Aneurysm Sac Pressure during Branched Endovascular Aneurysm Repair versus Multilayer Flow Modulator Implantation in Patients with Thoracoabdominal Aortic Aneurysm
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Branched Endovascular Aneurysm Repair
2.4. Multilayer Flow Modulator Implantation
2.5. Measurement Technique
2.6. Statistical Analysis
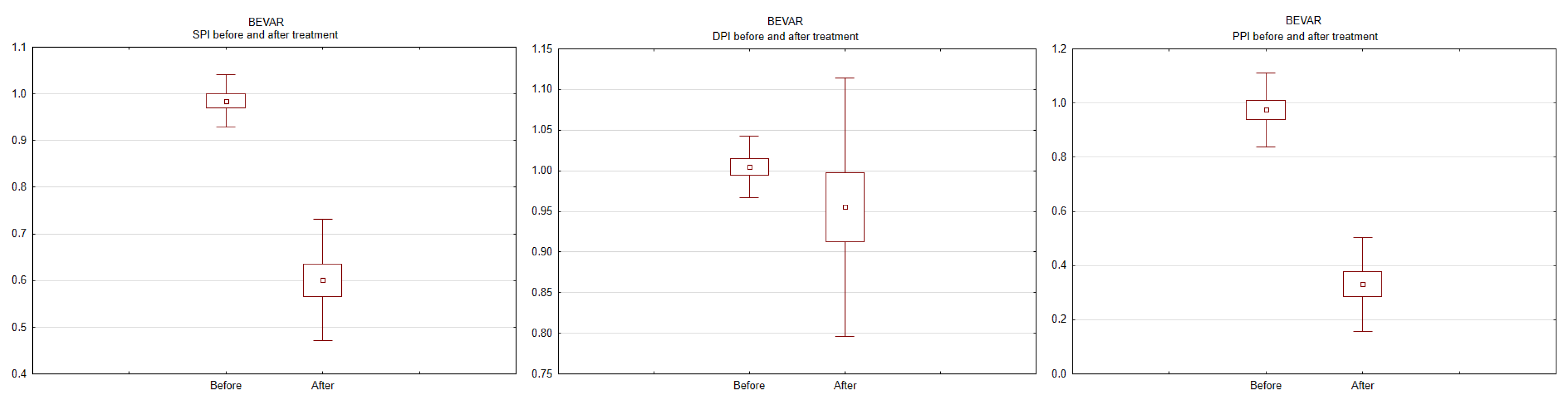
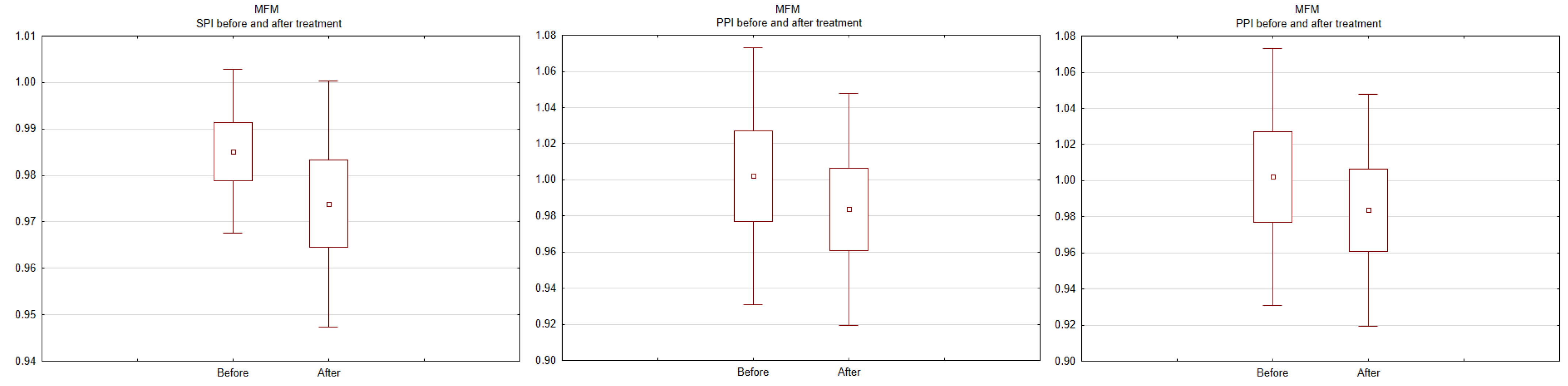
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelissen, B.G.L.; Herwaarden, J.A.; Pasterkamp, G.; Moll, F.L.; Vaartjes, I. Shifting abdominal aortic aneurysm mortality trends in The Netherlands. J. Vasc. Surg. 2015, 61, 642–647.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verhoeven, E.L.G.; Zeebregts, C.J.; Kapma, M.R.; Tielliu, I.F.J.; Prins, T.R.; van den Dungen, J.J.A.M. Fenestrated and branched endovascular techniques for thoraco-abdominal aneurysm repair. J. Cardiovasc. Surg. 2005, 46, 131–140. [Google Scholar]
- Verhoeven, E.L.G.; Tielliu, I.F.J.; Ferreira, M.; Zipfel, B.; Adam, D.J. Thoraco-abdominal aortic aneurysm branched repair. J. Cardiovasc. Surg. 2010, 51, 149–155. [Google Scholar] [PubMed]
- de Vries, J.-P.P.M. Treatment of complex thoracoabdominal or juxtarenal aortic aneurysms with a Multilayer stent. J. Endovasc. Ther. 2012, 19, 125–127. [Google Scholar] [CrossRef]
- Oderich, G.S. Evidence of use of multilayer flow modulator stents in treatment of thoracoabdominal aortic aneurysms and dissections. J. Vasc. Surg. 2017, 65, 935–937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baum, R.A.; Carpenter, J.P.; Cope, C.; Golden, M.A.; Velazquez, O.C.; Neschis, D.G.; Mitchell, M.E.; Barker, C.F.; Fairman, R.M. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J. Vasc. Surg. 2001, 33, 32–41. [Google Scholar] [CrossRef]
- Sonesson, B.; Dias, N.; Malina, M.; Olofsson, P.; Griffin, D.; Lindblad, B.; Ivancev, K. Intra-aneurysm pressure measurements in successfully excluded abdominal aortic aneurysm after endovascular repair. J. Vasc. Surg. 2003, 37, 733–738. [Google Scholar] [CrossRef]
- Dias, N.; Ivancev, K.; Malina, M.; Resch, T.; Lindblad, B.; Sonesson, B. Intra-aneurysm sac pressure measurements after endovascular aneurysm repair: Differences between shrinking, unchanged, and expanding aneurysms with and without endoleaks. J. Vasc. Surg. 2004, 39, 1229–1235. [Google Scholar] [CrossRef]
- Dias, N.; Ivancev, K.; Resch, T.; Malina, M.; Sonesson, B. Endoleaks after endovascular aneurysm repair lead to nonuniform intra-aneurysm sac pressure. J. Vasc. Surg. 2007, 46, 197–203. [Google Scholar] [CrossRef]
- Skillern, C.; Stevens, S.; Piercy, K.; Donnell, R.; Freeman, M.; Goldman, M. Endotension in an experimental aneurysm model. J. Vasc. Surg. 2002, 36, 814–817. [Google Scholar] [CrossRef]
- Kasprzak, P.M.; Gallis, K.; Cucuruz, B.; Pfister, K.; Janotta, M.; Kopp, R. Editor’s choice—Temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Hynes, N.; Sultan, M. When not to implant the multilayer flow modulator: Lessons learned from application outside the indications for use in patients with thoracoabdominal pathologies. J. Endovasc. Ther. 2014, 21, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Thakar, T.; Chaudhuri, A. Early experience with the multilayer aneurysm repair stent in the endovascular treatment of trans/infragenicular popliteal artery aneurysms: A mixed bag. J. Endovasc. Ther. 2013, 20, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Benjelloun, A.; Henry, M.; Taberkant, M.; Berrado, A.; Houati, R.E.; Semlali, A. Multilayer Flow Modulator Treatment of Abdominal and Thoracoabdominal Aortic Aneurysms with Side Branch Coverage: Outcomes from a Prospective Single-Center Moroccan Registry. J. Endovasc. Ther. 2016, 23, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Costache, V.S.; Yeung, K.K.; Solomon, C.; Popa, R.; Melnic, T.; Sandu, M.; Bucurenciu, C.; Candea, G.; Santa, A.; Costache, A. Aortic Remodeling After Total Endovascular Aortic Repair with Multilayer Stents: Computational Fluid Dynamics Analysis of Aortic Remodeling Over 3 Years of Follow-up. J. Endovasc. Ther. 2018, 25, 760–764. [Google Scholar] [CrossRef]
- Sultan, S.; Kavanagh, E.P.; Bonneau, M.; Kang, C.; Alves, A.; Hynes, N.M. Kinetics of endothelialization of the multilayer flow modulator and single-layer arterial stents. Vascular 2016, 24, 78–87. [Google Scholar] [CrossRef]
- Sultan, S.; Kavanagh, E.P.; Diethrich, E.; Costache, V.; Sultan, M.; Jordan, F.; Hynes, N. A clinical review of early outcomes from contemporary flow modulation versus open, fenestrated and branch technologies in the management of thoracoabdominal aortic aneurysm. Vascular 2018, 26, 209–215. [Google Scholar] [CrossRef]
- Lowe, C.; Worthington, A.; Serracino-Inglott, F.; Ashleigh, R.; McCollum, C. Multi-layer Flow-modulating Stents for Thoraco-abdominal and Peri-renal Aneurysms: The UK Pilot Study. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 225–231. [Google Scholar] [CrossRef]
- Vaislic, C.D.; Fabiani, J.N.; Chocron, S.; Robin, J.; Costache, V.S.; Villemot, J.-P.; Alsac, J.M.; Leprince, P.N.; Unterseeh, T.; Portocarrero, E.; et al. One-year outcomes following repair of thoracoabdominal aneurysms with the multilayer flow modulator: Report from the STRATO trial. J. Endovasc. Ther. 2014, 21, 85–95. [Google Scholar] [CrossRef]
- Vaislic, C.D.; Fabiani, J.N.; Chocron, S.; Robin, J.; Costache, V.S.; Villemot, J.-P.; Alsac, J.M.; Leprince, P.N.; Unterseeh, T.; Portocarrero, E.; et al. Three-Year Outcomes with the Multilayer Flow Modulator for Repair of Thoracoabdominal Aneurysms: A Follow-up Report from the STRATO Trial. J. Endovasc. Ther. 2016, 23, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.; Sultan, S.; Elhelali, A.; Diethrich, E.B.; Kavanagh, E.P.; Sultan, M.; Stefanov, F.; Delassus, P.; Morris, L. Systematic Review and Patient-Level Meta-analysis of the Streamliner Multilayer Flow Modulator in the Management of Complex Thoracoabdominal Aortic Pathology. J. Endovasc. Ther. 2016, 23, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Antkiewicz, M.; Kuliczkowski, W.; Protasiewicz, M.; Kobielarz, M.; Barć, P.; Malinowski, M.; Frączkowska, K.; Kulikowska, K.; Merenda, M.; Jacyna, K.; et al. Intra-aneurysm sac pressure measurement using a thin pressure wire during endovascular aneurysm repair. Adv. Clin. Exp. Med. 2021, 30, 309–313. [Google Scholar] [CrossRef] [PubMed]

| Variable | BEVAR | MFM |
|---|---|---|
| Gender | ||
| female | 2 | 2 |
| male | 12 | 6 |
| Age (M ± SD) | 73 ± 7 | 76 ± 4 |
| Risk factors | ||
| cerebral stroke | 1 | 1 |
| heart failure | 7 | 3 |
| diabetes mellitus | 1 | 2 |
| chronic obstructive pulmonary disease | 1 | 2 |
| renal insufficiency | 1 | 2 |
| arterial hypertension | 12 | 6 |
| obesity (BMI > 30 kg/cm2) | 5 | 4 |
| nicotinism | 14 | 8 |
| peripheral artery disease | 6 | 4 |
| cancer | - | 2 |
| Aneurysm diameter (mm) | 60.5 ± 9.9 | 69.9 ± 17.1 |
| Variable | BEVAR | MFM |
|---|---|---|
| Aneurysm diameter (mm) | 56.2 ± 11.7 | 70.9 ± 17.4 |
| Successful aneurysm occlusion | 9 (64.3%) | 1 (12.5%) |
| Visceral arteries patency | 55/56 (98.2%) | 30/32 (93.8%) |
| Severe complications | ||
| death | - | 1 (12.5%) (intestine ischemia) |
| myocardial infarction | - | 1 (12.5%) |
| acute kidney injury | 1 (7.1%) | 2 (25.0%) |
| acute limb ischemia | 2 (14.3%) | 1 (12.5%) |
| graft migration | - | 2 (25.0%) |
| Reinterventions | 4 (28.6%) | 3 (37.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antkiewicz, M.; Kuliczkowski, W.; Protasiewicz, M.; Zubilewicz, T.; Terlecki, P.; Kobielarz, M.; Janczak, D. Aneurysm Sac Pressure during Branched Endovascular Aneurysm Repair versus Multilayer Flow Modulator Implantation in Patients with Thoracoabdominal Aortic Aneurysm. Int. J. Environ. Res. Public Health 2022, 19, 14563. https://doi.org/10.3390/ijerph192114563
Antkiewicz M, Kuliczkowski W, Protasiewicz M, Zubilewicz T, Terlecki P, Kobielarz M, Janczak D. Aneurysm Sac Pressure during Branched Endovascular Aneurysm Repair versus Multilayer Flow Modulator Implantation in Patients with Thoracoabdominal Aortic Aneurysm. International Journal of Environmental Research and Public Health. 2022; 19(21):14563. https://doi.org/10.3390/ijerph192114563
Chicago/Turabian StyleAntkiewicz, Maciej, Wiktor Kuliczkowski, Marcin Protasiewicz, Tomasz Zubilewicz, Piotr Terlecki, Magdalena Kobielarz, and Dariusz Janczak. 2022. "Aneurysm Sac Pressure during Branched Endovascular Aneurysm Repair versus Multilayer Flow Modulator Implantation in Patients with Thoracoabdominal Aortic Aneurysm" International Journal of Environmental Research and Public Health 19, no. 21: 14563. https://doi.org/10.3390/ijerph192114563
APA StyleAntkiewicz, M., Kuliczkowski, W., Protasiewicz, M., Zubilewicz, T., Terlecki, P., Kobielarz, M., & Janczak, D. (2022). Aneurysm Sac Pressure during Branched Endovascular Aneurysm Repair versus Multilayer Flow Modulator Implantation in Patients with Thoracoabdominal Aortic Aneurysm. International Journal of Environmental Research and Public Health, 19(21), 14563. https://doi.org/10.3390/ijerph192114563








