Directions of Changes in the Profession of Hospital Pharmacist in Poland
Abstract
1. Introduction
- What is the satisfaction rating with the way a pharmacist currently performs his profession in a medical entity?
- What is the assessment of the need to introduce changes in the manner of performing the profession of pharmacist in medical entities?
- What are the areas of change in hospital pharmacy and clinical pharmacy?
- What are the problems related to the introduction of clinical pharmacy?
2. Status of Hospital Pharmacy
- Exercising pharmaceutical care;
- Providing pharmaceutical services;
- Performing professional tasks;
- Performing other activities specified in the Act.
3. Hospital Pharmacy Solutions
- Infrastructure, including information technology (IT);
- Optimization of work organization;
- Support for pharmaceutical staff and their development;
- Support for innovative solutions in the field of pharmacy in healthcare entities (R&D).
3.1. Infrastructure, including Information Technology (IT)
- Support in the development of hospital pharmacy premises, including the specification of requirements to be met by the pharmacy premises;
- Digitization of processes related to drug trading;
- Support in the development of automation.
3.2. Optimization of Work Organization
3.3. Support for Pharmaceutical Staff and Their Development
3.4. Activities in the Field of R&D
3.4.1. ATMP
3.4.2. 3D Printing
3.4.3. Innovations in the Field of Pharmacy
4. Materials and Methods
4.1. Data Collection and Procedures
4.2. Measures
4.3. Data Analysis
5. Results and Discussion
5.1. Job Satisfaction
- The size of the hospital (p < 0.05, Somers’ D = 0.153)—this is a weak correlation; the smaller the facility, the more often pharmacists indicated a low or medium level of satisfaction, and the larger the entity (especially over 600 beds), the more often they indicated a high and very high level of satisfaction;
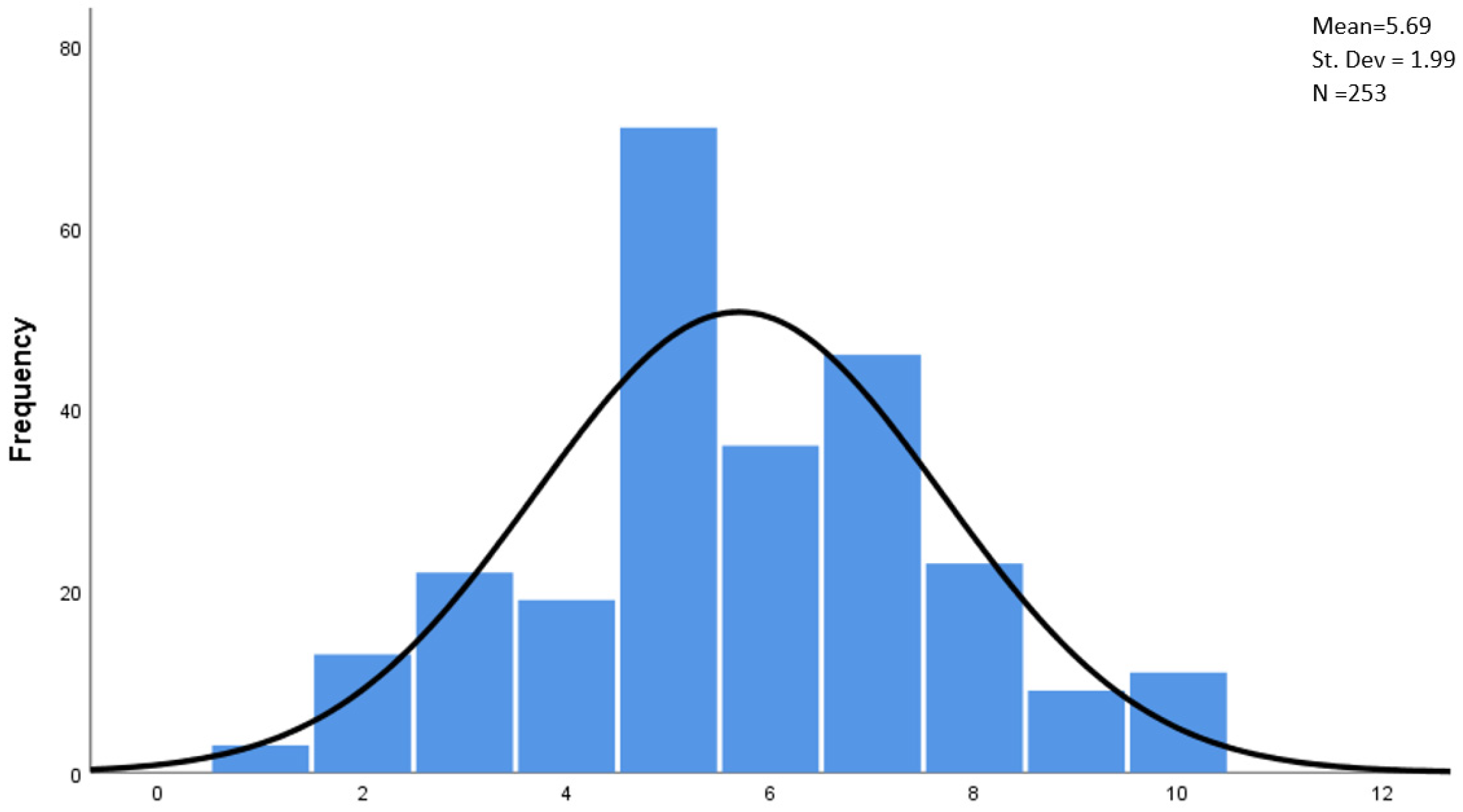
- The pharmacist’s age (p < 0.05; Pearson’s r = 0.177)—this is a weak correlation; the higher the age of the pharmacist, the higher the level of satisfaction;
- The number of years of work in a pharmacy (p < 0.05; Pearson’s r = 0.71)—this is a weak correlation; the longer the work experience, the higher the level of satisfaction.
5.2. Need for Changes and Readiness to Make Them
5.3. Areas of Change
5.3.1. Start of Research and Development Work
“Greater direct involvement of pharmacists (not only in the preparation of medications and their storage), but active participation in the collection of data and their analysis” (R185)
“Equipping pharmacies with laboratories for testing the durability and quality of medications prepared—HPLC, HPLC-MS, IR spectra” (R180)
“A clear indication that if the clinical trial entity is located in the same location as a hospital pharmacy, the medications must be registered with the pharmacy and additional funding is necessary for the pharmacist as a member of the research team” (R66)
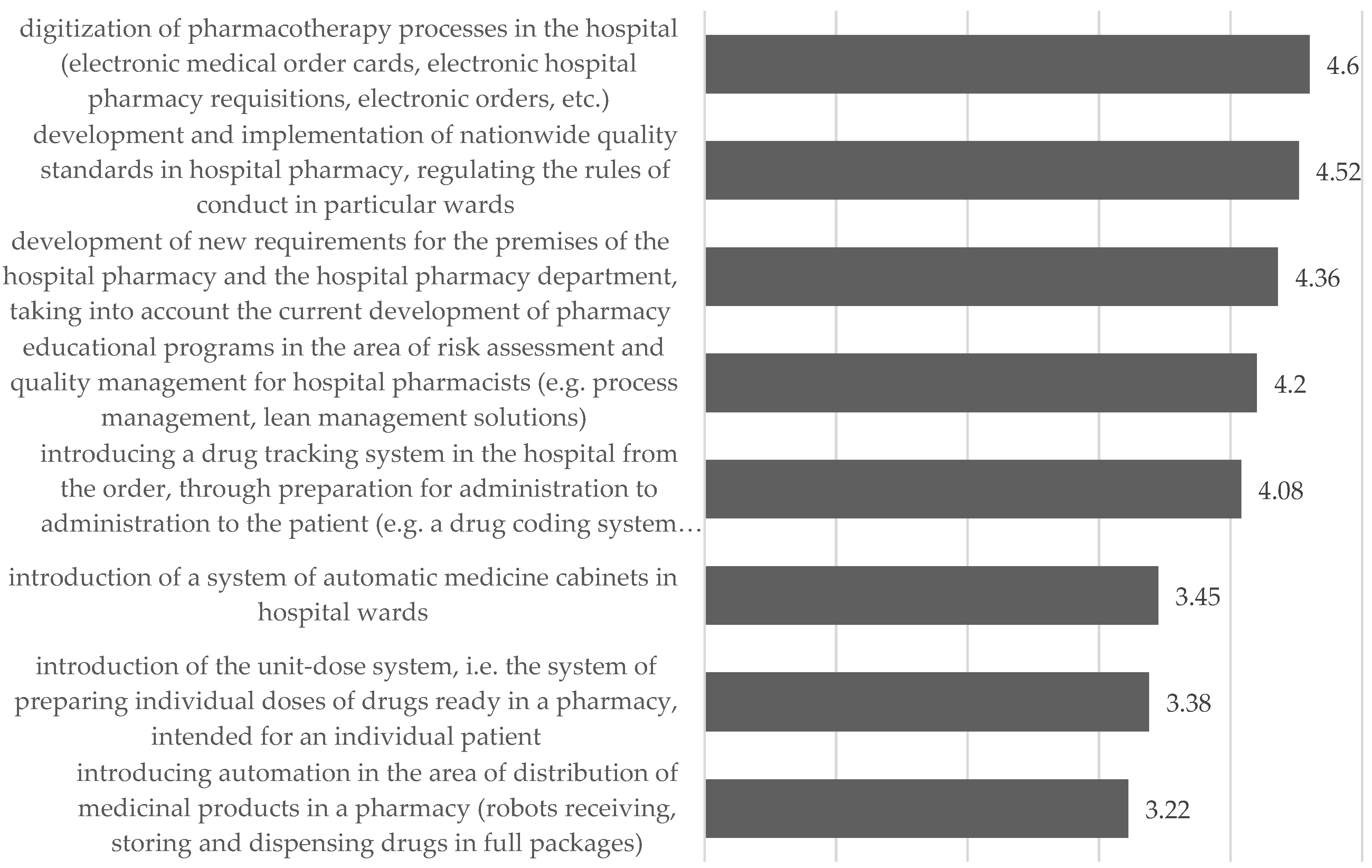
5.3.2. Changes in the Way Pharmacists Operate in Distribution of Medicinal Products and Medical Devices
- Communication (digitization of processes; medication tracking systems);
- Regulatory (development of nationwide standards regulating the rules of conduct);
- Management (educational programs in the field of quality management);
- Technical (new requirements for pharmacy premises).
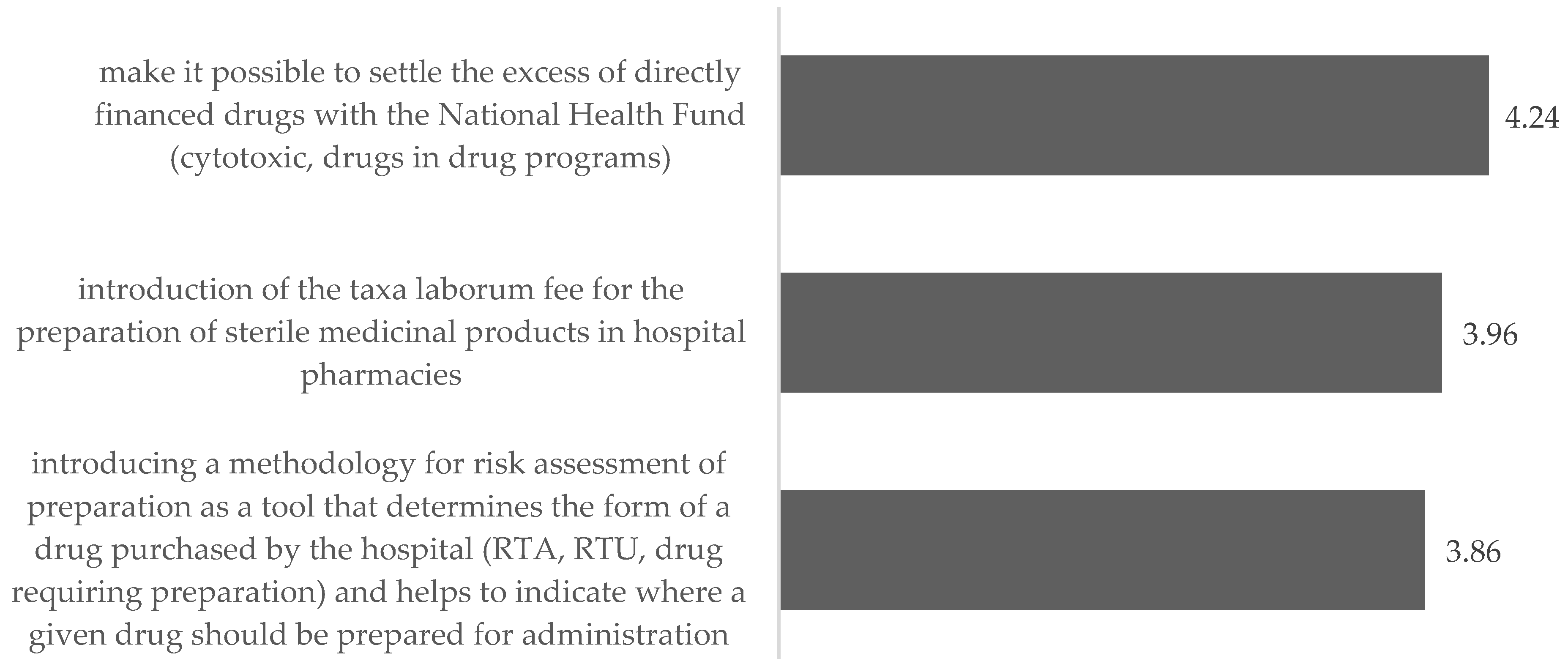
5.3.3. Changes in Preparation of Medicinal Products
“Guaranteeing, by means of a decree of the Minister of Health, appropriate local and staffing standards and the necessary equipment at a level that allows the preparation of medications in accordance with the current guidelines and standards; developing solutions facilitating the centralization of medication preparation for several entities in one place in order to optimize the management of medicinal products, reduce medication losses and guarantee the highest standards of medication preparation”. (R4)
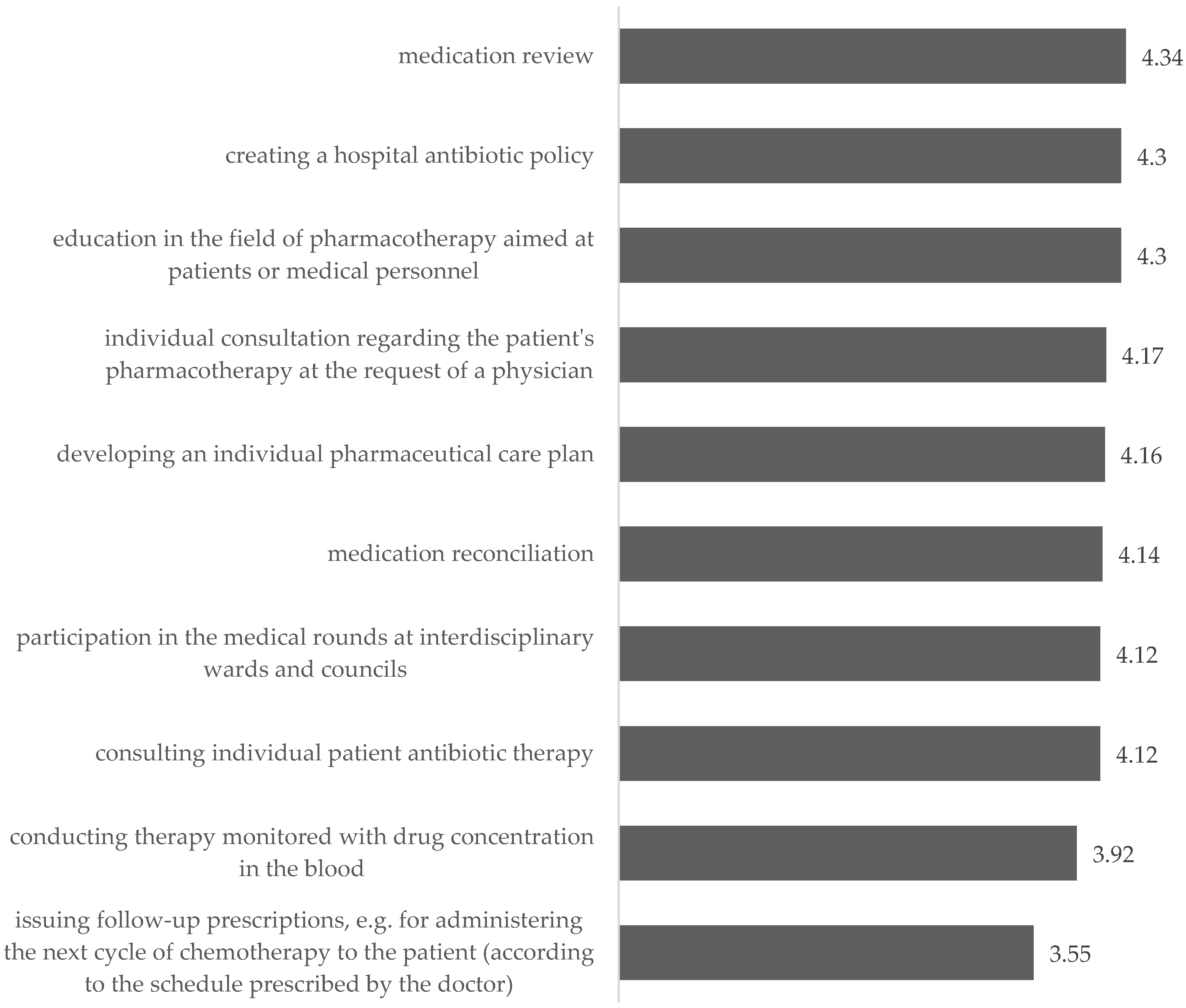
5.3.4. Changes in the Way Pharmacists Operate in Hospitals by Launching a Clinical Pharmacy Service
- Forms of patient education (especially patients qualifying for transplant);
- Running a medication information center;
- Monitoring of undesirable effects, analysis of complications and treatment failure, and analysis of prolongation of hospitalization;
- Pharmacotherapy supervision after discharge from the hospital.
“(…) targeted teaching of pharmacy students and practicing the profession of pharmacist for the safe and proper use of a medicinal product in a patient, thus placing the main emphasis on the importance of a medicinal product for the patient in the entire complexity of this issue, including the route of administration, form and formulation of the medicinal product, dosage, interactions with other medicinal products, food, and diagnostic tests”.
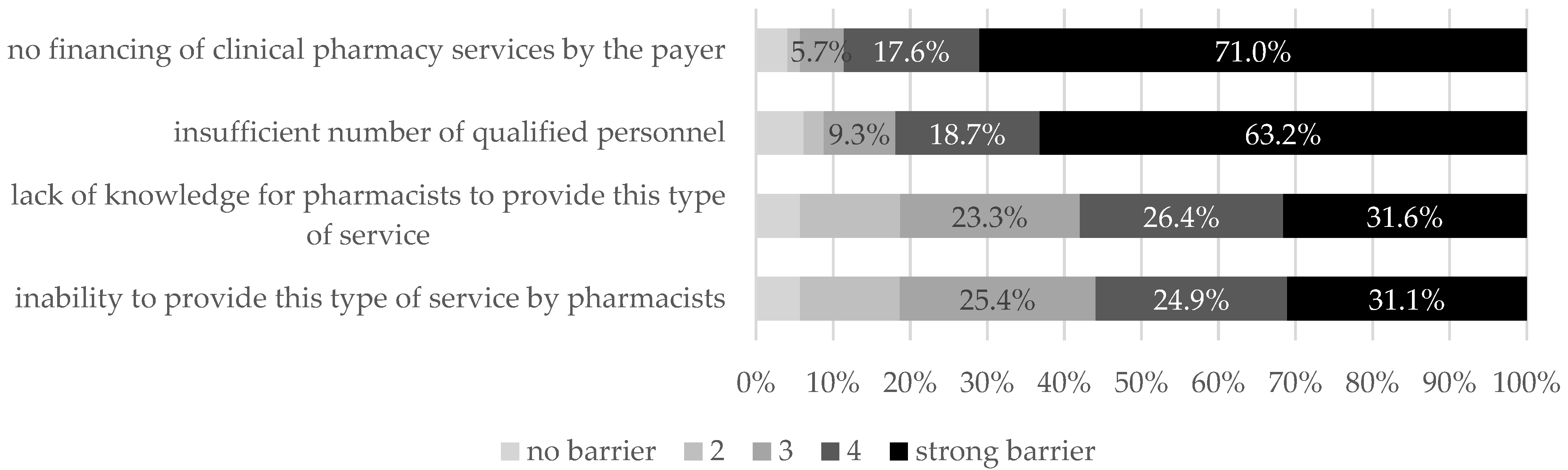
5.4. Problems Related to Clinical Pharmacy Implementation
- The opportunity to specialize:
“Very, very difficult access to specialization in both hospital and clinical pharmacy. A very small number of places, even though the specialization is payable about PLN 10,000. There are only 3 training centers for the whole of Poland”. (R145)
“No (co)funding from hospitals for pharmacists wishing to deepen their knowledge. No possibility of periodic training and educational leaves”. (R158)
- The opportunity to gain experience:
“Currently, the very limited presence of pharmacists in the field of even the basic clinical aspects of the work of hospital wards, which causes a lack of understanding of people working there (doctors/physicians, nurses) of the need to involve pharmacists in this area. It is necessary to involve pharmacists on the largest possible scale, initially primarily in areas not implemented or reluctantly implemented by other professional groups (e.g., medication conciliation, medication reviews, patient, and staff education) to broadly demonstrate the benefits of involving pharmacists in the process of therapy and care of patient. Consequently, this is leading to the natural willingness of doctors and nurses to involve more pharmacists at the higher levels of clinical pharmacy services and to build confidence in such changes”. (R4)
“Lack of clinical experience—necessary support of medical staff, no specialization in a given field of medicine, e.g., oncology and pediatric hematology, science takes a lot of private time, no defined responsibilities, competences, and powers of a clinical pharmacist”. (R248)
“Due to the shortage of staff and the number of tasks in the activities of the basic hospital pharmacy, the chances of delegating a pharmacist strictly to work in the ward are almost zero”. (R206)
“Still a big problem is the lack of financing for services and too few masters who deal with, for example, tenders daily (because it is important for the Directorate). Without funding, it will be difficult to get through. The hospital pharmacy must start earning money so that it is profitable and noticeable in the eyes of the management in the way we wish”. (R153)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FIP. Revised FIP Basel Statements on the Future of Hospital Pharmacy. Approved September 2014, Bangkok, Thailand, as Revisions of the Initial 2008 Version; Available online: https://fiphsa.com/docs/assessments/fip-basel-statements-en.pdf (accessed on 1 August 2022).
- Bragazzi, N.L.; Mansour, M.; Bonsignore, A.; Ciliberti, R. The Role of Hospital and Community Pharmacists in the Management of COVID-19: Towards an Expanded Definition of the Roles, Responsibilities, and Duties of the Pharmacist. Pharmacy 2020, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Yfantopoulos, N.; Yfantopoulos, P.; Yfantopoulos, J. Pharmaceutical Policies under Economic Crisis: The Greek case Authors. JHPOR 2016, 2, 4–17. [Google Scholar]
- Goodrick, E.; Reay, T. Constellations of Institutional Logics: Changes in the Professional Work of Pharmacists. Work. Occup. 2011, 38, 372–416. [Google Scholar] [CrossRef]
- Farmaceuta w Polsce. Ogólnopolskie Badania Wizerunkowe; Fundacja Aflofarm i Naczelna Izba Aptekarska: Warszawa, Poland, 2019. [Google Scholar]
- Deloitte. Jak Wprowadzić w Polsce Opiekę Farmaceutyczną; Rola i Wyzwania Współczesnej Apteki; Deloitte: Warszawa, Poland, 2018. [Google Scholar]
- Ustawa z Dnia 10 Grudnia 2020 r. o Zawodzie Farmaceuty (Dz.U. 2021 Poz. 97). (In English: The Act on the Pharmacist Profession). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20210000097/U/D20210097Lj.pdf (accessed on 1 August 2022).
- Sørensen, K.; Pelikan, J.M.; Röthlin, F.; Ganahl, K.; Slonska, Z.; Doyle, G.; Fullam, J.; Kondilis, B.; Agrafiotis, D.; Uiters, E.; et al. Health literacy in Europe: Comparative results of the European health literacy survey (HLS-EU). Eur. J. Public Health 2015, 25, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Merks, P.; Świeczkowski, D.; Kazimierczak, J.; Białoszewska, K.; Olszewska, A.; Krysiński, J. Apteka szpitalna jako prawdziwe miejsce sprawowania opieki farmaceutycznej. Nauka Praktyka. Czas. Aptek. 2015, 5, 46–51. [Google Scholar]
- Żak, K. Opieka farmaceutyczna czy profesjonalne doradztwo? Bariery wdrażania opieki farmaceutycznej w Polsce. Ekon.—Wroc. Econ. Rev. 2018, 24, 65–81. [Google Scholar] [CrossRef][Green Version]
- Ayalew, M.; Taye, K.; Asfaw, D.; Lemma, B.; Dadi, F.; Solomon, H.; Tazeze, H.; Tsega, B. Patients’/clients’ expectation toward and satisfaction from pharmacy services. J. Res. Pharm. Pract. 2017, 6, 21. [Google Scholar] [CrossRef]
- Berenguer, B.; La Casa, C.; de la Matta, M.J.; Martin-Calero, M.J. Pharmaceutical Care: Past, Present and Future. Curr. Pharm. Des. 2005, 10, 3931–3946. [Google Scholar] [CrossRef]
- Gupchup, G.V.; Singhal, P.K.; Dole, E.J.; Lively, B.T. Burnout in a sample of HMO pharmacists using the Maslach Burnout Inventory. J. Manag. Care Pharm. 1998, 4, 495–503. [Google Scholar] [CrossRef]
- Nelson, D.L.; Simmons, B.L. Health Psychology and Work Stress: A More Positive Approach. In Handbook of Occupational Health Psychology; In, J.C., Quick, L.E., Tetrick, Eds.; American Psychological Association: Washington, DC, USA, 2003; pp. 97–119. [Google Scholar] [CrossRef]
- Opieka Farmaceutyczna. Kompleksowa Analiza Procesu Wdrożenia; Raport z Prac Zespołu ds. Opieki Farmaceutycznej Powołanego Przez Ministra Zdrowia Na Podstawie Zarządzenia z Dnia 8 Lipca 2020 r. (Dziennik Urzędowy Ministra Zdrowia z dnia 9 lipca 2020 r., Warszawa); Ministerstwo Zdrowia: Warszawa, Poland, 2020.
- Rejestr Aptek (a database Register of Pharmacies including data of all pharmacies registered in Poland). Available online: https://rejestrymedyczne.ezdrowie.gov.pl/ra/search/public (accessed on 1 August 2022).
- NIK. Funkcjonowanie Aptek Szpitalnych i Działów Farmacji Szpitalnej; NIK: Warszawa, Poland, 2018. Available online: https://www.nik.gov.pl/kontrole/P/17/093 (accessed on 1 August 2022).
- Uchwała nr 196/2021 Rady Ministrów z Dnia 27 Grudnia 2021, Zdrowa Przyszłość. Ramy Strategiczne Rozwoju Systemu Ochrony Zdrowia Na Lata 2021–2027, z Perspektywą do 2030 r. Ministerstwo Zdrowia. Available online: https://www.gov.pl/attachment/4a9bd160-e052-4a52-8fd4-b7c546d556f8 (accessed on 1 August 2022).
- De Goede, A. Presentation, Hospital pharmacists involved in APTM and in Risk Assessment. In Proceedings of the 23rd Congress of the EAHP, Gothenburg, Sweden, 21–23 March 2018. [Google Scholar]
- Guiu, J.M. Advancing Hospital Pharmacy Practice through New Competences in Advanced Therapy Medicinal Products. Am. J. Pharm. Educ. 2014, 78, 22. [Google Scholar] [CrossRef]
- Stępniak, P.; Bialik, W.; Żuk, A. Systemy automatycznej dystrybucji leków (unit dose) w szpitalach w Polsce oraz w wybranych krajach świata. Ann. Acad. Med. Silesiensis 2017, 71, 304–313. [Google Scholar] [CrossRef]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Cho, E. Making Reliability Reliable. Organ. Res. Methods 2016, 19, 651–682. [Google Scholar] [CrossRef]
- Somers, R.H. A new asymmetric measure of association for ordinal variables. Am. Sociol. Rev. 1962, 27, 799. [Google Scholar] [CrossRef]
- Newson, R. Parameters behind “nonparametric” statistics: Kendall’s tau, Somers’ D and median differences. Stata J. 2002, 2, 45–64. [Google Scholar] [CrossRef]
- Rodgers; Nicewander, Thirteen ways to look at the correlation coefficient. Am. Stat. 1988, 42, 59–66. [CrossRef]
- Iorga, M.; Dondaș, C.; Soponaru, C.; Antofie, I. Determinants of Hospital Pharmacists’ Job Satisfaction in Romanian Hospitals. Pharmacy 2017, 5, 66. [Google Scholar] [CrossRef]
- Carvajal, M.J.; Popovici, I.; Hardigan, P.C. Gender and Pharmacists’ Career Satisfaction in the United States. Pharmacy 2021, 9, 173. [Google Scholar] [CrossRef]
- Mattsson, S.; Gustafsson, M. Job Satisfaction among Swedish Pharmacists. Pharmacy 2020, 8, 127. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Dang, V.T.; Yen, H.-Y.; Lai, K.-M. Influence of Risk of Drug–Drug Interactions and Time Availability on Patient Trust, Satisfaction, and Cooperation with Clinical Pharmacists. Int. J. Environ. Res. Public Health 2019, 16, 1566. [Google Scholar] [CrossRef]
- Policarpo, V.; Romano, S.; António, J.H.C.; Correia, T.S.; Costa, S. A new model for pharmacies? Insights from a quantitative study regarding the public’s perceptions. BMC Health Serv. Res. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; White, L. Key determinants of hospital pharmacy staff’s job satisfaction. Res. Soc. Adm. Pharm. 2011, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Sansgiry, S.S.; Ngo, C. Factors affecting job satisfaction among hospital pharmacists. Hosp. Pharm. 2003, 38, 1037–1046. [Google Scholar] [CrossRef]
- Stavrou, G.; Siskou, O.C.; Talias, M.A.; Galanis, P. Assessing Job Satisfaction and Stress among Pharmacists in Cyprus. Pharmacy 2022, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Zięcik, P.; Miłowska, K. O Roli Farmaceuty w Prowadzeniu Badań Klinicznych—Wybrane Zagadnienia. Available online: https://zflegal.pl/aktualnosci/o-roli-farmaceuty-w-prowadzeniu-badan-klinicznych-wybrane-zagadnienia (accessed on 1 August 2022).
- Learn About Clinical Studies—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/about-studies/learn (accessed on 1 August 2022).
- Dlaczego Prowadzi Się Badania Kliniczne. Available online: https://www.badaniaklinicznewpolsce.pl/o-badaniach-klinicznych/podstawowe-informacje/dlaczego-prowadzi-sie-badania-kliniczne/ (accessed on 1 August 2022).
- Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16th April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC. Dz.U. L 158 z 27.5.2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0536&qid=1667573910313&from=EN (accessed on 1 August 2022).
- Regulska, K. Leki Innowacyjne w Badaniach Klinicznych. Available online: https://www.woia.pl/news/3346/leki-innowacyjne-w-badaniach-klinicznych.html (accessed on 1 August 2022).
- Chudziński, W. Badania Kliniczne w Aptece Szpitalnej, Aptekaszpitalna.pl. Available online: https://www.aptekaszpitalna.pl/farmaceuta-w-szpitalu/badania-kliniczne-w-aptece-szpitalnej/2018-11-02 (accessed on 1 August 2022).
- Bele, N.; Kumar, S.; Singay, J. Conceptual Framework of Digitization of Hospital Services and Operations. Int. J. Health Edu. Med. Inf. 2018, 5, 1–5. [Google Scholar]
- Zarowitz, B.J. Emerging Pharmacotherapy and Health Care Needs of Patients in the Age of Artificial Intelligence and Digitalization. Ann. Pharm. 2020, 54, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, H.; Khosravi, S.H. Hospitals pharmacy quality assurance system assessment in tehran university of medical sciences, iran. Iran. J. Public Health 2010, 39, 102–113. [Google Scholar]
- American Society of Health-System Pharmacists. ASHP guidelines: Minimum standard for pharmacies in hospitals. Am. J. Health-Syst. Pharm. 2013, 70, 1619–1630. [Google Scholar] [CrossRef]
- Piction, C. Professional Standards for Hospital Pharmacy Services. Optimising Patient Outcomes from Medicines. England, Scotland and Wales; Royal Pharmaceutical Society: London, UK, 2014; 20p, Available online: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Professional%20standards/Professional%20standards%20for%20Hospital%20pharmacy/rps-professional-standards-for-hospital-pharmacy.pdf (accessed on 1 August 2022).
- Scheepers, H.P.; Langedijk, J.; Handlos, V.N.; Walser, S.; Schutjens, M.H.; Neef, C. Legislation on the preparation of medicinal products in European pharmacies and the Council of Europe Resolution. Eur. J. Hosp. Pharm. 2017, 24, 224–229. [Google Scholar] [CrossRef]
- Bouwman, Y. Andersen, LMGMP and preparation in hospital pharmacies. Eur. J. Hosp. Pharm. Sci. Pract. 2012, 19, 469–473. [Google Scholar] [CrossRef]
- Piecuch, A.; Kozłowska-Wojciechowska, M.; Jaszewska, E.; Makarewicz-Wujec, M. Farmaceuta kliniczny—Odpowiedź na zmieniające się potrzeby społeczne (In English: Clinical pharmacist—A response to changing social need). Farm. Pol. 2014, 70, 395–399. [Google Scholar]
- WHO/FIP. Developing Pharmacy Practice. A Focus on Patient Care Handbook—2006 Edition; WHO/FIP: Geneva, Switzerland, 2006. [Google Scholar]
- NIA/PTFarm. Strategia wdrażania opieki farmaceutycznej w Polsce. Biul. Nacz. Rady Aptek. 2007, 4, 1–12. Available online: http://www.nia.org.pl/dat/magazyn/BiuletynNRA_IV17.pdf (accessed on 1 August 2022).
- American College of Clinical Pharmacy. Standards of practice for clinical pharmacists. Pharmacotherapy 2014, 34, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.I.; Rodrígez-González, C.G.; Martín-Barbero, M.L.; Tovar-Pozo, M. CP-085 The Impact of Pharmacist Interventions on Safety and Cost Savings. Eur. J. Hosp. Pharm. 2016, 23, A37. [Google Scholar] [CrossRef]
- Gallagher, J.; Byrne, S.; Woods, N.; Lynch, D.; McCarthy, S. Cost-outcome description of clinical pharmacist interventions in a university teaching hospital. BMC Health Serv. Res. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Karimzadeh, I.; Mirzabeigi, P.; Dashti-Khavidaki, S. Evaluation of clinical pharmacist’s interventions in an infectious diseases ward and impact on patient’s direct medication cost. Eur. J. Intern. Med. 2013, 24, 227–233. [Google Scholar] [CrossRef]
- Khalili, H.; Farsaei, S.; Rezaee, H.; Dashti-Khavidaki, S. Role of clinical pharmacists’ interventions in detection and prevention of medication errors in a medical ward. Int. J. Clin. Pharm. 2011, 33, 281–284. [Google Scholar] [CrossRef]
- Alderman, C.P.; Farmer, C. A brief analysis of clinical pharmacy interventions undertaken in an Australian teaching hospital. J. Qual. Clin. Pract. 2001, 21, 99–103. [Google Scholar] [CrossRef]
- Krämer, I. Hospital Pharmacy in Germany. Available online: https://hospitalpharmacyeurope.com/news/editors-pick/hospital-pharmacy-in-germany (accessed on 1 August 2022).
- Ronan, S.; Shannon, N.; Cooke, K.; McKeon, T.; Walsh, E.K.; Kearney, A.; Sahm, L.J. The Role of the Clinical Pharmacist in an Irish University Teaching Hospital: A Mixed-Methods Study. Pharmacy 2020, 8, 14. [Google Scholar] [CrossRef]
- NHS Cumbria Clinical Commissioning Group. Medication Review. A Practice Guide. 2013. Available online: https://medicines.necsu.nhs.uk/cumbria-practice-resources (accessed on 1 August 2022).
- Corsonello, A.; Pedone, C.; Incalzi, R.A. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr. Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef]
- Beers, M.H. Aging as a Risk Factor for Medication-Related Problems. Consult. Pharm. 1999, 14, 1337–1341. [Google Scholar]
- FIP. Medicines Reconciliation. A Toolkit for Pharmacists. 2021. Available online: https://www.fip.org/file/4949 (accessed on 1 August 2022).
- Kearney, A.; Halleran, C.; Walsh, E.; Byrne, D.; Haugh, J.; Sahm, L.J. Medication Reviews by a Clinical Pharmacist at an Irish University Teaching Hospital. Pharmacy 2017, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Bem, A. Public financing of healthcare services. Efinanse—Financ. Internet Q. 2013, 9, 1–12. [Google Scholar]




| Demographic Variables | Description | n (%) |
|---|---|---|
| Gender | Female | 207 (74.8%) |
| Male | 70 (25.2%) | |
| Position | Pharmacy manager in public hospital | 127 (46%) |
| Pharmacist | 122 (44.2%) | |
| Pharmacy manager in private hospital | 17 (6.2%) | |
| Other including pharmacy owner or partner | 10 (3.6%) | |
| Sector | Pharmacist working in public sector | 253 (91.2%) |
| Pharmacist working in private sector | 24 (8.8%) | |
| Year of working experience | Up to 5 years | 36 (13%) |
| Between 6 and 10 years | 49 (17.7%) | |
| Between 11 and 20 years | 95 (34.3%) | |
| Over 21 years | 97 (35%) | |
| Size of hospital | Up to 100 beds | 28 (10.1%) |
| Between 101 and 300 beds | 103 (37.2%) | |
| Between 301 and 600 beds | 81 (29.2%) | |
| Between 601 and 1000 beds | 42 (15.2%) | |
| Over 1000 beds | 23 (8.3%) | |
| Educational qualification | Master of Pharmacy (MPharm) | 261 (93.9%) |
| PhD in Pharmaceutical Sciences (PSC) | 17 (6.1%) |
| Job Satisfaction | No Specialization | Specialization | ||
|---|---|---|---|---|
| Hospital Pharmacy | Clinical Pharmacy | Community Pharmacy | ||
| Very low (1–2) | 6.5% | 8.3% | 7.1% | 3.8% |
| Low (3–4) | 17.0% | 11.7% | 28.6% | 12.5% |
| Medium (5–6) | 37.9% | 30.0% | 47.6% | 36.2% |
| High (7–8) | 30.1% | 36.7% | 16.7% | 35.0% |
| Very high (9–10) | 8.5% | 13.3% | 0.0% | 12.5% |
| Overall (N) | 100 | 60 | 42 | 80 |
| Need to Make Changes | Yes (%) | N |
|---|---|---|
| In the model of functioning of pharmacists in medical entities | 96.1 | 259 |
| In the way pharmacists operate in distribution of medicinal products and medical devices | 83.5 | 243 |
| In preparation of medicinal products | 85.9 | 227 |
| In the way pharmacists operate in a hospital by launching a clinical pharmacy service | 95.4 | 216 |
| Launching R&D work (in cooperation with a university or a private company) in the areas of medication form technology, new medication technologies | 82.4 | 222 |
| Please Rate on a Scale of 1–10 Your General Readiness to Start Work On: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | Mean | SD | ||||||||||
| Making changes to the way pharmacists operate in your hospital | 2.3 | 1.6 | 3.9 | 1.6 | 15.2 | 9.3 | 14.0 | 16.0 | 8.2 | 28.0 | 7.35 | 2.37 |
| Introducing new solutions in the distribution area | 3.8 | 0.4 | 3.8 | 2.5 | 17.6 | 10.0 | 13.8 | 16.3 | 8.4 | 23.4 | 7.11 | 2.39 |
| Introducing new solutions in the field of preparation of medicinal products | 4.1 | 1.8 | 1.4 | 4.6 | 18.7 | 10.0 | 11.0 | 14.2 | 5.9 | 28.3 | 7.11 | 2.52 |
| Introducing new solutions in R&D | 4.7 | 5.1 | 3.3 | 5.1 | 15.4 | 7.0 | 11.7 | 16.4 | 7.0 | 24.3 | 6.83 | 2.70 |
| Starting work on the launch of clinical pharmacy services in your hospital | 4.9 | 1.9 | 3.4 | 7.3 | 15.0 | 7.8 | 9.2 | 12.6 | 8.7 | 29.1 | 7.05 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochniarz, M.; Inglot-Brzęk, E.; Lewandowska, A.; Podgórska, J. Directions of Changes in the Profession of Hospital Pharmacist in Poland. Int. J. Environ. Res. Public Health 2022, 19, 14522. https://doi.org/10.3390/ijerph192114522
Bochniarz M, Inglot-Brzęk E, Lewandowska A, Podgórska J. Directions of Changes in the Profession of Hospital Pharmacist in Poland. International Journal of Environmental Research and Public Health. 2022; 19(21):14522. https://doi.org/10.3390/ijerph192114522
Chicago/Turabian StyleBochniarz, Marcin, Elżbieta Inglot-Brzęk, Anna Lewandowska, and Joanna Podgórska. 2022. "Directions of Changes in the Profession of Hospital Pharmacist in Poland" International Journal of Environmental Research and Public Health 19, no. 21: 14522. https://doi.org/10.3390/ijerph192114522
APA StyleBochniarz, M., Inglot-Brzęk, E., Lewandowska, A., & Podgórska, J. (2022). Directions of Changes in the Profession of Hospital Pharmacist in Poland. International Journal of Environmental Research and Public Health, 19(21), 14522. https://doi.org/10.3390/ijerph192114522








