Studying the Research–Practice Gap in Physical Therapies for Cerebral Palsy: Preliminary Outcomes Based on a Survey of Spanish Clinicians
Abstract
1. Introduction
2. Materials and Methods
- General information about the participants, including age, education or professional career, number of years of experience and working institution.
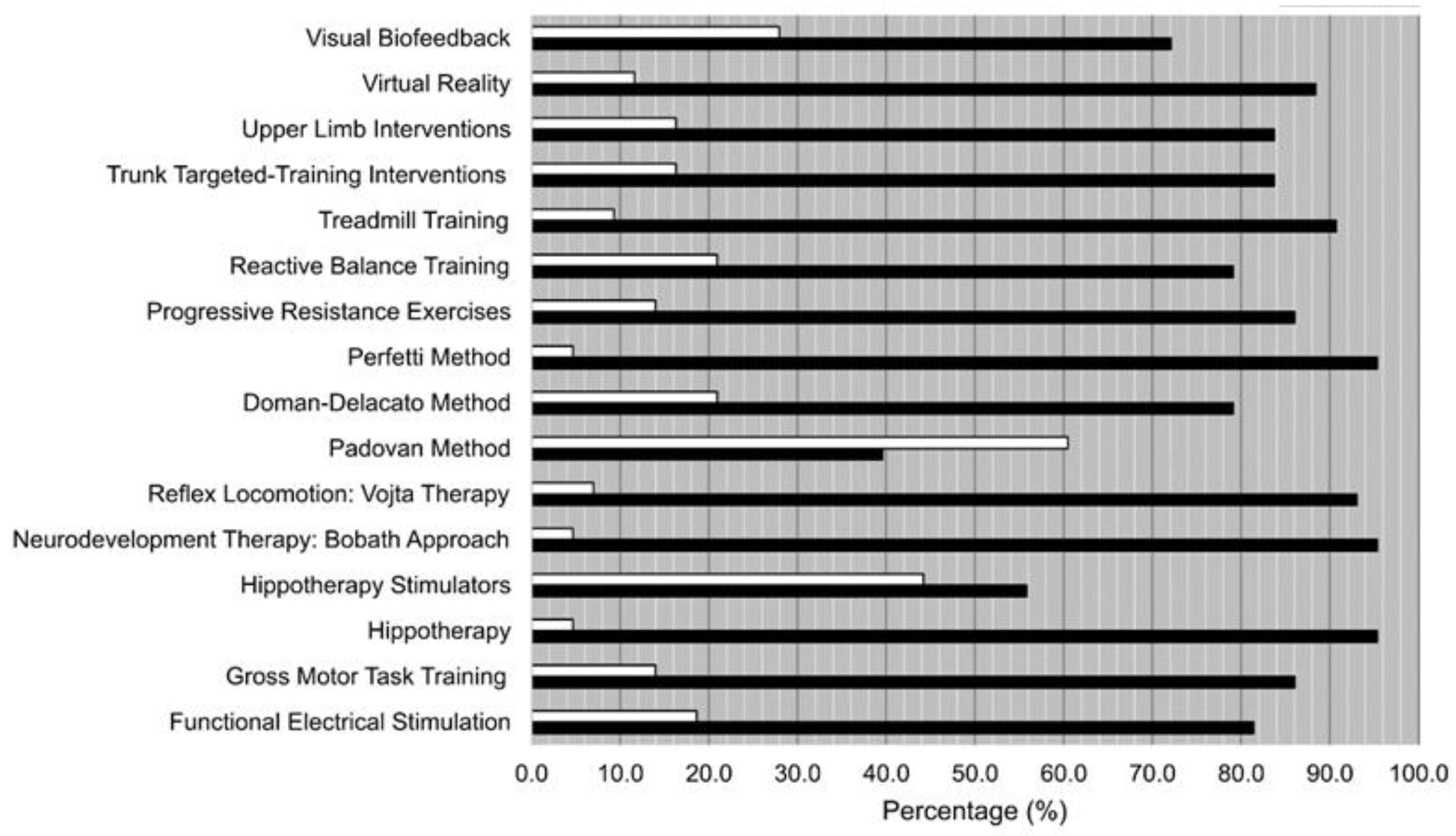
- Knowledge of the different treatments. Participants answered if they knew (Yes) or not (No) about each one of the listed techniques, therapies, and interventions.
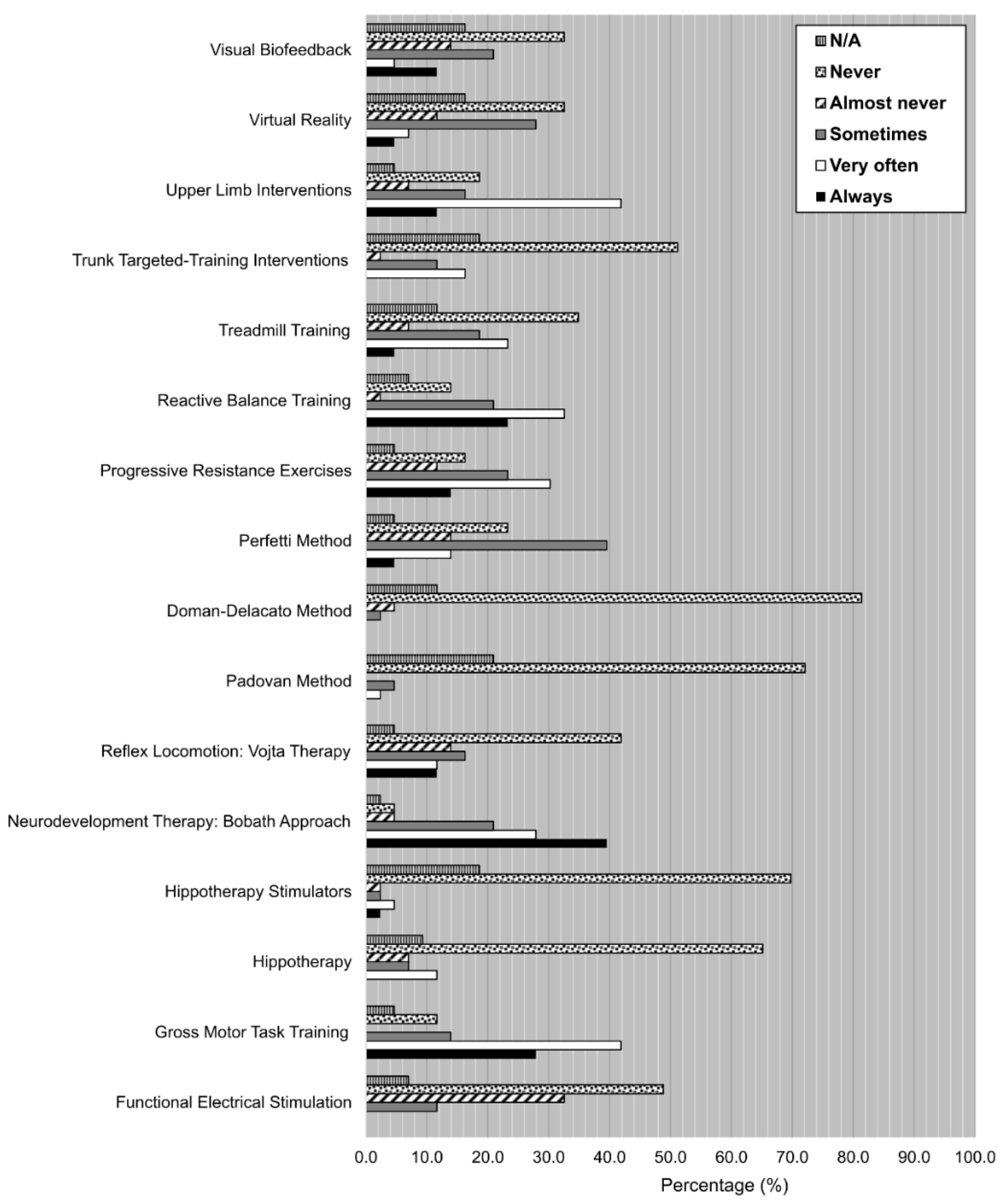
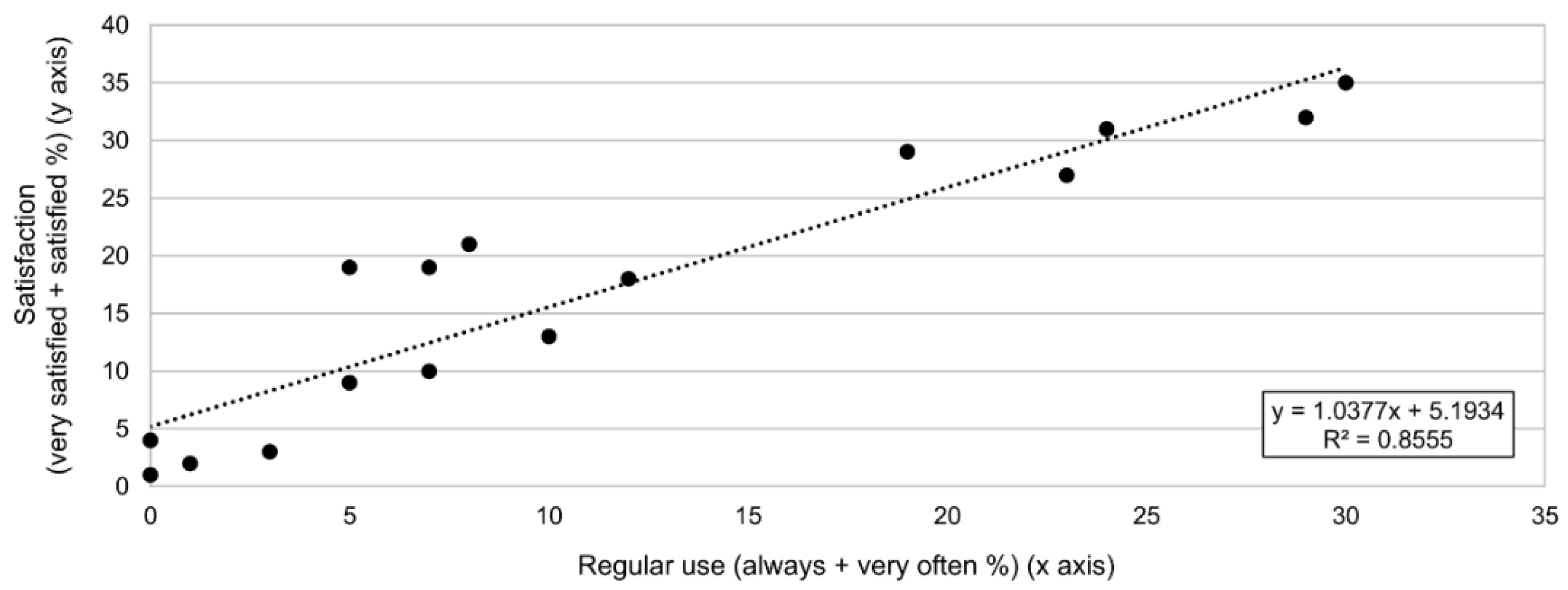
- Degree of use of the treatments using a Likert scale. Participants had to choose among six different options depending on how often they had used each one of the listed techniques, therapies, and interventions throughout their careers, according to the following scale: always, very often, sometimes, almost never, never, N/A.
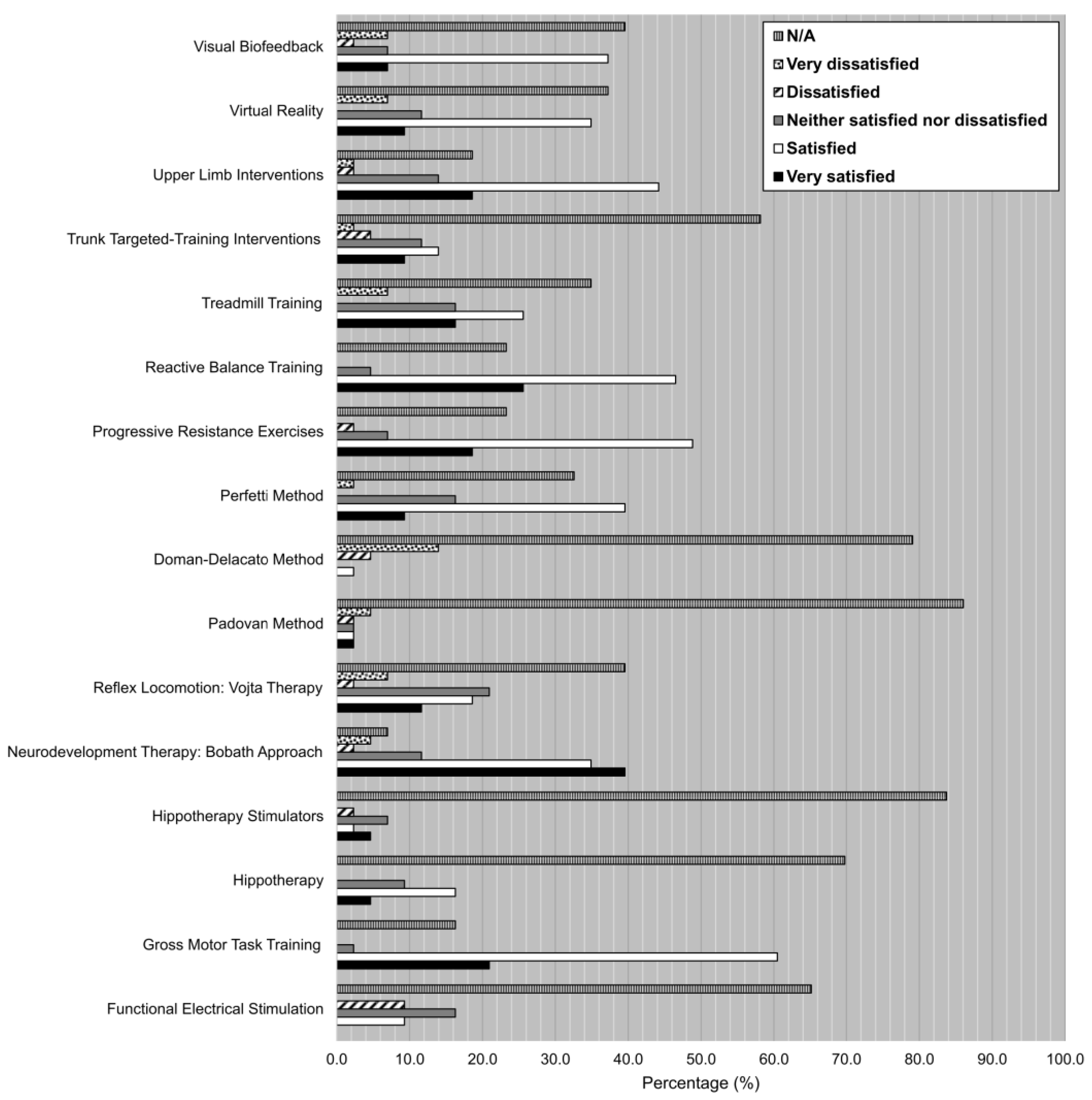
- Degree of satisfaction of the treatments using a Likert scale. Participants had to choose among six different options based on their degree of satisfaction with each one of the listed techniques, therapies, and interventions after its use on children with cerebral palsy throughout their careers, according to the following scale: very satisfied, satisfied, neither satisfied nor dissatisfied, dissatisfied, very dissatisfied, N/A.
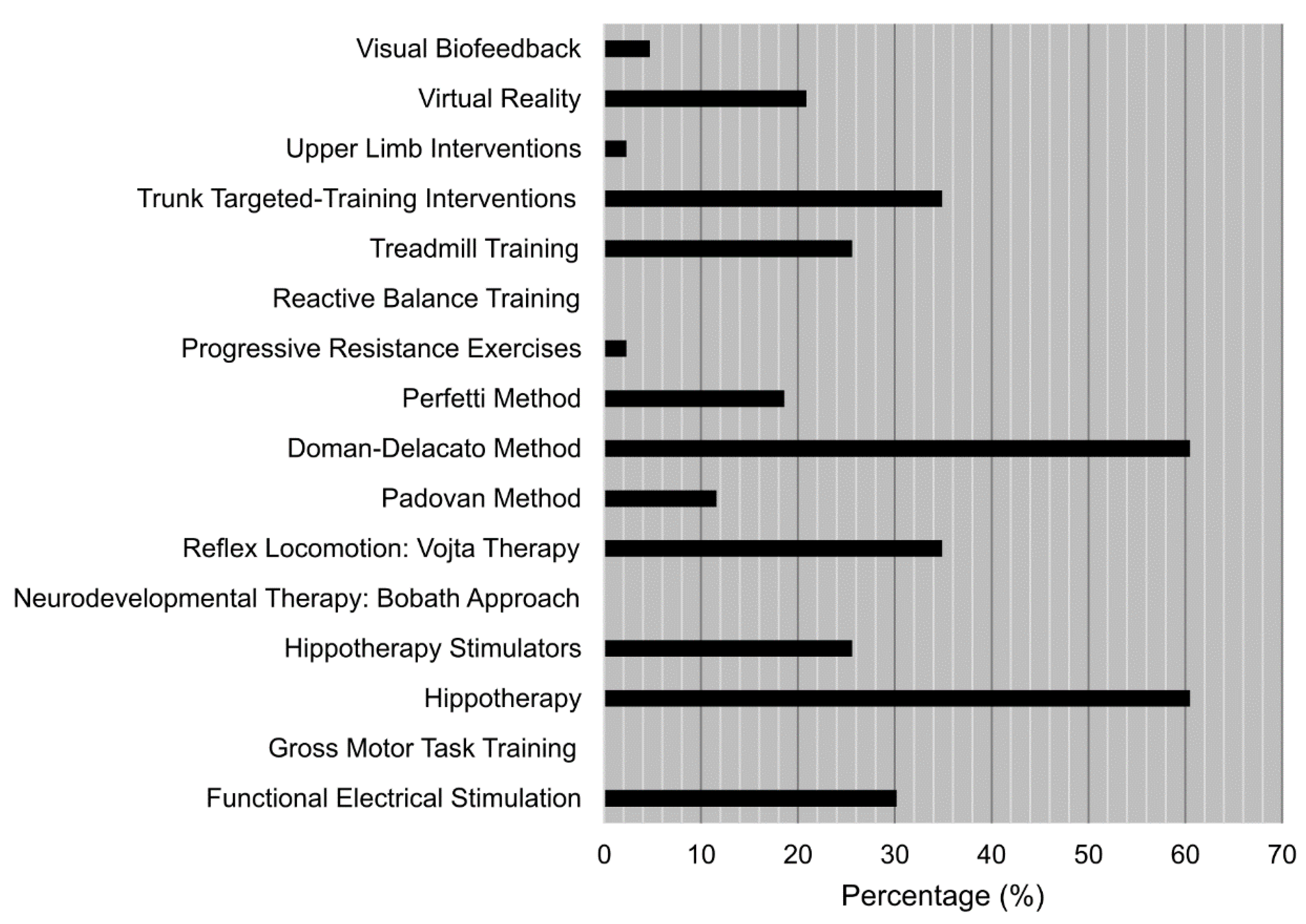
- The last part of the survey was an open-ended question asking participants to state which treatments, both from the listed ones or from other sources according to their professional experience, they thought had more future in the rehabilitation of this type of patients. Participants had a comment box with no specific pre-set to answer the previous question, and an extra comment box to add extra observations if considered.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- General information:
- Current occupation
- Age
- Years of experience in the field
- Knowledge of the techniques/therapies:
- Choose the option that best describes your knowledge of these techniques/therapies
| I Know About It and Have Used It | I Know About It but Never Have used It | I Do Not Know About This Technique | |
| Functional Electrical Stimulation | |||
| Gross Motor Task Training | |||
| Hippotherapy Simulators | |||
| Hippotherapy | |||
| Neurodevelopmental Therapy: Bobath Approach | |||
| Reflex Locomotion: Votja Therapy | |||
| Padovan Method | |||
| Doman Delacato Method | |||
| Perfetti Method | |||
| Progressive Resistance Exercises | |||
| Reactive Balance Training | |||
| Treadmill Training | |||
| Trunk-Targeted Training Interventions | |||
| Upper Limb Interventions | |||
| Virtual Reality | |||
| Visual Biofeedback |
- 3.
- Degree of use of the techniques/therapies:
- Choose the option that best describes your degree of use of these techniques/therapies
| Always | Very Often | Sometimes | Almost Never | Never | DK/DA | |
| Functional Electrical Stimulation | ||||||
| Gross Motor Task Training | ||||||
| Hippotherapy Simulators | ||||||
| Hippotherapy | ||||||
| Neurodevelopmental Therapy: Bobath Approach | ||||||
| Reflex Locomotion: Votja Therapy | ||||||
| Padovan Method | ||||||
| Doman Delacato Method | ||||||
| Perfetti Method | ||||||
| Progressive Resistance Exercises | ||||||
| Reactive Balance Training | ||||||
| Treadmill Training | ||||||
| Trunk-Targeted Training Interventions | ||||||
| Upper Limb Interventions | ||||||
| Virtual Reality | ||||||
| Visual Biofeedback |
- 4.
- Degree of satisfaction with the techniques/therapies:
- Choose the option that best describes your degree of satisfaction with the following techniques/therapies after its use on children with cerebral palsy throughout your career
| Very Satisfied | Satisfied | Neither Satisfied Nor Dissatisfied | Dissatisfied | Very Dissatisfied | DK/DA | |
| Functional Electrical Stimulation | ||||||
| Gross Motor Task Training | ||||||
| Hippotherapy Simulators | ||||||
| Hippotherapy | ||||||
| Neurodevelopmental Therapy: Bobath Approach | ||||||
| Reflex Locomotion: Votja Therapy | ||||||
| Padovan Method | ||||||
| Doman Delacato Method | ||||||
| Perfetti Method | ||||||
| Progressive Resistance Exercises | ||||||
| Reactive Balance Training | ||||||
| Treadmill Training | ||||||
| Trunk-Targeted Training Interventions | ||||||
| Upper Limb Interventions | ||||||
| Virtual Reality | ||||||
| Visual Biofeedback |
- 5.
- Open-ended questions:
- Which one of the previous techniques/therapies (or other you know but are not mentioned here) has, in your opinion, a better and more promising future?
- Observations or comments
References
- Rosenbaum, P.; Paneth, N.; Leviton, A. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child 2007, 109 (Suppl. 109), 8–14. [Google Scholar]
- Riquelme, I.; Montoya, P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin. Neurophysiol. 2010, 121, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.; Silva, D.; Funayama, C. Classification of cerebral palsy: Association between gender, age, motor type, topography and Gross Motor Function. Arq. Neuro. 2009, 67, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Cans, C. Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurology 2010, 42, 816–824. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Brodwin, M. Medical, Psychosocial, and Vocational Aspects of Disability; Elliott & Fitzpatrick, Inc.: Athens, GA, USA, 1992. [Google Scholar]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; ISBN 9780781766913. [Google Scholar]
- Dewar, R.; Love, S.; Johnston, L.M. Exercise interventions improve postural control in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2015, 57, 504–520. [Google Scholar] [CrossRef]
- Pierce, S.R.; Paremski, A.C.; Skorup, J.; Stergiou, N.; Senderling, B.; Prosser, L.A. Linear and Nonlinear Measures of Postural Control in a Toddler with Cerebral Palsy: Brief Report: Brief Report. Pediatr. Phys. Ther. 2020, 32, 80–83. [Google Scholar] [CrossRef]
- Kyvelidou, A.; Harbourne, R.T.; Willett, S.L.; Stergiou, N. Sitting Postural Control in Infants with Typical Development, Motor Delay, or Cerebral Palsy. Pediatr. Phys. Ther. 2013, 25, 46–51. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Shumway-Cook, A. Postural dysfunction during standing and walking in children with cerebral palsy: What are the underlying problems and what new therapies might improve balance? Neural Plast. 2005, 12, 211–219. [Google Scholar] [CrossRef]
- Morgan, C.; Darrah, J.; Gordon, A.M.; Harbourne, R.; Spittle, A.; Johnson, R.; Fetters, L. Effectiveness of motor interventions in infants with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2016, 58, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Cassidy, E.E.; Noorduyn, S.G.; O’Connell, N.E. Exercise interventions for cerebral palsy. Cochrane Database Syst. Rev. 2017, 6, CD011660. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, V.F.P.; Vriezekolk, J.E.; Aarts, P.B.M.; Geurts, A.C.H.; Van den Ende, C.H.M. Interventions to improve upper limb function for children with bilateral cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2019, 61, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Mcintyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.A.; Goldsmith, S. A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev. Med. Child Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef] [PubMed]
- Rodger, S.; Brown, G.T.; Brown, A. Profile of paediatric occupational therapy practice in Australia. Aust. Occup. Ther. J. 2005, 52, 311–325. [Google Scholar] [CrossRef]
- Saleh, M.N.; Korner-Bitensky, N.; Snider, L.; Malouin, F.; Mazer, B.; Kennedy, E.; Roy, M.A. Actual vs. best practices for young children with cerebral palsy: A survey of paediatric occupational therapists and physical therapists in Quebec, Canada. Dev. Neurorehabil. 2008, 11, 60–80. [Google Scholar] [CrossRef]
- Larivière-Bastien, D.; Racine, E. Ethics in Health Care Services for Young Persons With Neurodevelopmental Disabilities: A Focus on Cerebral Palsy Springer Book Series: Advances in Neuroethics View project. Artic. J. Child Neurol. 2011, 26, 1221–1229. [Google Scholar] [CrossRef]
- Weisleder, P. Unethical Prescriptions: Alternative Therapies for Children With Cerebral Palsy. Clin. Pediatr. 2009, 49, 7–11. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence-CEBM. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (accessed on 29 July 2020).
- Oxman, A.D. Grading quality of evidence and strength of recommendations. Br. Med. J. 2004, 328, 1490–1494. [Google Scholar]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Oxman, A.D.; Guyatt, G.H. Validation of an index of the quality of review articles. J. Clin. Epidemiol. 1991, 44, 1271–1278. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, A.N.; Hausdorff, A.J.M. Treadmill training for the treatment of gait disturbances in people with Parkinson’s disease: A mini-review. J. Neural Transm. 2009, 116, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.L.; Dejong, S.L. A Systematic Review of the Effectiveness of Treadmill Training and Body Weight Support in Pediatric Rehabilitation. J. Neurol. Phys. Ther. 2009, 33, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S. Treadmill training with partial body weight support after stroke: A review. NeuroRehabilitation 2008, 23, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.; Krosschell, K.; Spira, D. Treadmill training with partial body-weight support in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2009, 51, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Franki, I.; Desloovere, K.; De Cat, J.; Feys, H.; Molenaers, G.; Calders, P.; Vanderstraeten, G.; Himpens, E.; Van Den Broeck, C. The evidence-base for conceptual approaches and additional therapies targeting lower limb function in children with cerebral palsy: A systematic review using the international clasification of functioning, disability and health as a framework. J. Rehabil. Med. 2012, 44, 396–405. [Google Scholar] [CrossRef]
- Lim, H.; Kim, T. Effects of Vojta Therapy on Gait of Children with Spastic Diplegia. J. Phys. Ther. Sci. 2014, 25, 1605–1608. [Google Scholar] [CrossRef]
- Paci, M. Physiotherapy based on the Bobath concept for adults with post-stroke hemiplegia: A review of effectiveness studies. J. Rehabil. Med. 2003, 35, 2–7. [Google Scholar] [CrossRef]
- Tekin, F.; Kavlak, E.; Cavlak, U.; Altug, F. Effectiveness of Neuro-Developmental Treatment (Bobath Concept) on postural control and balance in Cerebral Palsied children. J. Back Musculoskelet. Rehabil. 2018, 31, 397–403. [Google Scholar] [CrossRef]
- Díaz-Arribas, M.J.; Martín-Casas, P.; Cano-de-la-Cuerda, R.; Plaza-Manzano, G. Effectiveness of the Bobath concept in the treatment of stroke: A systematic review. Disabil. Rehabil. 2020, 42, 1636–1649. [Google Scholar] [CrossRef]
- Novak, I.; Honan, I. Effectiveness of paediatric occupational therapy for children with disabilities: A systematic review. Aust. Occup. Ther. J. 2019, 66, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Bronson, C.; Brewerton, K.; Ong, J.; Palanca, C.; Sullivan, S.J. Does hippotherapy improve balance in persons with multiple sclerosis: A systematic review. Eur. J. Phys. Rehabil. Med. 2010, 46, 347–353. [Google Scholar] [PubMed]
- Zadnikar, M.; Kastrin, A. Effects of hippotherapy and therapeutic horseback riding on postural control or balance in children with cerebral palsy: A meta-analysis. Dev. Med. Child Neurol. 2011, 53, 684–691. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, A.; Vignais, N.; Biddiss, E. Biofeedback interventions for people with cerebral palsy: A systematic review protocol. Syst. Rev. 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Hutchinson, S.; Kartin, D.; MSME, R.P.; Woollacott, M. Effect of balance training on recovery of stability in children with cerebral palsy. Dev. Med. Child Neurol. 2007, 45, 591–602. [Google Scholar] [CrossRef]
- El-Shamy, S.M.; Abd El Kafy, E.M. Effect of balance training on postural balance control and risk of fall in children with diplegic cerebral palsy. Disabil. Rehabil. 2014, 36, 1176–1183. [Google Scholar] [CrossRef]
- El-gohary, T.M.; Emara, H.A.; Al-Shenqiti, A.; Hegazy, F.A. Biodex balance training versus conventional balance training for children with spastic diplegia. J. Taibah Univ. Med. Sci. 2017, 12, 534–540. [Google Scholar] [CrossRef]
- Lintanf, M.; Bourseul, J.-S.; Houx, L.; Lempereur, M.; Brochard, S.; Pons, C. Effect of Ankle-Foot Orthoses on Gait, Balance and Gross Motor Functions in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2018, 61, e317. [Google Scholar] [CrossRef]
- Harris, S.R.; Atwater, S.W.; Crowe, T.K. Accepted and controversial neuromotor therapies for infants at high risk for cerebral palsy. J. Perinatol. 1988, 8, 3–13. [Google Scholar]
- Ziring, P.R.; Brazdziunas, D.; Cooley, W.C.; Kastner, T.A.; Kummer, M.E.; De Pijem, L.G.; Quint, R.D.; Ruppert, E.S.; Sandler, A.D. The treatment of neurologically impaired children using patterning. Pediatrics 1999, 104, 1149–1151. [Google Scholar]
- Liptak, G.S. Complementary and alternative therapies for Cerebral Palsy. MRDD Res. Rev. 2005, 11, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Nickel, R.E.; Child, I.Y. Controversial Therapies for Young Children with Developmental Disabilities. Infants Young Child. 1996, 8, 29–40. [Google Scholar] [CrossRef]
- Medeiros Pereira, L.; Correa Vileicar, D.; Bezerra Sales, S.; Landim Rodrigues Alves, M.d.S.; de Melo Casado Pereira, J.; Lourenço Alves, L.M.; Rodrigues Figueiredo, C.J.; Teixeira Batista, H.M.; Rodrigues Pinheiro, W.; Pinheiro Bezerra, I.M.; et al. Padovan Method Of Neurofunctional Reorganization As A Way For Neurological Recovery in Newborns. Int. Arch. Med. 2015, 8. [Google Scholar] [CrossRef]
- Delmondes, E.D.L.; Albuquerque, L.T.C.d.; Pereira, L.M. Neurorehabilitation with Padovan Method in a Newborn with Treacher Collins Syndrome: A Case Report. Amadeus Int. Multidiscip. J. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Rahbar, M.; Salekzamani, Y.; Jahanjou, F.; Eslamian, F.; Niroumand, A.; Dolatkhah, N. Effect of hippotherapy simulator on pain, disability and range of motion of the spinal column in subjects with mechanical low back pain: A randomized single-blind clinical trial. J. Back Musculoskelet. Rehabil. 2018, 31, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; You, J.H. Innovative robotic hippotherapy improves postural muscle size and postural stability during the quiet stance and gait initiation in a child with cerebral palsy: A single case study. NeuroRehabilitation 2018, 42, 247–253. [Google Scholar] [CrossRef]
- Hemachithra, C.; Meena, N.; Ramanathan, R.; Felix, A.J.W. Effect of Hippotherapy Simulator on Spasticity in Children with Cerebral Palsy (A Quasi-experimental Pilot Study). Indian J. Physiother. Occup. Ther.-Int. J. 2019, 13, 23. [Google Scholar] [CrossRef]
- Unger, M.; Jelsma, J.; Stark, C. Effect of a trunk-targeted intervention using vibration on posture and gait in children with spastic type cerebral palsy: A randomized control trial. Dev. Neurorehabil. 2013, 16, 79–88. [Google Scholar] [CrossRef]
- Gajewska, E.; Neukirch, B. Vojta Therapy for a 12 year-old Child with Cerebral Palsy. J. Phys. Ther. Sci. 2012, 24, 783–785. [Google Scholar] [CrossRef]
- Ha, S.Y.; Sung, Y.H. Effects of Vojta approach on diaphragm movement in children with spastic cerebral palsy. J. Exerc. Rehabil. 2018, 14, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.W.; Landenberger, M.; Jung, T.; Lindenthal, T.; Philippi, H. Vojta therapy and neurodevelopmental treatment in children with infantile postural asymmetry: A randomised controlled trial. J. Phys. Ther. Sci. 2017, 29, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.A.; Rose, J. A Scoping Review of Neuromuscular Electrical Stimulation to Improve Gait in Cerebral Palsy: The Arc of Progress and Future Strategies. Front. Neurol. 2019, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Prosser, L.A.; Curatalo, L.A.; Alter, K.E.; Damiano, D.L. Acceptability and potential effectiveness of a foot drop stimulator in children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 2012, 54, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Pool, D.; Valentine, J.; Blackmore, A.M.; Colegate, J.; Bear, N.; Stannage, K.; Elliott, C. Daily functional electrical stimulation during everyday walking activities improves performance and satisfaction in children with unilateral spastic cerebral palsy: A randomized controlled trial. Arch. Physiother. 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.P.; Pagnussat, A.S.; Pereira, G.A.; Scopel, G.; Lukrafka, J.L. Neuromuscular electrical stimulation to improve gross motor function in children with cerebral palsy: A meta-analysis. Braz. J. Phys. Ther. 2019, 23, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Blanche, E.; Botticelli, T.; Hallway, M. Combining Neuro-Developmental Treatment and Sensory Integration Principles: An Approach to Pediatric Therapy; Therapy Skill Builders: Perth, Australia, 1995. [Google Scholar]
- Graham, J.V.; Eustace, C.; Brock, K.; Swain, E.; Irwin-Carruihers, S. The bobath concept in contemporary clinical practice. Top. Stroke Rehabil. 2009, 16, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Meadows, L.; Lynch-Ellerington, M. (Eds.) Bobath Concept: Theory and Clinical Practice in Neurological Rehabilitation, 1st ed.; Wiley-Blackwell: Chichester, UK, 2013; ISBN 9781405170413. [Google Scholar]
- Abuin-Porras, V.; Pedersini, P.; Berjano, P.; Villafañe, J.H. The efficacy of physical therapy on the improvement of the motor components of visual attention in children with cerebral palsy: A case series study. J. Exerc. Rehabil. 2019, 15, 103–108. [Google Scholar] [CrossRef]
- Zanon, M.A.; Pacheco, R.L.; Latorraca, C.d.O.C.; Martimbianco, A.L.C.; Pachito, D.V.; Riera, R. Neurodevelopmental Treatment (Bobath) for Children With Cerebral Palsy: A Systematic Review. J. Child Neurol. 2019, 34, 679–686. [Google Scholar] [CrossRef]
- Golomb, M.R.; Garg, B.P.; Saha, C.; Azzouz, F.; Williams, L.S. Cerebral palsy after perinatal arteril ischemic stroke. J. Child Neurol. 2008, 23, 279–286. [Google Scholar] [CrossRef]
- Gordon, A.M.; Schneider, J.A.; Chinnan, A.; Charles, J.R. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: A randomized control trial. Dev. Med. Child Neurol. 2007, 49, 830–838. [Google Scholar] [CrossRef]
- Fedrizzi, E.; Rosa-Rizzotto, M.; Turconi, A.C.; Pagliano, E.; Fazzi, E.; Pozza, L.V.D.; Facchin, P. Unimanual and Bimanual Intensive Training in Children With Hemiplegic Cerebral Palsy and Persistence in Time of Hand Function Improvement. J. Child Neurol. 2013, 28, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sakzewski, L.; Ziviani, J.; Boyd, R.N. Efficacy of Upper Limb Therapies for Unilateral Cerebral Palsy: A Meta-Analysis. Pediatrics 2014, 133, e175–e204. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, I.J. Progressive Resistance Exercise and Its Application to Health and Clinical Problems; University of London-Imperial College London: London, UK, 2006. [Google Scholar]
- Scholtes, V.A.; Becher, J.G.; Comuth, A.; Dekkers, H.; Van Dijk, L.; Dallmeijer, A.J. Effectiveness of functional progressive resistance exercise strength training on muscle strength and mobility in children with cerebral palsy: A randomized controlled trial. Dev. Med. Child Neurol. 2010, 52, e107–e113. [Google Scholar] [CrossRef] [PubMed]
- Fosdahl, M.A.; Jahnsen, R.; Kvalheim, K.; Holm, I. Stretching and Progressive Resistance Exercise in Children With Cerebral Palsy: A Randomized Controlled Trial. Pediatr. Phys. Ther. 2019, 31, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-Y.; Kim, W.-H. Meta-Analysis of the Effect of Strengthening Interventions in Individuals with Cerebral Palsy. Res. Dev. Disabil. 2014, 35, 239–249. [Google Scholar] [CrossRef]
- Merino-Andrés, J.; García de Mateos-López, A.; Damiano, D.L.; Sánchez-Sierra, A. Effect of Muscle Strength Training in Children and Adolescents with Spastic Cerebral Palsy: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2022, 36, 4–14. [Google Scholar] [CrossRef]
- Myrhaug, H.T.; ØstensjØ, S.; Larun, L.; Odgaard-Jensen, J.; Jahnsen, R. Intensive training of motor function and functional skills among young children with cerebral palsy: A systematic review and meta-analysis. BMC Pediatr. 2014, 14, 292. [Google Scholar] [CrossRef]
- Maharaj, S.S.; Lallie, R. Does a physiotherapy programme of gross motor training influence motor function and activities of daily living in children presenting with developmental coordination disorder? S. Afr. J. Physiother. 2016, 72, 304. [Google Scholar] [CrossRef]
- Toovey, R.; Bernie, C.; Harvey, A.R.; McGinley, J.L.; Spittle, A.J. Task-specific gross motor skills training for ambulant school-aged children with cerebral palsy: A systematic review. BMJ Paediatr. Open 2017, 1, e000078. [Google Scholar] [CrossRef]
- Lee, S.; Bae, S.; Jeon, D.; Kim, K.Y. The effects of cognitive exercise therapy on chronic stroke patients’ upper limb functions, activities of daily living and quality of life. J. Phys. Ther. Sci. 2015, 27, 2787–2791. [Google Scholar] [CrossRef]
- Chanubol, R.; Wongphaet, P.; Chavanich, N.; Werner, C.; Hesse, S.; Bardeleben, A.; Merholz, J. A randomized controlled trial of Cognitive Sensory Motor Training Therapy on the recovery of arm function in acute stroke patients Article. Clin. Rehabil. 2012, 26. [Google Scholar] [CrossRef] [PubMed]
- Albiol-Pérez, S.; Pruna-Panchi, E.-P.; Escobar-Anchaguano, I.-P.; Pilatasig-Panchi, M.-A.; Mena-Mena, L.-E.; Segovia-Chávez, J.; Bernis, A.; Zumbana, P. A Neurocognitive Virtual Rehabilitation System for Children with Cerebral Palsy: A Preliminary Usability Study. In New Advances in Information Systems and Technologies; Springer International Publishing: Cham, Switzerland, 2016; pp. 1057–1063. ISBN 9783319312316. [Google Scholar]
- Pruna, E.; Escobar, I.; Montaluisa, J.; Pilatásig, M.; Mena, L.; Zumbana, P.; Guamán, A.; Galarza, E.D. VRAndroid system based on cognitive therapeutic exercises for stroke patients. In Proceedings of the Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2017; Volume 570, pp. 657–663. [Google Scholar]
- Jacho-Guanoluisa, N.; Albiol-Pérez, S.; Collazos, C.A.; Fardoun, H.M.; Cano, S. Cognitive rehabilitation using virtual reality for children with rare diseases. In Proceedings of the ACM International Conference Proceeding Series; Association for Computing Machinery: New York, NY, USA, 2019; pp. 183–186. [Google Scholar]
- Balci, N.Ç. Current Rehabilitation Methods for Cerebral Palsy. In Cerebral Palsy-Current Steps; InTech: Houston, TX, USA, 2016; ISBN 9789535126485. [Google Scholar]
- Holden, M.K. Virtual environments for motor rehabilitation: Review. Cyberpsychology Behav. 2005, 8, 187–211. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, A.; Lam, E.; Vigneron, V.; Vignais, N.; Biddiss, E. Biofeedback Interventions for Individuals with Cerebral Palsy: A Systematic Review. Disabil. Rehabil. 2019, 41, 2369–2391. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Lee, D.R.; Cha, Y.J.; You, S.H. Augmented effects of EMG biofeedback interfaced with virtual reality on neuromuscular control and movement coordination during reaching in children with cerebral palsy. NeuroRehabilitation 2017, 40, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Laufer, Y.; Weiss, P.; Tamar, L. Virtual Reality in the Assessment and Treatment of Children With Motor Impairment: A Systematic Review. J. Phys. Ther. Educ. 2011, 25, 59–71. [Google Scholar] [CrossRef]
- Tatla, S.K.; Sauve, K.; Virji-Babul, N.; Holsti, L.; Butler, C.; Van Der Loos, H.F.M. Evidence for outcomes of motivational rehabilitation interventions for children and adolescents with cerebral palsy: An american academy for cerebral palsy and developmental medicine systematic review. Dev. Med. Child Neurol. 2013, 55, 593–601. [Google Scholar] [CrossRef]
- Laforme, F.; Alyssa, C.; Effgen, S.K. Outcomes for young children with disabilities associated with the use of partial, body-weight-supported, treadmill training: An evidence-based review. Phys. Ther. Rev. 2006, 1, 179–189. [Google Scholar] [CrossRef]
- Grecco, L.A.C.; Tomita, S.M.; Christovão, T.C.L.; Pasini, H.; Sampaio, L.M.M.; Oliveira, C.S. Effect of treadmill gait training on static and functional balance in children with cerebral palsy: A randomized controlled trial. Braz. J. Phys. Ther. 2013, 17, 17–23. [Google Scholar] [CrossRef]
- Cho, C.; Hwang, W.; Hwang, S.; Chung, Y. Treadmill Training with Virtual Reality Improves Gait, Balance, and Muscle Strength in Children with Cerebral Palsy. Tohoku J. Exp. Med. 2016, 238, 213–218. [Google Scholar] [CrossRef]
- Han, Y.G.; Yun, C.K. Effectiveness of treadmill training on gait function in children with cerebral palsy: Meta-analysis. J. Exerc. Rehabil. 2020, 16, 10–19. [Google Scholar] [CrossRef]
- Koscielny, I.; Therasuit®. Soft Dynamic Proprioceptive Orthotic. Cereb. Palsy Mag. Treat. Method 2004. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiynIe0gZb7AhViTWwGHc8EB80QFnoECA4QAQ&url=https%3A%2F%2Frevivo.ca%2Fpdf%2FTheraSuit%2520Article.pdf&usg=AOvVaw31SJd4k20UhlkVqnpiHTQX (accessed on 30 July 2022).
- Koscielny, I.; Koscielny, R. Effectiveness of Therasuit Method® and Therasuit®. Pilot study. Results of the Intensive Exercise Program in the Pediatric Cerebral Palsy Population. Am. Assoc. Intensive Pediatr. Phys. Ther. 2004. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjUr5TfgZb7AhU163MBHdbnDH0QFnoECBwQAQ&url=http%3A%2F%2Fwww.allaboutkidstherapyservices.com%2Fassets%2FEFFECTIVENESS_OF_THERASUIT_M6903.doc&usg=AOvVaw0EQZ9fo-XusFjKgIo9t4-L (accessed on 30 July 2022).
- Bailes, A.F.; Greve, K.; Schmitt, L.C. Changes in Two Children with Cerebral Palsy After Intensive Suit Therapy: A Case Report. Pediatr. Phys. Ther. 2010, 22, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Cordovil, R.; Oliveira, R.; Letras, S.; Lourenço, S.; Pereira, I.; Ferro, A.; Lopes, I.; Silva, C.R.; Marques, M. Efficacy of suit therapy on functioning in children and adolescents with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2016, 58, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.M.; Fonseca, S.T.; Figueiredo, P.R.P.; Aquino, A.A.; Mancini, M.C. Effects of interventions with therapeutic suits (clothing) on impairments and functional limitations of children with cerebral palsy: A systematic review. Braz. J. Phys. Ther. 2017, 21, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Karadağ-Saygı, E.; Giray, E. The clinical aspects and effectiveness of suit therapies for cerebral palsy: A systematic review. Turk. J. Phys. Med. Rehabil. 2019, 65, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.B.; Price, K.S.; Feldman, H.M. Myofascial Structural Integration: A Promising Complementary Therapy for Young Children With Spastic Cerebral Palsy. J. Evid. Based. Complement. Altern. Med. 2012, 17, 131–135. [Google Scholar] [CrossRef]
- Price, K.S.; Buysse, C.A.; Loi, E.C.; Hansen, A.B.; Jaramillo, T.M.; Pico, E.L.; Feldman, H.M. Gait improvement in children with cerebral palsy after Myofascial Structural Integration therapy. J. Bodyw. Mov. Ther. 2016, 20, 152. [Google Scholar] [CrossRef]
- Paul, J.; Nathan, S.C.; Kumar, P.; Remya, R.K. Efectiveness of myofascial release in reduction of hamstrigns spasticiy among diplegic cerebral palsy children. IJMAES 2018, 4, 453–458. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.B.; George, S.Z. Unraveling the mechanisms of manual therapy: Modeling an approach. J. Orthop. Sport. Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Taylor, R.L.; O’Brien, L.; Brown, T. A scoping review of the use of elastic therapeutic tape for neck or upper extremity conditions. J. Hand Ther. 2014, 27, 235–246. [Google Scholar] [CrossRef]
- Shamsoddini, A.; Rasti, Z.; Kalantari, M.; Hollisaz, M.T.; Sobhani, V.; Dalvand, H.; Bakhshandeh-Bali, M.K. The impact of Kinesio taping technique on children with cerebral palsy. Iran. J. Neurol. 2016, 15, 219–227. [Google Scholar]
- Karabay, I.; Doğan, A.; Ekiz, T.; Köseoğlu, B.F.; Ersöz, M. Training postural control and sitting in children with cerebral palsy: Kinesio taping vs. neuromuscular electrical stimulation. Complement. Ther. Clin. Pract. 2016, 24, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.B.; Lima-Alvarez, C.D.d.; Rocha, A.C.; Tudella, E. Effects of Elastic Therapeutic Taping on Motor Function in Children with Motor Impairments: A Systematic Review. Disabil. Rehabil. 2018, 40, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Junior, R.R.; De Lima, P.; Da Silva, J.N.; Vaz, D.V. Effects of Kinesiology Taping in Children with Cerebral Palsy: A Systematic Review. Fisioter. Mov. 2017, 30, 373–382. [Google Scholar] [CrossRef]
- Ortiz Ramirez, J.; Perez de la Cruz, S. Therapeutic Effects of Kinesio Taping in Children with Cerebral Palsy: A Systematic Review. Arch. Argent. Pediatr. 2017, 115, e356–e361. [Google Scholar] [CrossRef]
- Monge Pereira, E.; Molina Rueda, F.; Alguacil Diego, I.M.; Cano De La Cuerda, R.; De Mauro, A.; Miangolarra Page, J.C. Use of virtual reality systems as proprioception method in cerebral palsy: Clinical practice guideline. Neurología 2014, 29, 550–559. [Google Scholar] [CrossRef]
- Hussein, Z.A.; Salem, I.A.; Ali, M.S. Effect of simultaneous proprioceptive-visual feedback on gait of children with spastic diplegic cerebral palsy. JMNI 2019, 19, 500. [Google Scholar]
- Aman, J.E.; Elangovan, N.; Yeh, I.L.; Konczak, J. The effectiveness of proprioceptive training for improving motor function: A systematic review. Front. Hum. Neurosci. 2015, 8, 1075. [Google Scholar] [CrossRef]
| Characteristic | Number (%) of Participants n = 43 |
|---|---|
| Current occupation | |
| Physiotherapist/Pediatric physiotherapist | 35 (81.4) |
| Occupational/Neuro-psychomotricity therapist | 8 (18.6) |
| Age | |
| <25 | 2 (4.7) |
| 25–29 | 14 (32.6) |
| 30–34 | 9 (20.9) |
| 35–39 | 10 (2.3) |
| ≥40 | 8 (18.6) |
| Years of experience in the field | |
| <5 | 10 (23.2) |
| 5–9 | 12 (27.9) |
| 10–14 | 7 (16.3) |
| 15–19 | 7 (16.3) |
| ≥20 | 7 (16.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, C.; Lerma-Lara, S.; Garcia-Carmona, R.; Urendes, E.; Laccourreye, P.; Raya, R. Studying the Research–Practice Gap in Physical Therapies for Cerebral Palsy: Preliminary Outcomes Based on a Survey of Spanish Clinicians. Int. J. Environ. Res. Public Health 2022, 19, 14535. https://doi.org/10.3390/ijerph192114535
Sanchez C, Lerma-Lara S, Garcia-Carmona R, Urendes E, Laccourreye P, Raya R. Studying the Research–Practice Gap in Physical Therapies for Cerebral Palsy: Preliminary Outcomes Based on a Survey of Spanish Clinicians. International Journal of Environmental Research and Public Health. 2022; 19(21):14535. https://doi.org/10.3390/ijerph192114535
Chicago/Turabian StyleSanchez, Cristina, Sergio Lerma-Lara, Rodrigo Garcia-Carmona, Eloy Urendes, Paula Laccourreye, and Rafael Raya. 2022. "Studying the Research–Practice Gap in Physical Therapies for Cerebral Palsy: Preliminary Outcomes Based on a Survey of Spanish Clinicians" International Journal of Environmental Research and Public Health 19, no. 21: 14535. https://doi.org/10.3390/ijerph192114535
APA StyleSanchez, C., Lerma-Lara, S., Garcia-Carmona, R., Urendes, E., Laccourreye, P., & Raya, R. (2022). Studying the Research–Practice Gap in Physical Therapies for Cerebral Palsy: Preliminary Outcomes Based on a Survey of Spanish Clinicians. International Journal of Environmental Research and Public Health, 19(21), 14535. https://doi.org/10.3390/ijerph192114535









