Action. International Journal of Environmental Research and Public Health. 2022; 19(3):1097. https://doi.org/10.3390/ijerph19031097
Chicago/Turabian StylePeprah, Emmanuel, Bronwyn Myers, Andre-Pascal Kengne, Nasheeta Peer, Omar El-Shahawy, Temitope Ojo, Barbara Mukasa, Oliver Ezechi, Juliet Iwelunmor, Nessa Ryan, and et al. 2022. "Using a Syndemics Framework to Understand How Substance Use Contributes to Morbidity and Mortality among People Living with HIV in Africa: A Call to Action" International Journal of Environmental Research and Public Health 19, no. 3: 1097. https://doi.org/10.3390/ijerph19031097
APA StylePeprah, E., Myers, B., Kengne, A.-P., Peer, N., El-Shahawy, O., Ojo, T., Mukasa, B., Ezechi, O., Iwelunmor, J., Ryan, N., Sakho, F., Patena, J., & Gyamfi, J. (2022). Using a Syndemics Framework to Understand How Substance Use Contributes to Morbidity and Mortality among People Living with HIV in Africa: A Call to Action. International Journal of Environmental Research and Public Health, 19(3), 1097. https://doi.org/10.3390/ijerph19031097










