Learning Curve for Metastatic Liver Tumor Open Resection in Patients with Primary Colorectal Cancer: Use of the Cumulative Sum Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Procedures Characteristics
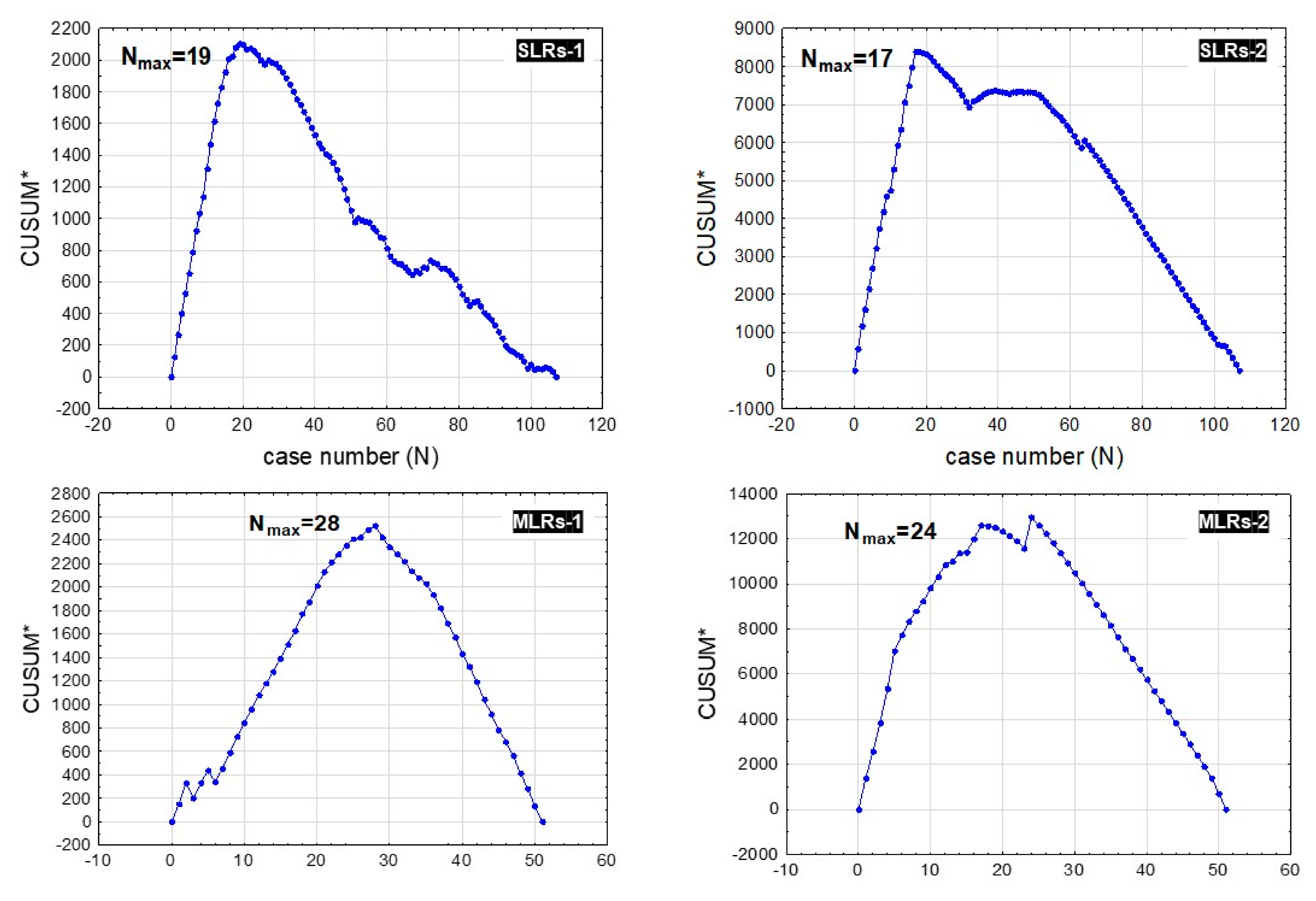
2.2. Statistical Analysis
3. Results
3.1. Patients and Procedure
3.2. Learning Curve Endpoints
3.3. Patient Safety
4. Discussion
5. Conclusions
- As expected, SLR procedures were performed faster and with less intraoperative blood loss levels, shorter postoperative stays, and fewer MAEs and SAEs than MLR procedures.
- Fewer procedures were needed to gain stabilization and repeatability in operating times and intraoperative blood loss levels in SLRs compared to MLR procedures.
- Operative time and intraoperative blood loss cannot be surrogates for SAE risk in MLRs, as they present significantly different learning curves.
- In MLR procedures, SAE reduction is gained significantly later than operative time and intraoperative blood loss level stabilization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horn, S.R.; Stoltzfus, K.C.; Lehrer, E.J.; Dawson, L.A.; Tchelebi, L.; Gusani, N.J.; Sharma, N.K.; Chen, H.; Trifiletti, D.M.; Zaorsky, N.G. Epidemiology of liver metastases. Cancer Epidemiol. 2020, 67, 101760. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [Green Version]
- Riihimäki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic sites and survival in lung cancer. Lung Cancer 2014, 86, 78–84. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitanidis, A.; Alevizakos, M.; Tsaroucha, A.; Simopoulos, C.; Pitiakoudis, M. Incidence and predictors of synchronous liver metastases in patients with gastrointestinal stromal tumors (GISTs). Am. J. Surg. 2018, 216, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, K.; Yang, C.; Tan, Q.; Song, W.; Lu, M.; Zhao, W.; Lou, G.; Li, Z.; Li, K.; Hou, Y. Sites of distant metastases and overall survival in ovarian cancer: A study of 1481 patients. Gynecol. Oncol. 2018, 150, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef]
- Merola, G.; Cavallaro, G.; Iorio, O.; Frascio, M.; Pontecorvi, E.; Corcione, F.; Andreuccetti, J.; Pignata, G.; Stabilini, C.; Bracale, U. Learning curve in open inguinal hernia repair: A quality improvement multicentre study about Lichtenstein technique. Hernia 2020, 24, 651–659. [Google Scholar] [CrossRef]
- Barrie, J.; Jayne, D.G.; Wright, J.; Murray, C.J.; Collinson, F.J.; Pavitt, S.H. Attaining surgical competency and its implications in surgical clinical trial design: A systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann. Surg. Oncol. 2014, 21, 829–840. [Google Scholar] [CrossRef]
- Chaput de Saintonge, D.M.; Vere, D.W. Why don’t doctors use cusums? Lancet 1974, 1, 120–121. [Google Scholar] [CrossRef]
- Wohl, H. The cusum plot: Its utility in the analysis of clinical data. N. Engl. J. Med. 1977, 296, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Cheng, K.C.; Yeung, Y.P.; Ho, K.M. Learning Curve for Laparoscopic Major Hepatectomy: Use of the Cumulative Sum Method. Surg. Laparosc. Endosc. Percutan Tech. 2016, 26, e41–e45. [Google Scholar] [CrossRef]
- Nomi, T.; Fuks, D.; Kawaguchi, Y.; Mal, F.; Nakajima, Y.; Gayet, B. Learning curve for laparoscopic major hepatectomy. Br. J. Surg. 2015, 102, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Woo, J.W.; Lee, J.K.; Park, J.H.; Kim, J.Y.; Kwag, S.J.; Park, T.; Jeong, S.H.; Ju, Y.T.; Jeong, E.J.; et al. Comparison of Learning Curves for Major and Minor Laparoscopic Liver Resection. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Halls, M.C.; Alseidi, A.; Berardi, G.; Cipriani, F.; Van der Poel, M.; Davila, D.; Ciria, R.; Besselink, M.; D’Hondt, M.; Dagher, I.; et al. A Comparison of the Learning Curves of Laparoscopic Liver Surgeons in Differing Stages of the IDEAL Paradigm of Surgical Innovation: Standing on the Shoulders of Pioneers. Ann. Surg. 2019, 269, 221–228. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, C.W.; Cho, M.S.; Baik, S.H.; Kim, D.W.; Min, B.S.; Lee, K.Y.; Kim, N.K. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg. Endosc. 2014, 28, 2821–2831. [Google Scholar] [CrossRef]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef]
- Morino, M.; Morra, I.; Rosso, E.; Miglietta, C.; Garrone, C. Laparoscopic vs. open hepatic resection: A comparative study. Surg. Endosc. 2003, 17, 1914–1918. [Google Scholar] [CrossRef]
- Memeo, R.; de’Angelis, N.; Compagnon, P.; Salloum, C.; Cherqui, D.; Laurent, A.; Azoulay, D. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World J. Surg. 2014, 38, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Sandro, S.; Bagnardi, V.; Najjar, M.; Buscemi, V.; Lauterio, A.; De Carlis, R.; Danieli, M.; Pinotti, E.; Benuzzi, L.; De Carlis, L. Minor laparoscopic liver resection for Hepatocellular Carcinoma is safer than minor open resection, especially for less compensated cirrhotic patients: Propensity score analysis. Surg. Oncol. 2018, 27, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Laurent, A.; Tayar, C.; Tomatis, M.; Ponti, A.; Cherqui, D. The learning curve in laparoscopic liver resection: Improved feasibility and reproducibility. Ann. Surg. 2009, 25, 772–782. [Google Scholar] [CrossRef]

| Total (n = 158) | SLRs % (n = 107) | MLRs %% (n = 51) | p * | |
|---|---|---|---|---|
| Mean age (±SD $) [years] | 57.60 (±13.03) | 58.20 (±13.89) | 56.31 (±11.03) | 0.407 |
| Mean BMI $$ (±SD $) [kg/m2] | 26.88 (±4.57) | 26.80 (±4.61) | 27.08 (±4.52) | 0.731 |
| Male (%)/Female (%) | 82 (51.90%)/ 76 (48.10%) | 55 (51.40%)/ 52 (49.60%) | 27 (52.94%)/ 24 (47.06%) | 0.856 |
| Median operating time (IQR $$$) [min.] | 205 (IQR: 165) | 170 (IQR: 600) | 400 (IQR: 195) | <0.001 ** |
| Median postoperative hospital stay (IQR $$$) [days] | 6 (IQR: 3) | 5 (IQR: 3) | 8 (IQR: 7) | 0.016 |
| Median intraoperative blood loss (IQR $$$) [mL] | 330 (IQR: 540) | 170 (IQR: 155) | 450 (IQR: 980) | <0.001 ** |
| Number of minor adverse effects (%) | 71 (44.94%) | 46 (42.99%) | 25 (49.02%) | 0.476 |
| Number of severe adverse effects (%) | 28 (17.72%) | 15 (14.02%) | 13 (25.49%) | 0.077 |
| Incidence of Pringle’s manouver (%) | 81 (51.27%) | 58 (54.21%) | 23 (45.10%) | 0.284 |
| Median time of Pinard’s manouver (IQR $$$) [min.] | 15 (IQR: 30) | 15 (IQR: 30) | 30 (IQR: 15) | 0.534 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banas, B.; Gwizdak, P.; Zabielska, P.; Kolodziejczyk, P.; Richter, P. Learning Curve for Metastatic Liver Tumor Open Resection in Patients with Primary Colorectal Cancer: Use of the Cumulative Sum Method. Int. J. Environ. Res. Public Health 2022, 19, 1068. https://doi.org/10.3390/ijerph19031068
Banas B, Gwizdak P, Zabielska P, Kolodziejczyk P, Richter P. Learning Curve for Metastatic Liver Tumor Open Resection in Patients with Primary Colorectal Cancer: Use of the Cumulative Sum Method. International Journal of Environmental Research and Public Health. 2022; 19(3):1068. https://doi.org/10.3390/ijerph19031068
Chicago/Turabian StyleBanas, Bartlomiej, Piotr Gwizdak, Paulina Zabielska, Piotr Kolodziejczyk, and Piotr Richter. 2022. "Learning Curve for Metastatic Liver Tumor Open Resection in Patients with Primary Colorectal Cancer: Use of the Cumulative Sum Method" International Journal of Environmental Research and Public Health 19, no. 3: 1068. https://doi.org/10.3390/ijerph19031068
APA StyleBanas, B., Gwizdak, P., Zabielska, P., Kolodziejczyk, P., & Richter, P. (2022). Learning Curve for Metastatic Liver Tumor Open Resection in Patients with Primary Colorectal Cancer: Use of the Cumulative Sum Method. International Journal of Environmental Research and Public Health, 19(3), 1068. https://doi.org/10.3390/ijerph19031068






