Transvenous Lead Extraction in Adult Patient with Leads Implanted in Childhood-Is That the Same Procedure as in Other Adult Patients?
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Lead Extraction Procedure
2.3. Definitions
2.4. Statistical Analysis
2.5. Approval of the Bioethics Committee
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macr, R.; Redaelli, S.; Eufrate, S. Permanent pacemaker implantation in children after open heart cardiac surgery (author’s transl). G. Ital. Cardiol. 1978, 8 (Suppl. 1), 240–244. [Google Scholar]
- Hayes, D.L.; Holmes, D.R., Jr.; Maloney, J.D.; Neubauer, S.A.; Ritter, D.G.; Danielson, G.K. Permanent endocardial pacing in pediatric patients. J. Thorac. Cardiovasc. Surg. 1983, 85, 618–624. [Google Scholar] [CrossRef]
- Janson, C.M.; Patel, A.R.; Bonney, W.J.; Smoots, K.; Shah, M.J. Implantable cardioverter-defibrillator lead failure in children and young adults: A matter of lead diameter or lead design? J. Am. Coll. Cardiol. 2014, 63, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Fortescue, E.B.; Berul, C.I.; Cecchin, F.; Walsh, E.P.; Triedman, J.K.; Alexander, M.E. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm. 2004, 1, 150–159. [Google Scholar] [CrossRef]
- Atallah, J.; Erickson, C.E.; Cecchin, F.; Dubin, A.M.; Law, I.H.; Cohen, M.I.; LaPage, M.J.; Cannon, B.C.; Chun, T.U.H.; Freedenberg, V.; et al. Multi-institutional study of implantable defibrillator lead performance in children and young adults: Results of the Pediatric Lead Extractability and Survival Evaluation (PLEASE) Study. Circulation 2013, 127, 2393–2402. [Google Scholar] [CrossRef]
- Cooper, J.M.; Stephenson, E.A.; Berul, C.I.; Walsh, E.P.; Epstein, L.M. Implantable cardioverter defibrillator lead complications and laser extraction in children and young adults with congenital heart disease: Implications for implantation and management. J. Cardiovasc. Electrophysiol. 2003, 14, 344–349. [Google Scholar] [CrossRef]
- Silvetti, M.S.; Drago, F.; Grutter, G.; De Santis, A.; Diciommo, V.; Rava, L. Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace 2006, 8, 530–536. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H., 3rd; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef]
- McCanta, A.C.; Schaffer, M.S.; Collins, K.K. Pediatric and Adult Congenital Endocardial Lead Extraction or Abandonment Decision (PACELEAD) survey of lead management. Pacing Clin. Electrophysiol. 2011, 34, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Suga, C.; Hayes, D.L.; Hyberger, L.K.; Lloyd, M.A. Is there an adverse outcome from abandoned pacing leads? J. Interv. Card. Electrophysiol. 2000, 4, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Silvetti, M.S.; Drago, F. Outcome of young patients with abandoned, nonfunctional endocardial leads. Pacing Clin. Electrophysiol. 2008, 31, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.A.; Van Zandt, H.; Collins, E.; LeGras, M.; Perry, J. Lead extraction in young patients with and without congenital heart disease using the subclavian approach. Pacing Clin. Electrophysiol. 1996, 19, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Freedenberg, V.; Ramwell, C.; Skeete, A. Effectiveness of excimer laser-assisted pacing and ICD lead extraction in children and young adults. Pacing Clin. Electrophysiol. 2006, 29, 461–466. [Google Scholar] [CrossRef]
- Dilber, E.; Karagöz, T.; Celiker, A. Lead extraction in children and young adults using different techniques. Med. Princ. Pract. 2009, 18, 356–359. [Google Scholar] [CrossRef]
- Cecchin, F.; Atallah, J.; Walsh, E.P.; Triedman, J.K.; Alexander, M.E.; Berul, C.I. Lead extraction in pediatric and congenital heart disease patients. Circ. Arrhythm. Electrophysiol. 2010, 3, 437–444. [Google Scholar] [CrossRef]
- Zartner, P.A.; Wiebe, W.; Toussaint-Goetz, N.; Schneider, M.B. Lead removal in young patients in view of lifelong pacing. Europace 2010, 12, 714–718. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous Lead Extraction SAFeTY Score for Risk Stratification and Proper Patient Selection for Removal Procedures Using Mechanical Tools. J. Clin. Med. 2020, 9, 361. [Google Scholar] [CrossRef]
- Byrd, C.L.; Schwartz, S.J.; Hedin, N.B.; Goode, L.B.; Fearnot, N.E.; Smith, H.J. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin. Electrophysiol. 1990, 13, 1871–1875. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Soldati, E.; Zucchelli, G.; Di Cori, A.; Segreti, L.; De Lucia, R.; Solarino, G.; Balbarini, A.; Marzilli, M.; Mariani, M. Transvenous removal of pacing and implantable cardiac defibrillating leads using single sheath mechanical dilatation and multiple venous approaches: High success rate and safety in more than 2000 leads. Eur. Heart J. 2008, 29, 2886–2893. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.; Epstein, L.M.; Carrillo, R.G.; Love, C.; Adler, S.W.; Riggio, D.W.; Karim, S.S.; Bashir, J.; Greenspon, A.J.; DiMarco, J.P.; et al. Lead extraction in the contemporary setting: The LExICon study: An observational retrospective study of consecutive laser lead extractions. J. Am. Coll. Cardiol. 2010, 55, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm. 2014, 11, 799–805. [Google Scholar] [CrossRef]
- Bashir, J.; Fedoruk, L.M.; Ofiesh, J.; Karim, S.S.; Tyers, G.F. Classification and Surgical Repair of Injuries Sustained During Transvenous Lead Extraction. Circ. Arrhythm. Electrophysiol. 2016, 9, e003741. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Tarakji, K.G.; Martin, D.O.; Gadre, A.; Fraser, T.; Kim, A.; Brunner, M.P.; Barakat, A.F.; Saliba, W.I.; Kanj, M.; et al. Cardiac Implantable Electronic Device Infections: Added Complexity and Suboptimal Outcomes with Previously Abandoned Leads. JACC Clin. Electrophysiol. 2017, 3, 1–9. [Google Scholar]
- Kutarski, A.; Czajkowski, M.; Pietura, R.; Obszanski, B.; Polewczyk, A.; Jachec, W.; Polewczyk, M.; Mlynarczyk, K.; Grabowski, M.; Opolski, G. Effectiveness, safety, and long-term outcomes of non-powered mechanical sheaths for transvenous lead extraction. Europace 2018, 20, 1324–1333. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- Sood, N.; Martin, D.T.; Lampert, R.; Curtis, J.P.; Parzynski, C.; Clancy, J. Incidence and Predictors of Perioperative Complications with Transvenous Lead Extractions: Real-World Experience with National Cardiovascular Data Registry. Circ. Arrhythm. Electrophysiol. 2018, 11, e004768. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Segreti, L.; Bongiorni, M.G.; Kutarski, A. To abandon or not to abandon: Late consequences of pacing and ICD lead abandonment. Pacing Clin. Electrophysiol. 2019, 42, 1006–1017. [Google Scholar] [CrossRef]
- Segreti, L.; Giannotti Santoro, M.; Di Cori, A.; Fiorentini, F.; Zucchelli, G.; Bernini, G.; De Lucia, R.; Viani, S.; Paperini, L.; Barletta, V.; et al. Safety and efficacy of transvenous mechanical lead extraction in patients with abandoned leads. Europace 2020, 22, 1401–1408. [Google Scholar] [CrossRef]
- Starck, C.T.; Gonzalez, E.; Al-Razzo, O.; Mazzone, P.; Delnoy, P.P.; Breitenstein, A.; Steffel, J.; Eulert-Grehn, J.; Lanmüller, P.; Melillo, F.; et al. Results of the Patient-Related Outcomes of Mechanical lead Extraction Techniques (PROMET) study: A multicentre retrospective study on advanced mechanical lead extraction techniques. Europace 2020, 22, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Giannotti Santoro, M.; Segreti, L.; Zucchelli, G.; Barletta, V.; Fiorentini, F.; Di Cori, A.; De Lucia, R.; Bongiorni, M.G. Transvenous lead extraction: Efficacy and safety of the procedure in octogenarian patients. Pacing Clin. Electrophysiol. 2020, 43, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Klug, D.; Vaksmann, G.; Jarwe, M.; Wallet, F.; Francart, C.; Kacet, S.; Rey, C. Pacemaker lead infection in young patients. Pacing Clin. Electrophysiol. 2003, 26, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Gabbarini, F.; Golzio, P.G.; Bordese, R.; Agnoletti, G. Reforming of intracardiac lead loops in pediatric patients. Int. J. Cardiol. 2014, 171, e86–e87. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Gould, J.; Sieniewicz, B.; Porter, B.; Rinaldi, C.A. The role of transvenous lead extraction in the management of redundant or malfunctioning pacemaker and defibrillator leads post ELECTRa. Europace 2018, 20, 1733–1740. [Google Scholar] [CrossRef]
- Mori, H.; Kato, R.; Ikeda, Y.; Tsutsui, K.; Hoya, H.; Tanaka, S.; Iwanaga, S.; Nakano, S.; Muramatsu, T.; Sumitomo, N.; et al. Transvenous lead performance of implantable cardioverter-defibrillators and pacemakers. Pacing Clin. Electrophysiol. 2021, 44, 481–489. [Google Scholar] [CrossRef]
- Sterns, L.D. Pacemaker lead surveillance and failure: Is there a signal in the noise? Heart Rhythm. 2019, 16, 579–580. [Google Scholar] [CrossRef]
- Mendenhall, G.S.; Saba, S. Prophylactic lead extraction at implantable cardioverter-defibrillator generator change. Circ. Arrhythm. Electrophysiol. 2014, 7, 330–336. [Google Scholar] [CrossRef][Green Version]
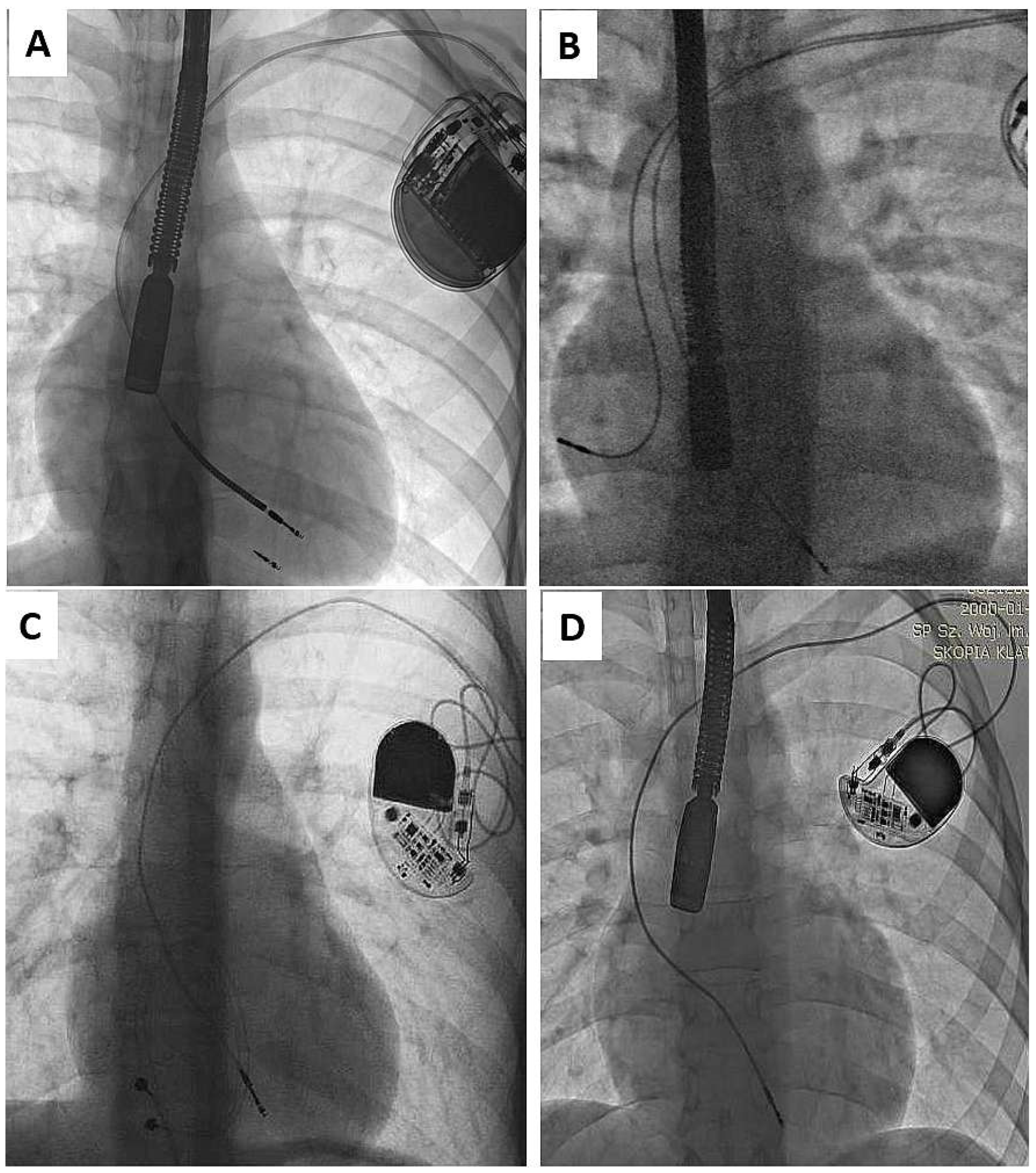
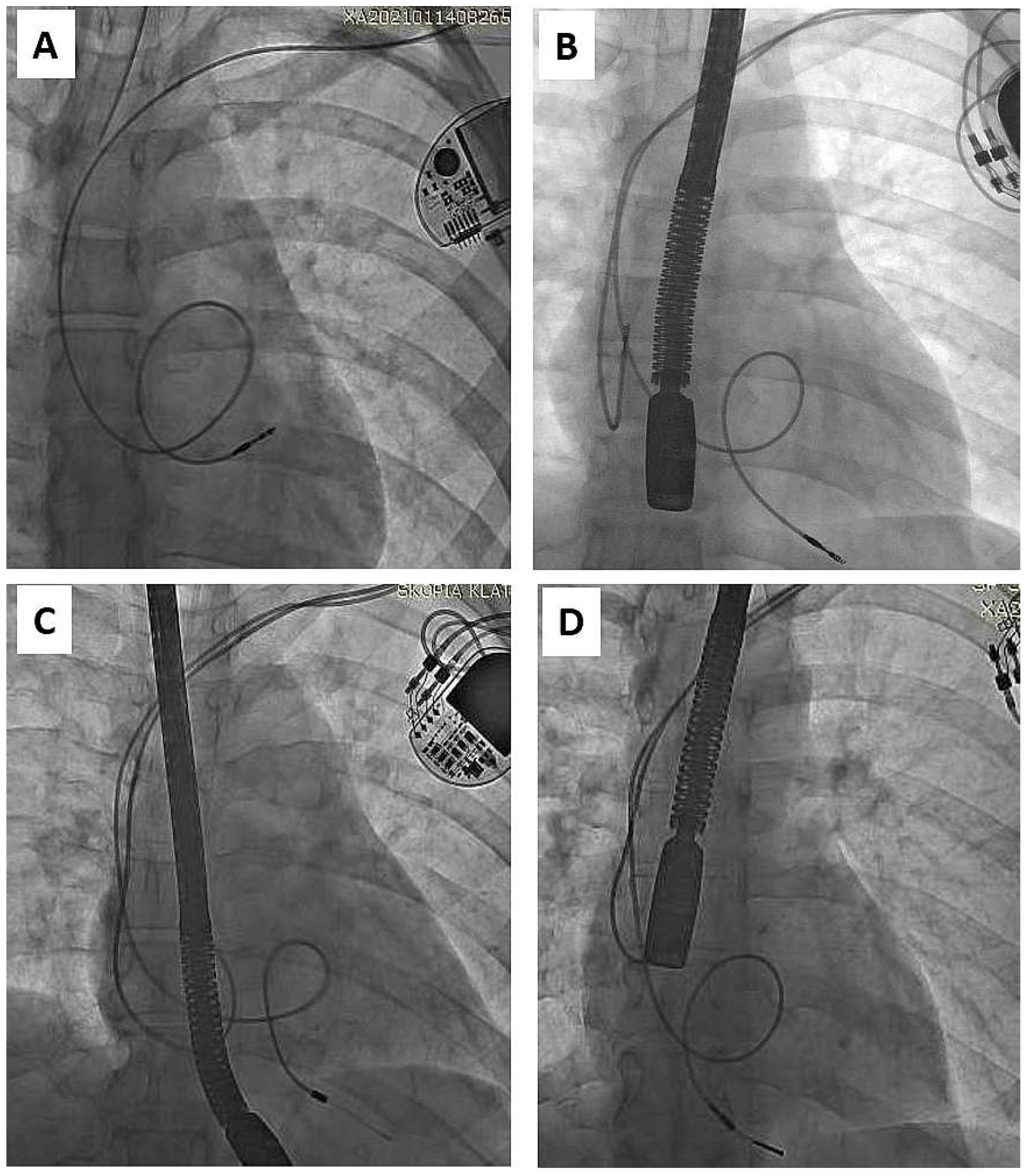
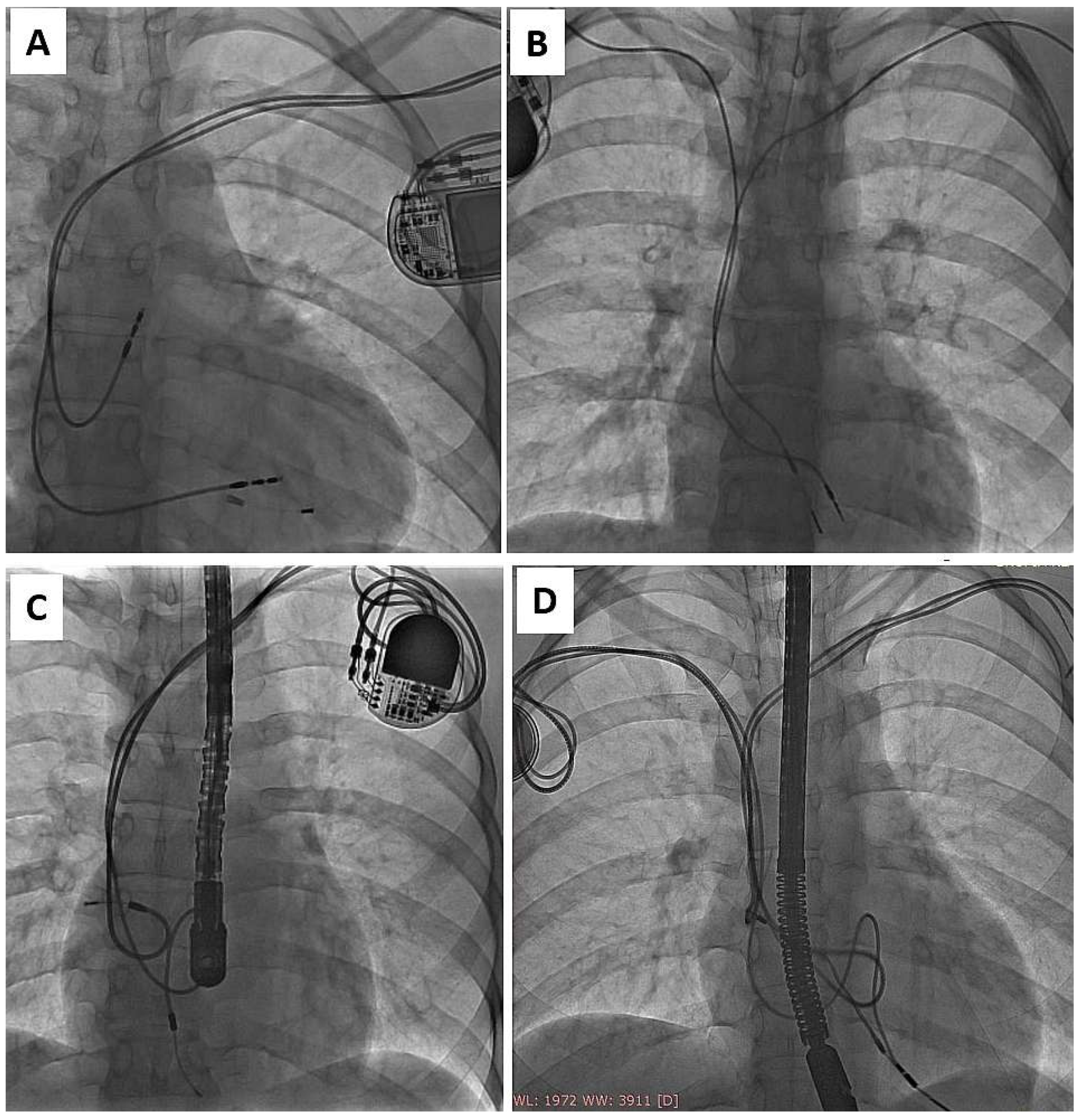
| Groups | CIP (A) | AIP (B) | A vs. B | ||
|---|---|---|---|---|---|
| Number | 98 | 2659 | |||
| Mean ± SD n (%) | Mean ± SD n (%) | p | |||
| Patient characteristics | |||||
| Patient age at TLE [years] | 27.46 | 6.77 | 66.49 | 9.38 | <0.001 |
| Patient age at first system implantation [years] | 13.04 | 4.47 | 58.44 | 11.51 | <0.001 |
| Sex (female patients) | 48 | 48.98% | 994 | 37.38 | 0.009 |
| Etiology: (ischemic heart disease) | 1 | 1.025% | 1507 | 56.68 | <0.001 |
| Etiology: cardiomyopathy | 5 | 5.10% | 465 | 17.49 | 0.002 |
| Etiology: congenital, channelopathy, neurocardiogenic, or cardiac surgery | 92 | 93.88% | 696 | 26.18 | <0.001 |
| NYHA class III and IV | 0 | 0.00% | 401 | 15.08 | <0.001 |
| LVEF average [%] | 60.00 | 8.69 | 47.81 | 15.48 | <0.001 |
| Tricuspid regurgitation before TLE: significant | 13 | 13.27% | 362 | 13.61% | 0.957 |
| Tricuspid regurgitation before TLE: severe | 3 | 3.06% | 89 | 3.35% | 0.895 |
| Diabetes (any) | 2 | 2.04% | 561 | 21.10% | <0.001 |
| Renal failure (any) | 0 | 0.00% | 543 | 20.42% | <0.001 |
| Creatinine level [mg/dL] | 0.83 | 0.17 | 1.24 | 1.84 | <0.001 |
| BMI [kg/m2] | 24.34 | 4.40 | 28.31 | 5.40 | <0.001 |
| Previous sternotomy | 20 | 21.04% | 400 | 15.04% | 0.191 |
| Valve prosthesis | 5 | 5.10% | 216 | 8.12% | 0.372 |
| Long-term anticoagulation | 8 | 8.16% | 1076 | 40.47% | <0.001 |
| Long-term antiplatelet treatment | 7 | 7.14% | 1229 | 46.22% | <0.001 |
| Charlson comorbidity index [points] | 0.20 | 1.01 | 4.73 | 3.52 | <0.001 |
| Indications for TLE (main, predominant) | |||||
| Systemic infection | 12 | 12.24% | 595 | 22.38% | 0.024 |
| Local (pocket) infection | 6 | 6.12% | 263 | 9.89% | 0.289 |
| Mechanical lead damage (electrical failure) | 43 | 43.87% | 684 | 25.72% | <0.001 |
| Lead dysfunction (exit/entry block, dislodgement, or extracardiac pacing) | 8 | 8.16% | 331 | 12.45% | 0.266 |
| Lead dysfunction caused by (usually dry) perforation | 6 | 6.12% | 289 | 10.87% | 0.185 |
| Change of pacing mode/upgrading, downgrading | 4 | 4.08% | 163 | 6.13% | 0.536 |
| Abandoned lead/prevention of abandonment (AF, superfluous leads) | 2 | 2.04% | 87 | 3.27% | 0.699 |
| Threating/potentially threatening lead (loops, free ending, left heart, or LDTVD) | 9 | 9.18% | 80 | 3.01% | 0.002 |
| Other (MRI indications, cancer, painful pocket, or pacing/ICD no longer necessary) | 5 | 5.10% | 66 | 2.48% | 0.199 |
| Re-establishing venous access (symptomatic occlusion, SVC syndrome, or lead replacement/upgrading) | 3 | 3.06% | 101 | 3.80% | 0.915 |
| Groups | CIP (A) | AIP (B) | A vs. B | ||
|---|---|---|---|---|---|
| Number | 98 | 2659 | |||
| Mean ± SD n (%) Median * IQR * | Mean ± SD n (%) Median * IQR * | p | |||
| Patient/system/procedure information | |||||
| System removal—infection | 17 | 17.35% | 779 | 29.30% | 0.014 |
| Upgrading | 18 | 18.36% | 294 | 11.06% | 0.037 |
| Downgrading | 0 | 0.00% | 100 | 3.76% | 0.093 |
| Lead replacement | 48 | 48.97% | 1289 | 48.48% | 0.943 |
| Superfluous lead extraction | 7 | 7.14% | 86 | 3.23% | 0.069 |
| Complete device system removal | 4 | 4.08% | 38 | 1.43% | 0.092 |
| System removal—reimplantation deferred | 4 | 4.08% | 73 | 2.75% | 0.643 |
| System and history of pacing | |||||
| PM—AAI | 3 | 3.06% | 192 | 7.22% | 0.169 |
| PM—DDD | 62 | 63.27% | 1185 | 44.57% | <0.001 |
| PM—VDD | 2 | 2.04% | 53 | 1.99% | 0.738 |
| PM—VVI | 22 | 22.45% | 268 | 10.08% | <0.001 |
| PM—CRT-P | 0 | 0.00% | 74 | 2.78% | 0.175 |
| Abandoned only PM lead (unit removed earlier) before TLE | 1 | 1.02% | 20 | 0.75% | 0.771 |
| ICD—VVI | 2 | 2.04% | 347 | 13.05% | 0.002 |
| ICD—DDD | 6 | 6.12% | 291 | 10.94% | 0.178 |
| ICD—CRT-D | 0 | 0.00% | 220 | 8.27% | 0.006 |
| Abandoned only ICD lead (unit removed earlier) before TLE | 0 | 0.00% | 8 | 0.30% | 0.680 |
| Leads before TLE | |||||
| Number of leads in the system | 1.69 | 0.48 | 1.83 | 0.65 | 0.105 |
| Patients with abandoned leads | 19 | 19.39% | 308 | 11.58% | 0.029 |
| Number of abandoned leads | 0.26 | 0.60 | 0.16 | 0.49 | 0.328 |
| Patients with multiple abandoned leads | 6 | 6.12% | 102 | 3.84% | 0.367 |
| Number of leads in the heart | 1.96 | 0.74 | 1.98 | 0.77 | 0.392 |
| ICD lead presence | 7 | 7.14% | 839 | 31.55% | <0.001 |
| One single-coil ICD lead | 3 | 3.06% | 344 | 12.94% | 0.006 |
| Dual-coil ICD lead | 2 | 2.04% | 443 | 16.66% | <0.001 |
| CS lead presence | 1 | 1.02% | 481 | 18.09% | <0.001 |
| Leads on the left side of the chest | 83 | 84.69% | 2512 | 94.47% | <0.001 |
| Leads on the right side of the chest | 5 | 5.10% | 67 | 2.52% | 0.201 |
| Leads on both sides of the chest | 10 | 10.20% | 80 | 3.01% | <0.001 |
| Previous TLE | 10 | 10.20% | 126 | 4.74% | 0.027 |
| Excessive lead slack on X ray | 16 | 16.33% | 137 | 5.15% | <0.001 |
| Number of procedures before lead extraction | 2.23 | 1.14 | 1.87 | 1.02 | <0.001 |
| Dwell time of oldest lead per patient [months] | 169.0 * | 109.0 * | 75.96 * | 81.96 * | <0.001 |
| Mean lead implant duration (per patient) [months] | 156.0 * | 85.68 * | 72.00 * | 73.68 * | <0.001 |
| Groups | CIP (A) | AIP (B) | A vs. B | ||
|---|---|---|---|---|---|
| Number | 98 | 2659 | |||
| Mean ± SD n (%) Median * IQR * | Mean ± SD n (%) Median * IQR * | p | |||
| Venue of the procedure | |||||
| Electrophysiology laboratory | 42 | 42.86% | 1405 | 52.84% | 0.066 |
| Cardiac surgery operating room | 18 | 18.37% | 504 | 18.99% | 0.989 |
| Hybrid room | 38 | 38.78% | 750 | 28.21% | 0.031 |
| The role of cardiac surgeon | |||||
| Co-operator | 51 | 52.04% | 1262 | 47.46% | 0.468 |
| Standby | 47 | 47,96% | 1397 | 52.54% | 0.468 |
| Type of anesthesia | |||||
| General anesthesia | 47 | 47.96% | 1162 | 43.70% | 0.465 |
| Local anesthesia + general sedation, analgesia | 51 | 52.04% | 1497 | 56.30% | 0.465 |
| TEE monitoring as mandatory standard (with rare exceptions) since 2015 y | |||||
| Routine TEE in monitoring lead extraction | 38 | 38.78% | 1022 | 38.44% | 0.970 |
| Lack of TEE monitoring during TLE procedure as the rule | 60 | 61.22% | 1637 | 61.56% | 0.970 |
| Procedure-related risk factors for major complications and increased procedure complexity | |||||
| Number of extracted leads in one patient | 1.84 | 0.93 | 1.67 | 0.77 | 0.328 |
| One or two leads were extracted | 89 | 90.82% | 2345 | 88.19% | 0.719 |
| Three or more leads were extracted | 9 | 9.18% | 312 | 11.73% | 0.562 |
| Leads extracted from both sides of the chest during the same TLE | 4 | 4.08% | 33 | 1.24% | 0.051 |
| Approach—left (side of the chest) | 79 | 80.61% | 2508 | 94.32% | <0.001 |
| Approach—right (side of the chest) | 6 | 6.12% | 48 | 1.81% | 0.008 |
| Approach—both (sides of the chest) | 4 | 4.08% | 19 | 0.71% | 0.002 |
| Approach—subclavian + femoral | 3 | 3.06% | 21 | 0.79% | 0.002 |
| Extraction of lead with endocardial excessive slack | 12 | 12.24% | 94 | 3.54% | <0.001 |
| Extraction of broken lead with endocardial excessive slack | 3 | 3.06% | 67 | 2.52% | 0.994 |
| Extraction of abandoned lead(s) (any) | 19 | 19.39% | 288 | 10.83% | 0.015 |
| Extraction of abandoned lead(s) (per patient) | 2.27 | 0.02 | 0.15 | 0.00 | 0.030 |
| ICD lead extracted | 7 | 7.14% | 788 | 29.64% | <0.001 |
| CS (LV pacing) lead extracted | 0 | 0.00% | 185 | 6.96% | 0.013 |
| Oldest extracted lead dwell time [months] | 169.0 * | 96.00 * | 75.00 * | 81.96 * | <0.001 |
| Mean (per patient) extracted lead dwell time [months] | 156.0 * | 89.04 * | 72.96 * | 87.76 * | <0.001 |
| Cumulative dwell time of extracted leads (sum of dwell times of extracted leads) [years] | 20.67 * | 15.00 * | 8.83 * | 12.25 * | <0.001 |
| SAFeTY score of MC risk [20]—number of points | 10.35 | 4.16 | 5.65 | 4.21 | <0.001 |
| Groups | CIP (A) | AIP (B) | A vs. B | ||
|---|---|---|---|---|---|
| Number | 98 | 2659 | |||
| Median * IQR * n (%) | Median * IQR * n (%) | p | |||
| Procedure complexity | |||||
| Procedure duration (“skin-to-skin”) [minutes] | 63.00 * | 22.00 * | 55.00 * | 20.00 * | <0.001 |
| Procedure duration (“sheath-to-sheath”) [minutes] | 21.00 * | 26.00 * | 8.00 * | 8.00 * | <0.001 |
| Mean time of single lead extraction (“sheath-to-sheath”/number of extracted leads) [minutes] All leads extracted | 12.50 * | 18.00 * | 4.50 * | 5.00 * | <0.001 |
| 84 | 88.42% | 2001 | 75.25% | 0.021 | |
| Functional leads left in place for continued use | 14 | 14.74% | 636 | 23.92% | <0.001 |
| Non-functional leads left in place | 0 | 0.00% | 16 | 0.60% | 0.926 |
| Non-functional, superfluous leads extracted | 19 | 20.00% | 288 | 10.83% | 0.013 |
| Procedure complexity/unexpected technical problems | |||||
| Technical problem during TLE (any) | 48 | 50.53% | 521 | 19.59% | <0.001 |
| Blockage in implant vein (subclavian region) | 16 | 16.84% | 188 | 7.07% | <0.001 |
| Lead-to-lead adhesion | 14 | 14.74% | 181 | 6.81% | 0.008 |
| Byrd dilator collapse/torsion/“fracture” | 15 | 15.79% | 77 | 2.90% | <0.001 |
| Extracted lead fracture/rupture during extraction | 22 | 23.16% | 150 | 5.64% | <0.001 |
| Need to use alternative approach | 14 | 14.74% | 103 | 3.87% | <0.001 |
| Number of technical problems | 1.61 | 1.00 | 1.35 | 0.67 | <0.001 |
| One technical problem only | 24 | 25.26% | 304 | 11.43% | <0.001 |
| Two technical problems | 13 | 13.68% | 74 | 2.78% | <0.001 |
| Three or more technical problems | 4 | 4.21% | 30 | 1.13% | 0.033 |
| Other minor technical problems | 14 | 14.74% | 126 | 4.74% | <0.001 |
| Use of additional tools | |||||
| Evolution (old and RL) or TightRail | 9 | 9.47% | 30 | 1.13% | <0.001 |
| Metal sheaths | 17 | 17.89% | 183 | 6.88% | <0.001 |
| Lasso catheters/snares/basket catheters | 14 | 14.74% | 86 | 3.23% | <0.001 |
| Loop created with a catheter, guidewire, and lasso | 3 | 3.16% | 47 | 1.77% | 0.478 |
| Groups | CIP (A) | AIP (B) | A vs. B p | ||
|---|---|---|---|---|---|
| Number | 98 | 2659 | |||
| Partial or lack of radiographic success | |||||
| Partial radiographic success (retained tip of lead) | 6 | 6.12% | 51 | 1.92% | 0.012 |
| Partial radiographic success (retained <4 cm lead fragment) | 9 | 9.18% | 42 | 1.58% | <0.001 |
| Lack of radiographic success (retained lead or long portion of lead) | 0 | 0.00% | 5 | 0.19% | 0.440 |
| Major complications | |||||
| Major complications (any) | 5 | 5.10% | 54 | 2.03% | 0.088 |
| Hemopericardium | 4 | 4.08% | 35 | 1.32% | 0.066 |
| Hemothorax | 0 | 0.00% | 5 | 0.19% | 0.667 |
| Tricuspid valve injury during TLE (severe) | 2 | 2.04% | 13 | 0.49% | 0.176 |
| Rescue cardiac surgery | 4 | 4.08% | 33 | 1.24% | 0.051 |
| Minor complications (any) | 12 | 12.24% | 201 | 7.56% | 0.130 |
| Procedure-related death (intra-, post-procedural) | 0 | 0.00% | 6 | 0.23% | 0.527 |
| Indication-related death (intra-, post-procedural | 0 | 0.00% | 2 | 0.08% | 0.101 |
| Clinical success | |||||
| Clinical success | 93 | 94.90% | 2545 | 95.71% | 0.891 |
| No; planned supplementary TLE or cardiac surgery | 0 | 0.00% | 90 | 3.38% | 0.118 |
| No; complication—death | 2 | 2.04% | 17 | 0.64% | 0.305 |
| Procedural success | |||||
| Complete procedural success | 80 | 81.63% | 2544 | 95.68% | <0.001 |
| No; lack of complete radiographic success | 16 | 16.33% | 97 | 3.65% | <0.001 |
| No; permanently disabling complication or death | 2 | 2.04% | 18 | 0.68% | 0.339 |
| Additional procedure information | |||||
| Pacemaker dependence | 35 | 35.71% | 442 | 16.62% | <0.001 |
| Condition of extracted leads (intracardiac lead abrasion) | |||||
| Probable abrasion (lead significantly damaged) | 12 | 12.24% | 77 | 2.90% | <0.001 |
| Certain lead abrasion | 30 | 30.61% | 451 | 16.96% | <0.001 |
| TV injury during TLE | |||||
| TR increase by 2 degrees | 4 | 4.08% | 40 | 1.50% | 0.112 |
| TR increase by 3 degrees | 1 | 1.02% | 9 | 0.34% | 0.805 |
| Reference Number | Year, Author, Journal | Number of Pts | Mean Age of Patients | Mean Dwell Time | Major Complications | Procedure-Related Death |
|---|---|---|---|---|---|---|
| Studies in Adults (Reporting > 1000 TLE Procedures) | ||||||
| [20] | 1999, Byrd, C.L., Pacing Clin Electrophysiol | 2338 | 64 | 47 | 1.40% | 0.40% |
| [21] | 2008, Bongiorni, M., Eur Heart J. | 1193 | 66 | 69 | 0.70% | 0.30% |
| [22] | 2010, Wazny, O., J Am Coll Cardiol. LEXICon Sudy | 1449 | 63 | 82 | 1.40% | 0.30% |
| [23] | 2014, Brunner, M.P., Heart Rhythm | 2999 | 67 | 61 | 1.80% | 0.20% |
| [24] | 2016, Bashir, J., Circ Arrhythm Electrophysiol | 1082 | 59 | 129 | 3.00% | 0.37% |
| [25] | 2017, Hussein, A.A., JACC Clin Electrophysiol | 1836 | 68 | 107.5 | 1.93% | 0.29% |
| [26] | 2017, Kutarski, A., Europace | 2049 | 65 | 89 | 1.80% | 0.36% |
| [27] | 2017, Bongiorni, M., Eur Heart Journal | 3555 | 65 | 76.8 | 1.70% | 0.50% |
| [28] | 2018, Sood, N., Circ Arrhythm Electrophysiol | 11,304 | 65 | 65 | 2.30% | 0.16% |
| [29] | 2019, Jacheć, W., Pacing Clin Electrophysiol | 3810 | 65 | 86.4 | 1.44% | 0.17% |
| [30] | 2020, Segreti, L., Europace | 1210 | 69 | 72 | 0.70% | 0.16% |
| [31] | 2020, Starck, C.T., Europace | 2205 | 66 | 74 | 1.00% | 0.18% |
| [32] | 2020, Giannotti Santoro, M., Pacing Clin Electrophysiol | 1316 | 65 | 72 | 0.70% | 0.00% |
| All studies in adults, summary | 36,346 | 65.2 | 74.16 | 1.75% | 0.24% | |
| Studies in children and juveniles (all available studies) | ||||||
| [14] | 1996, Friedman, R.A., 1, PacingClinElectrophysiol | 13 | 13.1 | 54 | 0.00% | 0.00% |
| [6] | 2003, Cooper, J.M., J CardiovascElectrophysiol | 14 | 17.9 | 42.4 | 0.00% | 0.00% |
| [15] | 2006, Moak, J.P., Pacing Clin Electrophysiol | 25 | 10 | 49.4 | 8.00% | 0.00% |
| [16] | 2009, Dilber, E., Med Princ Pract | 30 | 12 | 46 | 2.80% | 0.00% |
| [17] | 2010, Cecchin, F., Circ Arrhythm Electrophysiol | 144 | 21.5 | 86.8 | 2.80% | 0.00% |
| [18] | 2010, Zartner, P.A., Europace | 22 | 12.9 | 61.2 | 0.00% | 0.00% |
| [5] | 2013, Atallah, J., Circulation | 879 | 18.6 | 28.8 | 4.00% | 0.00% |
| Studies in children and juveniles, summary | 1127 | 18.42 | 38.22 | 3.73% | 0.00% | |
| Our group of patients with leads implanted in childhood | 98 | 27.5 | 171.8 | 5.26% | 0.00% | |
| Our control group of adult patients (40–80 years of age) | 2659 | 66.5 | 95.6 | 2.03% | 0.23% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutarski, A.; Jacheć, W.; Polewczyk, A.; Nowosielecka, D.; Miszczak-Knecht, M.; Brzezinska, M.; Bieganowska, K. Transvenous Lead Extraction in Adult Patient with Leads Implanted in Childhood-Is That the Same Procedure as in Other Adult Patients? Int. J. Environ. Res. Public Health 2022, 19, 14594. https://doi.org/10.3390/ijerph192114594
Kutarski A, Jacheć W, Polewczyk A, Nowosielecka D, Miszczak-Knecht M, Brzezinska M, Bieganowska K. Transvenous Lead Extraction in Adult Patient with Leads Implanted in Childhood-Is That the Same Procedure as in Other Adult Patients? International Journal of Environmental Research and Public Health. 2022; 19(21):14594. https://doi.org/10.3390/ijerph192114594
Chicago/Turabian StyleKutarski, Andrzej, Wojciech Jacheć, Anna Polewczyk, Dorota Nowosielecka, Maria Miszczak-Knecht, Monika Brzezinska, and Katarzyna Bieganowska. 2022. "Transvenous Lead Extraction in Adult Patient with Leads Implanted in Childhood-Is That the Same Procedure as in Other Adult Patients?" International Journal of Environmental Research and Public Health 19, no. 21: 14594. https://doi.org/10.3390/ijerph192114594
APA StyleKutarski, A., Jacheć, W., Polewczyk, A., Nowosielecka, D., Miszczak-Knecht, M., Brzezinska, M., & Bieganowska, K. (2022). Transvenous Lead Extraction in Adult Patient with Leads Implanted in Childhood-Is That the Same Procedure as in Other Adult Patients? International Journal of Environmental Research and Public Health, 19(21), 14594. https://doi.org/10.3390/ijerph192114594






