Abstract
The aim of this study was to determine the prevalence of non-communicable diseases and their correlation with COVID-19 disease severity among patients infected in Dubai. Clinical and demographic data were extracted from hospital records of 34,687 COVID-19 patients who visited or were admitted into Dubai hospitals between 28 January 2020 and 30 September 2020. Prevalence of co-morbidities in COVID-19 patients were assessed. The main risk factors associated with COVID-19 disease severity were also identified using three regression models. All co-morbidities were significantly associated with COVID-19 severity in the bivariate analysis (p-value ≤ 0.05) except for vitamin-D deficiency and chronic lower respiratory diseases. Patients with ischemic heart diseases (AOR: 2.08; 95% CI: 1.37, 3.15), pulmonary and other heart diseases (AOR: 2.13; 95% CI: 1.36, 3.32) and chronic kidney diseases (AOR: 1.81; 95% CI: 1.01, 3.25) had higher odds of severe COVID-19 symptoms. Suffering from multiple co-morbidities increased the odds of developing severe COVID-19 symptoms substantially in comparison to having only one co-morbidity i.e., (AOR: 1.52; 95% CI 1.76–2.60) to (AOR: 2.33; 95% CI: 1.37, 3.97). Identifying these risk factors could assist in the early recognition of high-risk populations and ensure the most appropriate preventive measures and required medical management during the pandemic.
1. Introduction
Since its first detection in Wuhan, China in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), later known as coronavirus disease 2019 (COVID-19) has spread worldwide to over 223 countries and territories including the United Arab Emirates (UAE) [1]. In the UAE there have been over 892,000 confirmed COVID-19 cases and 2302 total deaths as of 3rd April 2022 [1]. Research during the early phases of the pandemic indicates that demographic and behavioral factors such as sex, age and smoking may impact the severity of infections with COVID-19 [2,3,4,5,6].
Diabetes, hypertension and coronary heart disease have been reported as the most common comorbidities among COVID-19 patients [5,7,8,9]. Several studies, including those conducted in the Middle East and North Africa (MENA) region, have demonstrated that COVID-19 severity and clinical outcomes are worse for patients with pre-existing comorbidities such as pulmonary or kidney disease [4,5,10], cancer [11], nervous system diseases [12,13], diabetes [5,14,15], and hypertension or coronary heart disease [8,16]. Moreover, it has been reported that patients with multiple comorbidities experience worse COVID-19 symptoms and even mortality [17].
Among the total 9.89 million people living in the UAE in 2020, the majority (88%) were young adult expatriates [18]. However, the burden of diseases in the UAE is heavily dominated by non-communicable diseases (NCDs) with the UAE reporting the highest relative prevalence of diabetes in the MENA region (16.3% among adults aged 20 to 79 years) [19], and obesity prevalence of 32.3% among expatriate adults [20]. The positive association between NCDs and COVID-19 severity around the world combined with the high rate of NCDs in the UAE, places the country at higher risk of disease infection and worse clinical outcomes. Studies from Dubai and Al-Ain have demonstrated that patients with diabetes, chronic kidney disease, and vitamin-D Deficiency have more severe COVID-19 symptoms and mortality [21,22].
The World Health Organization (WHO) recently reported that pre-existing NCDs may increase COVID-19 severity [23]. Therefore, identifying patients at highest risk is crucial to improving health outcomes. However, studies from the UAE that have examined the association between pre-existing comorbidities and COVID-19 severity have been limited to small samples and single comorbidities. Therefore, the current study aims to fill this research gap by using a large sample of medical records from the Dubai Health Authority (DHA) to examine the prevalence of comorbidities among COVID-19 patients and the risk associated with existing comorbidities in causing severe COVID-19 symptoms. Furthermore, this study also examines the impact of multiple comorbidities on the severity of COVID-19 patient outcomes as compared to those with a single comorbidity or no comorbidity.
2. Methods
2.1. Study Design and Setting
We conducted a retrospective observational study using confirmed real-time polymerase chain reaction (RT-PCR) positive COVID-19 cases in the Emirate of Dubai, UAE. Data were extracted from the DHA health information system section. DHA is the governmental health authority responsible for providing medical services to the population of Dubai and governs four hospitals comprising approximately 1500 beds for adults and one 200 bed hospital for children. The EPIC electronic health record system is utilized throughout DHA facilities to document all medical information related to the patient’s hospital admission including patient information, symptoms, treatments, medical history and all relevant clinical data.
2.2. Participants and Study Size
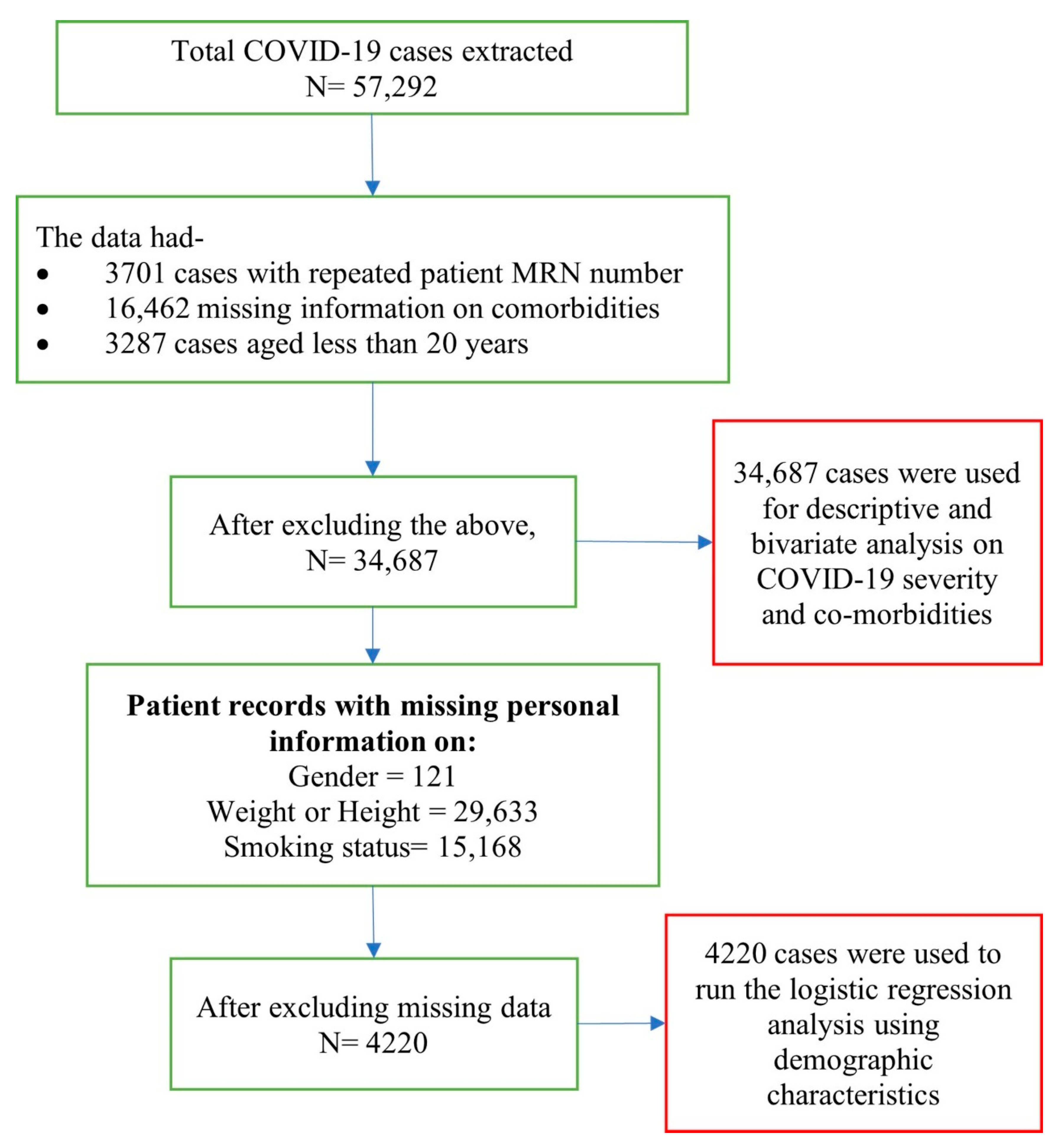
Data from a total of 57,292 patients of all ages and both sexes that were clinically diagnosed with COVID-19 and had tested positive in PCR laboratories for hospitalization, or were in isolation in Dubai DHA government hospitals, or quarantine centers under DHA between 28th January 2020 to 30th September 2020 were extracted from the DHA health information system. Inclusion criteria included, (i) adults aged 20 years and above, (ii) COVID-19 positive test results that met the case definition, and (iii) visited a DHA facility during the study period. A total of 34,687 patient data that fulfilled these criteria were included in the bivariate analysis. A detailed description and breakdown of included, missing, and repeated cases are presented in Figure 1.
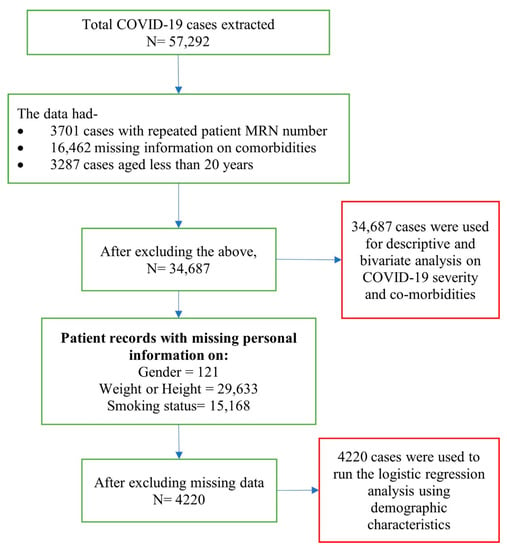
Figure 1.
Description and breakdown of patient data.
2.3. Defining Dataset Variables, Co-Morbidities, and Disease Severity
The dataset included: demographic variables (age, sex, residence, and smoking status); Body Mass Index (BMI) categorized as: underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9), and obese (>29.9) [24]; and pre-existing co-morbidities (grouped into 14 broad categories as per the International Classification of Diseases, Tenth Revision (ICD-10) (Table A1). Vitamin-D deficiency was classified as a serum 25-hydroxyvitamin D (25OHD) value less than 30 nmol/L as defined by the Institute of Medicine [25]. There were very few cases of tuberculosis and viral hepatitis, so these were excluded from further statistical analyses.
COVID-19 severity was classified into four categories following the WHO ISARIC classification [26] as; (i) Mild: symptomatic patients meeting the case definition for COVID-19 without evidence of viral pneumonia or hypoxia; (ii) Moderate: A Patients with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) but no signs of severe pneumonia, including SpO2 ≥ 90% in room air; (iii) Severe: A Patients with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) plus one of the following: respiratory rate > 30 breaths/min, severe respiratory distress, or SpO2 < 90% on room air); and (iv) Critical: Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction.
2.4. Statistical Analyses
Data were analyzed using Stata (16) [27]. Descriptive data are displayed in counts and percentages. Pearson Chi-Square (χ2) analysis identified associations between comorbidities and COVID-19 severity. Only those comorbidities that showed significant association in Chi-square analysis were further included in the binomial logistic regression analysis. COVID-19 severity was recoded into two groups for the regression model with mild and moderate severity classified as non-severe and severe and critical classified as severe. Three models were used for the multivariable logistic regression to identify predictors of COVID-19 severity. The first model controlled for comorbidity, the second model controlled for both comorbidity and sociodemographic characteristics, while the third model controlled for comorbidity, sociodemographic characteristics, and number of co-morbidities. The results are presented as adjusted odds ratios (aORs) with 95% confidence intervals (CI) after adjustment for the effects of potential confounders. A two-tailed p < 0.05 was considered statistically significant.
2.5. Ethical Approval
The study was approved by the DHA research ethics committee (DSREC-07/2020_18) on 22 July 2020 and the Emirates Institutional Review Board for COVID-19 Research DOH/CVDC/2020/1576 on 9 August 2020. Both committees waived the need for obtaining patient consent.
3. Results
The majority of COVID-19 patients were male (80%), aged 30–39 years (37%), and expatriates (90%) (Table 1). Almost two thirds of COVID-19 patients were either overweight (41%) or obese (25%) while 3% were current smokers and 1% former smokers. Most patients had mild COVID-19 severity (92%) followed by moderate (5%), and only 2% of patients were classified as severe and admitted to ICU (Table 1).

Table 1.
Sociodemographic and health characteristics of the study population.
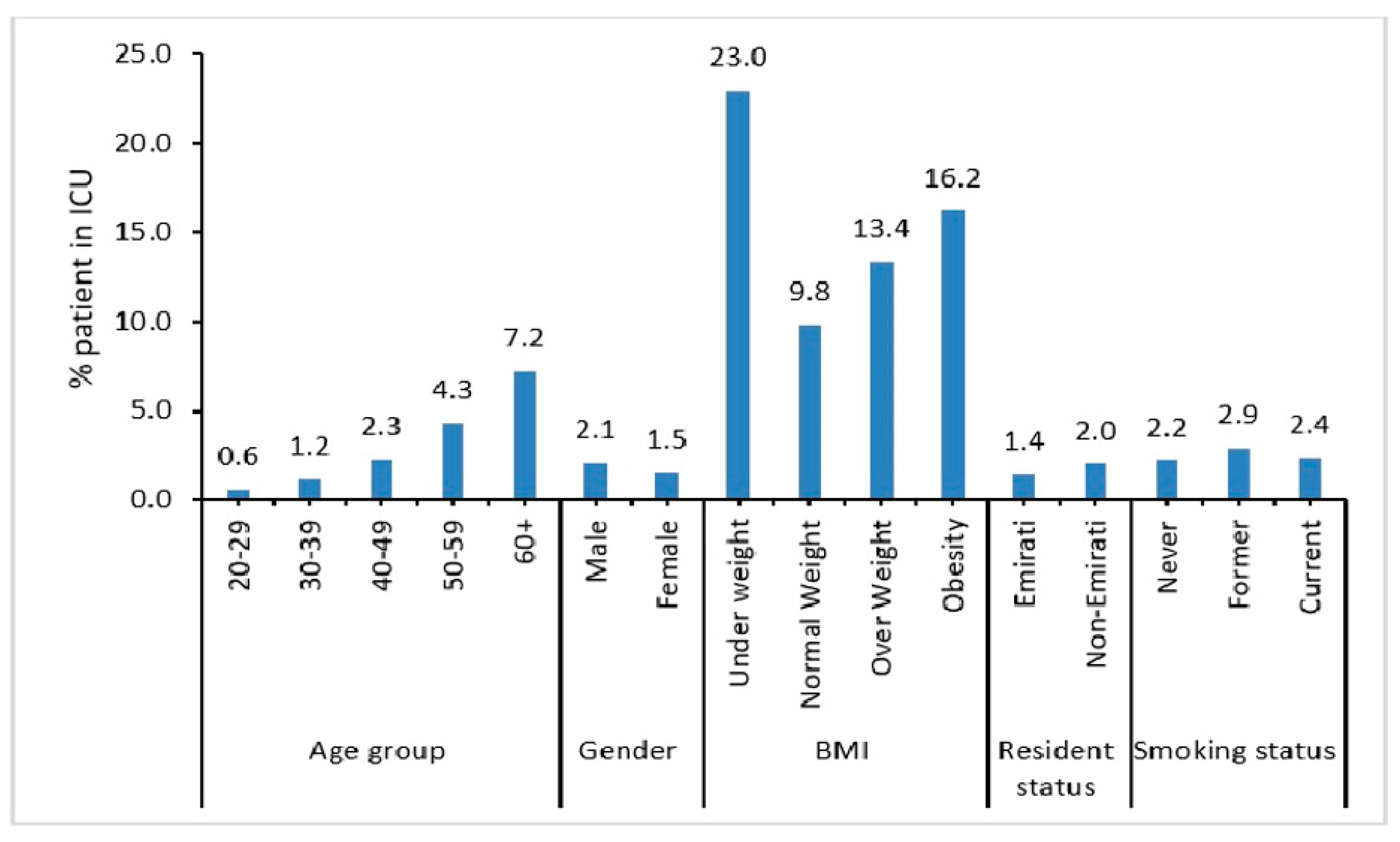
A higher percentage of elderly, i.e., aged 60 years and above (7.2%) and male patients (2.1%) were admitted into the ICU (Figure 2). Also, patients who did not maintain normal weight (i.e., underweight (23%), obese (13.4%), or overweight (16.2%)) were more likely to be admitted into the ICU.
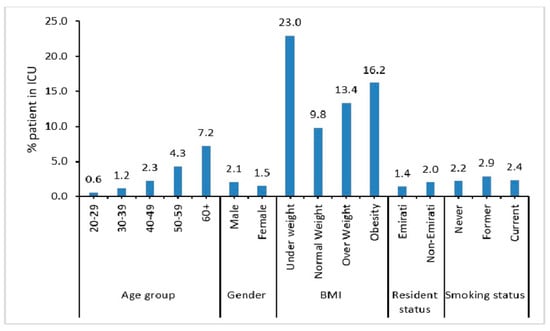
Figure 2.
Demographic characteristics of COVID-19 patients admitted to ICU.
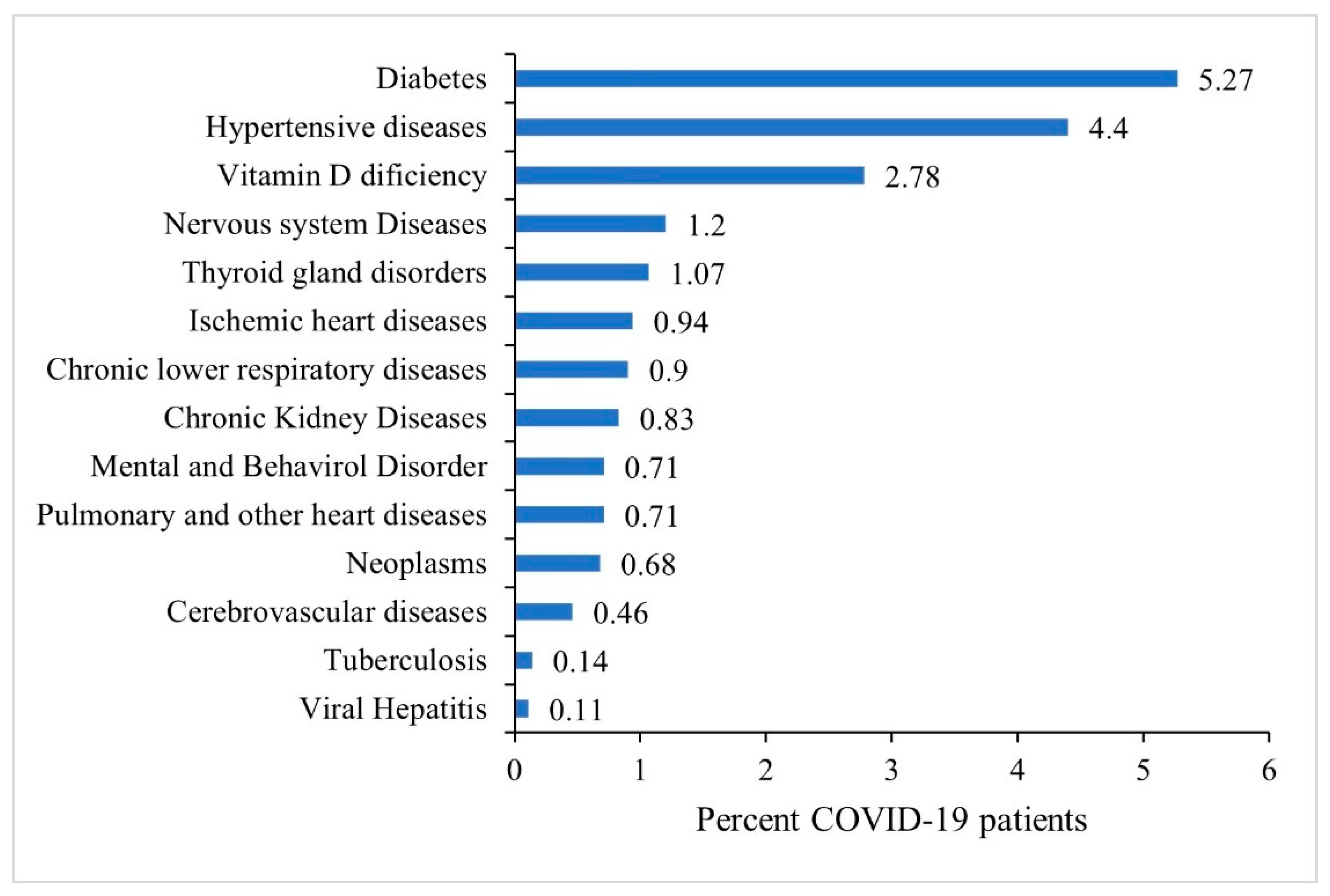
The most prevalent pre-existing comorbidities among COVID-19 patients were diabetes (5.27%) and hypertensive disorders (4.40%) followed by vitamin-D deficiency (2.78%), nervous system diseases (1.2%) and thyroid gland disorders (1.07%). Less than 1% of patients had other co-morbidities as shown in Figure 3.
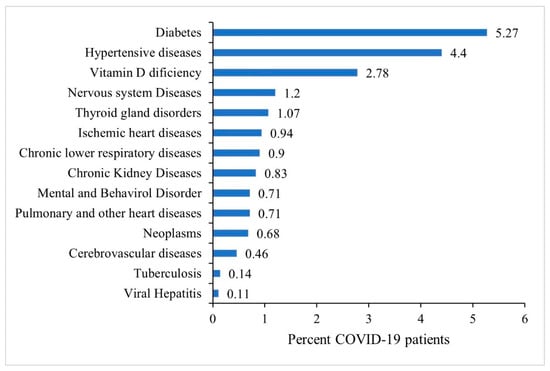
Figure 3.
Prevalence of existing co-morbidities among all COVID-19 patients, N = 34,687.
A significantly higher percentage of patients who had pulmonary and other heart diseases (27.64%), chronic kidney disease (27.08%), cerebrovascular diseases (25.95%), ischemic heart diseases (19.57%), and hypertensive disorders (7.87%) were reported as being critical COVID-19 cases (Table 2). There was significant association found between the number of existing co-morbidities and COVID-19 severity with patients reporting multiple co-morbidities having worse (critical) severity (11.46%).

Table 2.
Chi-square test for association between comorbidities and COVID-19 severity, N = 34,687.
The Chi-square test for association between comorbidities and COVID-19 severity for the 4220 patients included in the multivariable regression analysis are displayed in Table A2. Table 3 presents results from the multivariable logistic regression analysis. Among the three regression models, the Pseudo R2 values according to (McFadden estimates) were 0.062 for model one, 0.087 for model two, and 0.090 for model three, respectively, indicating that model three provided the best goodness of fit to the data.

Table 3.
Risk factors for COVID-19 severity using multivariable logistic regression, N = 4220.
In the first model, patients with diabetes (aOR: 2.32; 95% CI: 1.87, 2.88), ischemic heart diseases (aOR: 3.23; 95% CI: 2.19, 4.76), pulmonary and other heart diseases (aOR: 2.86; 95% CI: 1.88, 4.35), and chronic kidney diseases (aOR: 1.98; 95% CI: 1.10, 3.57) had higher odds of developing severe COVID-19 infection (Table 3; Model 1). However, once individual level characteristics such as age, gender, BMI, and smoking status were controlled for in the model, thyroid gland and mental and behavioral disorders also became significant predictors of worse COVID-19 severity. (Table 3; Model 2). In model 2, age, gender, BMI, and nationality were statistically significant predictors of worse COVID-19 severity. Increasing age was associated with increased likelihood of worse COVID-19 severity; 40–49 years (aOR: 2.04; 95% CI: 1.45, 2.86), 50–59 years (aOR: 2.47; 95% CI: 1.73, 3.52), 60 years and over (aOR: 3.28; 95% CI: 2.24, 4.80), and females had lower odds of developing severe COVID-19 infection compared to their male counterparts (aOR: 0.70; 95% CI: 0.55, 0.89). Patients who were underweight (aOR: 2.24; 95% CI: 1.18, 4.23); overweight (aOR: 1.39; 95% CI: 1.13, 1.72) or obese (aOR: 1.58; 95% CI: 1.25, 1.99) had higher odds of severe COVID-19 as compared to those within normal weight. After controlling for the number of comorbidities in the final model (Table 3; Model 3) the significance for diabetes, thyroid gland disorders and mental and behavioral disorders disappeared. Patients who had a pre-existing comorbidity, whether a single co-morbidity (aOR: 1.52; 95% CI: 1.13, 2.03) or multiple comorbidities (aOR: 2.33; 95% CI: 1.37, 3.97) had higher odds of developing severe COVID-19 infection as compared to those with no existing comorbidities.
4. Discussion
Almost two years have passed since the COVID-19 outbreak was declared a pandemic [28], and research continues to advance knowledge of disease prognosis, treatment and the long-term effects. Although COVID-19 treatment protocols have been proposed since the early stages of the pandemic [29,30,31,32], none have been widely adopted. In Dubai, hospitals are accredited by the Joint Commission International (JCI) accreditation body and JCI patient standards of care were maintained throughout the pandemic. However, in the absence of specific treatment protocols and the emergence of new variants, the identification of high-risk patients prone to clinical deterioration is critical for ensuring adequate and timely access to intensive care treatment and resource allocation.
The results from this study demonstrate that although most patients who were admitted to DHA facilities had mild COVID-19 severity, a significant number had moderate-to-critical health outcomes and required ICU admission. These results are consistent with prior reports concerning COVID-19 infections in China [33], which reported severe (14%) and critical (5%) symptoms and more recently in Abu Dhabi, UAE with similar reports of severe (1.8%) and critical (0.5%) outcomes [34]. COVID-19 infection was also observed to be highest among men and adults aged 20–50 years. Researchers from Abu Dhabi (UAE) who reported similar findings in 2021, indicated that one of the factors supporting these findings is that most expatriate men are of working age (20–50 years). This population is largely employed in high-risk occupations such as construction, hospitality, domestic work, and retail, and they live in shared accommodation where infections are quite likely to occur [35,36].
Compared with younger adults, older patients aged 60 years and over were more likely to experience severe COVID-19 symptoms. Several studies have shown similar results [6,37]. Interestingly, this association has also been observed in previous respiratory pandemics such as influenza (H7N9) [38,39]. Ageing as a risk factor for COVID-19 severity is particularly important given the rapid increase in the number and proportion of older people globally. The United Nations estimates that by 2050, one in six people worldwide will be 65 years of age or older, compared to one in eleven in 2019 [40]. Additionally, older patients with infections often present with atypical symptoms, making the diagnosis and treatment challenging [41]. With a median age of 30.3 years reported for Dubai [18], the UAE has a relatively young population. The disease trends observed in our study may be explained by these factors.
Another interesting finding in our study was the effect of weight on COVID-19 severity. Underweight patients had a 2.24 times higher likelihood of experiencing severe health outcomes than normal weight patients. Furthermore, obese patients had a 58% higher risk of experiencing poorer COVID-19 health outcomes. Studies from the MENA region have reported similar results including Kuwait [42], the Kingdom of Saudi Arabia (KSA) [5], and Iran [43]. In fact, the study of interactions between obesity and infectious respiratory diseases began even before the COVID-19 pandemic. Earlier research has shown that obesity can restrict ventilation and damage the immune system, both of which lead to an increased susceptibility to severe respiratory illness and adverse outcomes [44,45,46]. The exact mechanism by which obesity contributes to severe outcomes for COVID-19 is still unclear; however, one possible explanation might be the increased expression of the functional angiotensin-converting enzyme 2 (ACE2) receptor for SARS-CoV2 in obese individuals [47]. Further, obesity is associated with dysregulated lipid synthesis and clearance which results in increased pulmonary inflammation and injury [47,48]. The high prevalence of overweight and obesity in the UAE and other countries of the Gulf Cooperation Council (GCC) [20,49], emphasizes the need for future interventions and policies aimed at improving the health of underweight and obese patients.
Similar to other studies from the UAE, we also found that diabetes was the most common comorbidity among COVID-19 patients [34,50]. Higher mortality and worse disease outcomes have previously been reported in patients with diabetes [5,51]. Given the high prevalence of diabetes in the UAE in comparison to other countries [16,19], it is of critical importance to highlight the impact of COVID-19 on diabetes. Diabetes is known to have a negative impact on the innate immune response through insulin resistance and β-cell damage. Additionally, diabetes may impair the immune response by impairing macrophage and lymphocyte function, thus making patients more susceptible to disease complications and poor outcomes [52]. Based on our current observations and the literature cited previously, diabetes continues to be a major risk factor for poor prognosis among patients with COVID-19.
Although a relatively small percentage of patients in our study suffered from ischemic, pulmonary, and other heart diseases, those who did were more likely to develop severe COVID-19 outcomes, consistent with the literature [51,53]. The risk associated with COVID-19 severity among cardiac patients is relevant because cardiovascular patients are at an increased risk of cell damage due to direct viral damage, systemic inflammatory responses, destabilized coronary plaques, and aggravated hypoxia [54]. Moreover, cardiovascular patients often have increased ACE2 receptor expression, which facilitates the entry of viruses into the host body [53].
Chronic kidney disease has long been reported as a major public health problem and a significant risk factor for poor health outcomes [55]. Our finding that chronic kidney disease is a significant predictor of COVID-19 severity is consistent with previous observations in Spain and Mexico where studies found that kidney disease is significantly associated with severe COVID-19 symptoms and higher mortality compared with those without chronic kidney disorders [56,57]. Chronic kidney disease has become more prevalent over the past decade, with 13.4% of the population affected worldwide [58]. Among UAE nationals, the prevalence of kidney disease has been estimated to range from 2.8% to 4.6% [59]. These results may be underestimated due to the high prevalence of known kidney disease risk factors such as smoking and hypertension in the country [60,61]. There are several mechanisms responsible for kidney involvement during COVID-19 infection including cytokine damage (an exaggerated immune response), organ crosstalk (a mutual biological communication between distant organs mediated by signaling factors) and systemic effects (fluid expansion and hemodynamic instability) [62,63].
Our study also found that COVID-19 severity is strongly influenced by the presence of multiple comorbidities such that once these comorbidities were controlled in the model, the effects of diabetes, thyroid disorders, mental disorders, and behavioral disorders no longer appeared. These findings suggest that an increase in comorbidities is a significant predictor of COVID-19 severity, ICU admission, ventilator assistance, and even mortality-consistent with previous studies [64,65]. Additionally, multiple comorbidities are typically seen in older individuals. Therefore, aging and multiple comorbidities increase the likelihood of developing critical complications and outcomes from a novel coronavirus disease.
This study is particularly noteworthy for its comprehensive coverage of all DHA hospitals in Dubai and a large sample size of 34,687 patients collected over a period of nine months. The study provides further evidence to support the previous worldwide literature concerning the impact of age and pre-existing comorbidities on COVID-19 severity. Moreover, this data is from the first wave of the pandemic, which has two advantages. The first is that these results are not confounded by vaccination status, since vaccines were not available during 2020 [66] and secondly, these results are not confounded by serious second COVID-19 reinfection, because natural immunity held up throughout 2020. Nevertheless, one disadvantage of the first wave data is that the virus has since mutated substantially, so there can be some quantitative variability in the reported associations for the viral strains that are now circulating during 2022. However, qualitatively speaking, the associations can be reasonably expected to persist as reported. It is also important to note that the present study can only examine associations and cannot infer causality since it is retrospective. Additionally, the prevalence of NCDs such as diabetes, hypertension and obesity in the present study was lower than in previous studies conducted in the UAE. This may be due to the majority of our study population being relatively young and from Dubai, which comprises mostly expatriate males aged 20 to 40 years old. Moreover, since the comorbidity data were obtained from hospital records during admission, it is possible that some comorbidities were not detected and underreported.
5. Conclusions
The results of this study, in which data were extracted from a large dataset of patient medical records collected during the peak of the COVID-19 outbreak in Dubai, demonstrate that obese patients, those suffering from ischemic, pulmonary heart and kidney diseases and those afflicted with multiple co-morbidities are more likely to experience worse COVID-19 outcomes. Continually monitoring COVID-19 patients with underlying comorbidities will reduce the risk of severe outcomes. Due to the nature of the pandemic and the possibility of new variants emerging, it is imperative to improve healthcare providers’ awareness of vulnerable patients including expatriates and the elderly, so that specific strategies for their management of COVID-19 can be developed. Understanding the risk factors associated with COVID-19 infection will facilitate the identification of those at high risk, prompt their referral to hospitals at the earliest onset of symptoms, and prevent COVID-19 from progressing to severe levels. Furthermore, we recommend enhancing the systematic and real-time availability of epidemiological data for future more precise longitudinal and interventional studies.
Author Contributions
Conceptualization, N.A.A.B. and B.S.; Data curation, N.A.A.B.; Formal analysis, M.S.-H., A.S., N.A.-B. and B.S.; Funding acquisition, B.S.; Methodology, N.A.A.B. and M.S.-H.; Project administration, N.A.A.B.; Validation, A.S.; Visualization, N.A.-B.; Writing—original draft, A.S., N.A.-B. and B.S.; Writing—review & editing, N.A.A.B. and M.S.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Clinical Epidemiology Research Group operational grant (Grant code: 150389) University of Sharjah, UAE.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the DHA Research Ethics Committee (DSREC-07/2020_18) on 22 July 2020 and the Emirates Institutional Review Board for COVID-19 Research (DOH/CVDC/2020/1576) on 9 August 2020.
Informed Consent Statement
Informed consent was waived because of the retrospective nature of the study and the analysis of anonymous clinical data.
Data Availability Statement
Data used in this study were retrieved from hospital inpatient records and due to data protection policies cannot be shared at individual level.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Grouping of diseases according to ICD10 classification.
Table A1.
Grouping of diseases according to ICD10 classification.
| Disease Group | ICD Codes | |
|---|---|---|
| 1 | Tuberculosis | (A15–A19) |
| 2 | Viral hepatitis—hepatitis A, B, other acute viral, chronic viral, unspecified viral hepatitis | B15–B19 |
| 3 | Neoplasms—all malignant and benign cancers | C00–D48 |
| 4 | Thyroid gland disorders | E00–E07 |
| 6 | Diabetes mellitus—type 1, type 2 and other unspecified diabetes | E10–E14 |
| 7 | Mental and behavioral disorders | F00–F99 |
| 8 | Nervous system diseases | G00–G99 |
| 9 | Ischemic heart diseases—angina, myocardial infraction, other acute, and chronic ischemic heart diseases | I20–I25 |
| 10 | Pulmonary and other heart diseases—pulmonary embolism, other pulmonary heart diseases, diseases of pulmonary vessels, other forms of heart diseases | I26–I52 |
| 11 | Cerebrovascular diseases—hemorrhage, cerebral infarction, stroke, other cerebrovascular diseases | I60–I69 |
| 12 | Hypertensive disorders—primary and secondary hypertension, hypertensive heart disease, hypertensive kidney disease | I10–I15 |
| 13 | Chronic Kidney Diseases | N18 |
| 14 | Chronic lower respiratory diseases—chronic bronchitis, emphysema, asthma, other COPD, bronchiectasis | J40–47 |

Table A2.
Chi-square test for association between co-morbidities and COVID-19 severity, N = 4220.
Table A2.
Chi-square test for association between co-morbidities and COVID-19 severity, N = 4220.
| Mild | Moderate | Severe | Critical | Total | χ2 Value | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | N | |||
| Chronic lower respiratory diseases | |||||||||||
| No | 2462 | 59.8 | 1314 | 31.9 | 176 | 4.3 | 164 | 4 | 4116 | 7.94 | 0.047 |
| Yes | 75 | 72.1 | 20 | 19.2 | 4 | 3.8 | 5 | 4.8 | 104 | ||
| Diabetes | |||||||||||
| No | 2321 | 64.9 | 1020 | 28.5 | 120 | 3.4 | 117 | 3.3 | 3578 | 237.47 | ≤0.001 |
| Yes | 216 | 33.6 | 314 | 48.9 | 60 | 9.3 | 52 | 8.1 | 642 | ||
| Thyroid gland disorders | |||||||||||
| No | 2489 | 60.3 | 1307 | 31.7 | 177 | 4.3 | 154 | 3.7 | 4127 | 36.49 | ≤0.001 |
| Yes | 48 | 51.6 | 27 | 29 | 3 | 3.2 | 15 | 16.1 | 93 | ||
| Hypertension | |||||||||||
| No | 2342 | 62.5 | 1121 | 29.9 | 140 | 3.7 | 144 | 3.8 | 3747 | 86.10 | ≤0.001 |
| Yes | 195 | 41.2 | 213 | 45 | 40 | 8.5 | 25 | 5.3 | 473 | ||
| Ischemic heart diseases | |||||||||||
| No | 2499 | 61.2 | 1292 | 31.6 | 147 | 3.6 | 147 | 3.6 | 4085 | 209.47 | ≤0.001 |
| Yes | 38 | 28.1 | 42 | 31.1 | 33 | 24.4 | 22 | 16.3 | 135 | ||
| Pulmonary and other heart diseases | |||||||||||
| No | 2500 | 61 | 1289 | 31.4 | 161 | 3.9 | 151 | 3.7 | 4101 | 95.78 | ≤0.001 |
| Yes | 37 | 31.1 | 45 | 37.8 | 19 | 16 | 18 | 15.1 | 119 | ||
| Cerebrovascular diseases | |||||||||||
| No | 2511 | 60.3 | 1312 | 31.5 | 178 | 4.3 | 161 | 3.9 | 4162 | 17.19 | ≤0.001 |
| Yes | 26 | 44.8 | 22 | 37.9 | 2 | 3.4 | 8 | 13.8 | 58 | ||
| Kidney diseases | |||||||||||
| No | 2526 | 60.7 | 1301 | 31.3 | 178 | 4.3 | 154 | 3.7 | 4159 | 93.77 | ≤0.001 |
| Yes | 11 | 18 | 33 | 54.1 | 2 | 3.3 | 15 | 24.6 | 61 | ||
| Neoplasms | |||||||||||
| No | 2502 | 60.2 | 1317 | 31.7 | 176 | 4.2 | 160 | 3.9 | 4155 | 17.57 | ≤0.001 |
| Yes | 35 | 53.8 | 17 | 26.2 | 4 | 6.2 | 9 | 13.8 | 65 | ||
| Mental and behavioral disorders | |||||||||||
| No | 2495 | 60.4 | 1302 | 31.5 | 178 | 4.3 | 158 | 3.8 | 4133 | 20.18 | ≤0.001 |
| Yes | 42 | 48.3 | 32 | 36.8 | 2 | 2.3 | 11 | 12.6 | 87 | ||
| Nervous system diseases | |||||||||||
| No | 2459 | 60.1 | 1305 | 31.9 | 176 | 4.3 | 154 | 3.8 | 4094 | 23.71 | ≤0.001 |
| Yes | 78 | 61.9 | 29 | 23 | 4 | 3.2 | 15 | 11.9 | 126 | ||
| Musculoskeletal system diseases | |||||||||||
| No | 2403 | 59.7 | 1287 | 32 | 174 | 4.3 | 159 | 4 | 4023 | 7.39 | ≤0.001 |
| Yes | 134 | 68 | 47 | 23.9 | 6 | 3 | 10 | 5.1 | 197 | ||
| Number of existing morbidities | |||||||||||
| No co-morbidity | 1937 | 65.8 | 857 | 29.1 | 89 | 3 | 59 | 2 | 2942 | 251.25 | ≤0.001 |
| 1 co-morbidity | 383 | 51.6 | 274 | 36.9 | 33 | 4.4 | 52 | 7 | 742 | ||
| 2 or more co-morbidity | 217 | 40.5 | 203 | 37.9 | 58 | 10.8 | 58 | 10.8 | 536 | ||
| Total | 2537 | 60.1 | 1334 | 31.6 | 180 | 4.3 | 169 | 4 | 4220 | ||
References
- Worldometers COVID-19 Coronavirus Pandemic. Reported Cases and Deaths by Country or Territory. 2021. Available online: https://www.worldometers.info/coronavirus/ (accessed on 3 April 2022).
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, I.; Bonsignore, M.; Thürmann, P.; Hohenstein, S.; Jóźwiak, K.; Hauptmann, M.; Eifert, S.; Dengler, J.; Bollmann, A.; Groesdonk, H.V.; et al. Sex Differences in Clinical Course and Intensive Care Unit Admission in a National Cohort of Hospitalized Patients with COVID-19. J. Clin. Med. 2021, 10, 4954. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Alguwaihes, A.M.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alzahrani, S.H.; Sabico, S.; Al-Daghri, N.M.; Jammah, A.A. Diabetes and COVID-19 among hospitalized patients in Saudi Arabia: A single-centre retrospective study. Cardiovasc. Diabetol. 2020, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Htun, Y.M.; Win, T.T.; Aung, A.; Latt, T.Z.; Phyo, Y.N.; Tun, T.M.; Htun, N.S.; Tun, K.M.; Htun, K.A. Initial presenting symptoms, comorbidities and severity of COVID-19 patients during the second wave of epidemic in Myanmar. Trop. Med. Health 2021, 49, 62. [Google Scholar] [CrossRef]
- Nair, S.C.; Gasmelseed, H.I.; Khan, A.A.; Khafagy, I.N.; Sreedharan, J.; Saleem, A.A.; Abdrhman, H.I.; Alhosani, A.H.; Siddiqua, A.R.; Ahmed, A.R.; et al. Assessment of mortality from COVID-19 in a multicultural multi-ethnic patient population. BMC Infect. Dis. 2021, 21, 1115. [Google Scholar] [CrossRef]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef]
- Hernández-Galdamez, D.R.; González-Block, M.; Romo-Dueñas, D.K.; Lima-Morales, R.; Hernández-Vicente, I.A.; Lumbreras-Guzmán, M.; Méndez-Hernández, P. Increased Risk of Hospitalization and Death in Patients with COVID-19 and Pre-existing Noncommunicable Diseases and Modifiable Risk Factors in Mexico. Arch. Med, Res. 2020, 51, 683–689. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using Open SAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef]
- Romagnolo, A.; Balestrino, R.; Imbalzano, G.; Ciccone, G.; Riccardini, F.; Artusi, C.A.; Bozzali, M.; Ferrero, B.; Montalenti, E.; Montanaro, E.; et al. Neurological comorbidity and severity of COVID-19. J. Neurol. 2020, 268, 762–769. [Google Scholar] [CrossRef]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef]
- A Kass, D.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- Gutierrez, J.P.; Bertozzi, S.M. Non-communicable diseases and inequalities increase risk of death among COVID-19 patients in Mexico. PLoS ONE 2020, 15, e0240394. [Google Scholar] [CrossRef]
- Alzaabi, A.; Al-Kaabi, J.; Al-Maskari, F.; Farhood, A.F.; Ahmed, L.A. Prevalence of diabetes and cardio-metabolic risk factors in young men in the United Arab Emirates: A cross-sectional national survey. Endocrinol. Diabetes Metab. 2019, 2, e00081. [Google Scholar] [CrossRef]
- Ge, E.; Li, Y.; Wu, S.; Candido, E.; Wei, X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS ONE 2021, 16, e0258154. [Google Scholar] [CrossRef]
- GMI United Arab Emirates Population Statistics 2022. Available online: https://www.globalmediainsight.com/blog/uae-population-statistics/#:~:text=Majority%20of%20the%20UAE%20population,for%20Dubai%20is%2033.5%20years (accessed on 12 January 2022).
- International Diabetes Federation Diabetes Atlas, 9th ed. 2019. Available online: https://www.diabetesatlas.org/en/resources (accessed on 8 September 2021).
- Sulaiman, N.; Elbadawi, S.; Hussein, A.; Abusnana, S.; Madani, A.; Mairghani, M.; Alawadi, F.; Sulaiman, A.; Zimmet, P.; Huse, O.; et al. Prevalence of overweight and obesity in United Arab Emirates Expatriates: The UAE National Diabetes and Lifestyle Study. Diabetol. Metab. Syndr. 2017, 9, 88. [Google Scholar] [CrossRef]
- Elemam, N.M.; Hannawi, H.; Al Salmi, I.; Bin Naeem, K.; Alokaily, F.; Hannawi, S. Diabetes mellitus as a comorbidity in COVID-19 infection in the United Arab Emirates. Saudi Med. J. 2021, 42, 170–180. [Google Scholar] [CrossRef]
- AbuRuz, S.; Al-Azayzih, A.; ZainAlAbdin, S.; Beiram, R.; Al Hajjar, M. Clinical characteristics and risk factors for mortality among COVID-19 hospitalized patients in UAE: Does ethnic origin have an impact. PLoS ONE 2022, 17, e0264547. [Google Scholar] [CrossRef]
- WHO, Information Note on COVID-19 and NCDs. 2020. Available online: https://www.who.int/who-documents-detail/COVID-19-and-ncds (accessed on 6 September 2021).
- WHO, A Healthy Lifestyle-WHO Recommendations. 2010. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 19 September 2022).
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D status: United States 2001–2006. NCHS Data Brief Hyattsville MD Natl. Cent. Health Stat. 2011, 59, 1–8. [Google Scholar]
- ISARIC International Severe Acute Respiratory and Emerging Infection Consortium-COVID-19 Long Term Protocol. Available online: https://isaric.org/research/COVID-19-clinical-research-resources/COVID-19-long-term-follow-up-study/ (accessed on 15 July 2021).
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC.: College Station, TX, USA, 2019. [Google Scholar]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---11-march-2020 (accessed on 12 May 2021).
- Derwand, R.; Scholz, M.; Zelenko, V. COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study. Int. J. Antimicrob. Agents 2020, 56, 106214. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev. Cardiovasc. Med. 2020, 21, 517–530. [Google Scholar] [PubMed]
- Marik, P.E.; Kory, P.; Varon, J.; Iglesias, J.; Meduri, G.U. MATH+ protocol for the treatment of SARS-CoV-2 infection: The scientific rationale. Expert Rev. Anti-Infect. Ther. 2021, 19, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kory, P.; Meduri, G.U.; Iglesias, J.; Varon, J.; Cadegiani, F.A.; Marik, P.E. “MATH+” Multi-Modal Hospital Treatment Protocol for COVID-19 Infection: Clinical and Scientific Rationale. J. Clin. Med. Res. 2022, 14, 53–79. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Al Hosani, F.; Aden, B.; Al Memari, S.; Al Mazrouei, S.; Ajab, S.; Abid, M.; Alsuwaidi, A.R.; Grivna, M.; Paulo, M.S.; Sheek-Hussein, M. Epidemiology of asymptomatic and symptomatic Coronavirus Disease 2019 confirmed cases in the Emirate of Abu Dhabi, United Arab Emirates: Observational study. Medicine 2021, 100, e25219. [Google Scholar] [CrossRef]
- Statistics Center of Abu Dhabi, Population and Demography Population Vital Statistics. Abu Dhabi. Available online: https://www.scad.gov.ae/en/pages/default.aspx (accessed on 21 July 2022).
- Paulo, M.; Loney, T.; Lapão, L. The primary health care in the emirate of Abu Dhabi: Are they aligned with the chronic care model elements? BMC Health Serv. Res. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Setiati, S.; Harimurti, K.; Safitri, E.D.; Ranakusuma, R.W.; Saldi, S.R.F.; Azwar, M.K.; Marsigit, J.; Pitoyo, Y.; Widyaningsih, W. Risk factors and laboratory test results associated with severe illness and mortality in COVID-19 patients: A systematic review. Acta Med. Indones 2020, 52, 227–245. [Google Scholar]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Van Duin, D. Diagnostic challenges and opportunities in older adults with infectious diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 973–978. [Google Scholar] [CrossRef]
- United Nations World Population Ageing 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 23 December 2021).
- Cesari, M.; Montero-Odasso, M. COVID-19 and Older Adults. Lessons Learned from the Italian Epicenter. Can. Geriatr. J. 2020, 23, 155–159. [Google Scholar] [CrossRef]
- Al-Sabah, S.; Al-Haddad, M.; Al-Youha, S.; Jamal, M.; Almazeedi, S. COVID -19: Impact of obesity and diabetes on disease severity. Clin. Obes. 2020, 10, e12414. [Google Scholar] [CrossRef]
- Khorrami, Z.; Nili, S.; Sharifi, H.; Eybpoosh, S.; Shokoohi, M. Association of cigarette smoking, obesity, and underlying medical conditions with COVID-19 hospitalization and mortality in Iran: A nationwide retrospective ecological study. Med. J. Islam. Repub. Iran 2020, 34, 918–925. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Morgan, O.W.; Bramley, A.; Fowlkes, A.; Freedman, D.S.; Taylor, T.H.; Gargiullo, P.; Belay, B.; Jain, S.; Cox, C.; Kamimoto, L.; et al. Morbid Obesity as a Risk Factor for Hospitalization and Death Due to 2009 Pandemic Influenza A(H1N1) Disease. PLoS ONE 2010, 5, e9694. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity 2020, 28, 1187–1190. [Google Scholar] [CrossRef]
- Nguyen-Van-Tam, J.S.; Openshaw, P.J.M.; Hashim, A.; Gadd, E.M.; Lim, W.S.; Semple, M.G.; Read, R.C.; Taylor, B.L.; Brett, S.J.; McMenamin, J.; et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax 2010, 65, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Zaghloul, S.; Ali, H.I.; Harrison, G.; Popkin, B.M. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, S.; Hannawi, H.; Bin Naeem, K.; Elemam, N.M.; Hachim, M.Y.; Hachim, I.Y.; Darwish, A.S.; Al Salmi, I. Clinical and Laboratory Profile of Hospitalized Symptomatic COVID-19 Patients: Case Series Study From the First COVID-19 Center in the UAE. Front. Cell. Infect. Microbiol. 2021, 11, 632965. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2020, 109, 531–538. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Connecting Type 1 and Type 2 Diabetes through Innate Immunity. Cold Spring Harb. Perspect. Med. 2012, 2, a007724. [Google Scholar] [CrossRef]
- Alam Mahumud, R.; Kamara, J.K.; Renzaho, A.M.N. The epidemiological burden and overall distribution of chronic comorbidities in coronavirus disease-2019 among 202,005 infected patients: Evidence from a systematic review and meta-analysis. Infection 2020, 48, 813–833. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Tuttle, K.R.; Garcia-Garcia, G.; Gharbi, M.B.; Heerspink, H.J.; Johnson, D.W.; Liu, Z.-H.; Massy, Z.A.; Moe, O.; Nelson, R.G.; et al. Reducing major risk factors for chronic kidney disease. Kidney Int. Suppl. 2017, 7, 71–87. [Google Scholar] [CrossRef]
- Parra-Bracamonte, G.M.; Parra-Bracamonte, F.E.; Lopez-Villalobos, N.; Lara-Rivera, A.L. Chronic kidney disease is a very significant comorbidity for high risk of death in patients with COVID -19 in Mexico. Nephrology 2020, 26, 248–251. [Google Scholar] [CrossRef]
- Portolés, J.; Marques, M.; López-Sánchez, P.; de Valdenebro, M.; Muñez, E.; Serrano, M.L.; Malo, R.; García, E.; Cuervas, V. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. -Eur. Ren. Assoc. 2020, 35, 1353–1361. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Richards, N.; Hassan, M.; Saleh, A.K.; Dastoor, H.; Bernieh, B.; Abouchacra, S.; Al Jabri, O.; Fleischmann, A.; Richards, M.; Marcelli, D. Epidemiology and referral patterns of patients with chronic kidney disease in the Emirate of Abu Dhabi. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2015, 26, 1028–1034. [Google Scholar] [CrossRef]
- Al-Shamsi, S.; Al-Dhanhani, A.; Sheek-Hussein, M.M.; Bakoush, O. Provision of care for chronic kidney disease by non-nephrologists in a developing nation: A national survey. BMJ Open 2016, 6, e010832. [Google Scholar] [CrossRef]
- Al-Shamsi, S.; Regmi, D.; Govender, R.D. Chronic kidney disease in patients at high risk of cardiovascular disease in the United Arab Emirates: A population-based study. PLoS ONE 2018, 13, e0199920. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020, 16, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Armutcu, F. Organ crosstalk: The potent roles of inflammation and fibrotic changes in the course of organ interactions. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2019, 68, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.; Khalid, S.; Rahman, F.U.; Umar, M.; Ali, S.; Afridi, M.; Hassan, F.; Saleh Khader, Y.; Akhtar, N.; Khan, M.M.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among Patients With COVID-19 Hospitalized in Pakistan: Retrospective Observational Study. JMIR Public Health Surveill. 2021, 7, e32203. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Pivina, L.; Srinath, S.; Benahmed, A.G.; Semenova, Y.; Menzel, A.; Dadar, M.; Bjørklund, G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2020, 224, 108651. [Google Scholar] [CrossRef]
- Murchu, E.; Byrne, P.; Carty, P.G.; De Gascun, C.; Keogan, M.; O’Neill, M.; Harrington, P.; Ryan, M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev. Med. Virol. 2022, 32, e2260.1. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).