Validation Parameters of the Magnetic Stirrer Method for Pooled Sample Digestion for Trichinella spp. in Horse Meat Based on Proficiency Tests Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratories
2.2. Proficiency Testing
2.3. Data Analysis
3. Results
3.1. Qualitative Assessment
3.2. Quantitative Assessment
3.3. Evaluation of the Reported Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamble, H.R.; Bessonov, A.S.; Cuperlovic, K.; Gajadhar, A.A.; van Knapen, F.; Noeckler, K.; Schenone, H.; Zhu, X. International Commission on Trichinellosis: Recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet. Parasitol. 2000, 93, 393–408. [Google Scholar] [CrossRef]
- Karssin, A.; Remes, N.; Korge, K.; Viigipuu, M.; Stensvold, C.R.; Gomez-Morales, M.A.; Ludovisi, A.; Jokelainen, P.; Lassen, B. Herbivores as accidental hosts for Trichinella: Search for evidence of Trichinella infection and exposure in free-ranging moose (Alces alces) in a highly endemic setting. J. Wildl. Dis. 2021, 57, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Rozycki, M.; Bilska-Zajac, E.; Kochanowski, M.; Gradziel-Krukowska, K.; Zdybel, J.; Karamon, J.; Wisniewski, J.; Cencek, T. First case of Trichinella spiralis infection in beavers (Castor fiber) in Poland and Europe. Int. J. Parasitol. Parasites Wildl. 2020, 11, 46–49. [Google Scholar] [CrossRef]
- Lee, C.-E.; Seong, P.-N.; Oh, W.-Y.; Ko, M.-S.; Kim, K.-I.; Jeong, J.-H. Nutritional characteristics of horsemeat in comparison with those of beef and pork. Nutr. Res. Pract. 2007, 1, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Battaglia Richi, E.; Baumer, B.; Conrad, B.; Darioli, R.; Schmid, A.; Keller, U. Health risks associated with meat consumption: A Review of epidemiological studies. Int. J. Vitam. Nutr. Res. 2015, 85, 70–78. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bessa, R.J.B.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Horse-meat for human consumption—Current research and future opportunities. Meat Sci. 2015, 108, 74–81. [Google Scholar] [CrossRef]
- Stanisławczyk, R.; Rudy, M.; Rudy, S. The quality of horsemeat and selected methods of improving the properties of this raw material. Processes 2021, 9, 1672. [Google Scholar] [CrossRef]
- Kołodziejczyk, D.; Socik, M.; Socha, S. Importance of breeding and management of cold-blooded horses in terms of their meat utilization. Acta Sci. Pol. Zootech. 2019, 18, 63–72. [Google Scholar] [CrossRef]
- Jastrzębska, E.; Daszkiewicz, T.; Górecka-Bruzda, A.; Feliś, D. Current situation and prospects for the horse meat market in Poland and the world. Med. Weter 2019, 75, 196–202. [Google Scholar] [CrossRef]
- Forbes, L.B.; Gajadhar, A.A. A validated Trichinella digestion assay and an associated sampling and quality assurance system for use in testing pork and horse meat. J. Food Prot. 1999, 62, 1308–1313. [Google Scholar] [CrossRef]
- Dupouy-Camet, J.; Bougnoux, M.E.; Ancelle, T.; Fagard, R.; Lapierre, J. Antigenic characteristics of two strains of Trichinella spiralis isolated during the horsemeat-related outbreaks of 1985 in France. Parasitol. Res. 1988, 75, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E. New patterns of Trichinella infection. Vet. Parasitol. 2001, 98, 133–148. [Google Scholar] [CrossRef]
- Mantovani, A.; Filippini, I.; Bergomi, S. Indagini su un’epidemia di trichinellosi umana verificatasi in Italia. Parassitologia 1980, 22, 107–134. [Google Scholar]
- Pozio, E.; Tamburrini, A.; La Rosa, G. Horse trichinellosis, an unresolved puzzle. Parasite 2001, 8, S263–S265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blaga, R.; Cretu, C.M.; Gherman, C.; Draghici, A.; Pozio, E.; Noeckler, K.; Kapel, C.M.O.; Dida, I.; Cozma, V.; Boireau, P. Trichinella spp. infection in horses of Romania: Serological and parasitological survey. Vet. Parasitol. 2009, 159, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Ramisz, A.; Balicka, A. Remarks on horse trichinellosis in Poland. Wiad. Parazytol. 1994, 40, 381–384. [Google Scholar] [PubMed]
- Dupouy-Camet, J.; Soulé, C.; Ancelle, T. Recent news on trichinellosis: Another outbreak due to horsemeat consumption in France in 1993. Parasite 1994, 1, 99–103. [Google Scholar] [CrossRef]
- Scandrett, B.; Konecsni, K.; Lalonde, L.; Boireau, P.; Vallée, I. Detection of natural Trichinella murrelli and Trichinella spiralis infections in horses by routine post-slaughter food safety testing. Food Waterborne Parasitol. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Konecsni, K.; Scheller, C.; Scandrett, B.; Buholzer, P.; Gajadhar, A. Evaluation of the PrioCHECK Trichinella AAD Kit for the digestion and recovery of larvae in pork, horse meat and wild meat. Vet. Parasitol. 2017, 243, 267–271. [Google Scholar] [CrossRef]
- Neghina, R. Trichinellosis, a Romanian never-ending story. An overview of traditions, culinary customs, and public health conditions. Foodborne Pathog. Dis. 2010, 7, 999–1003. [Google Scholar] [CrossRef]
- Oivanen, L.; Mikkonen, T.; Haltia, L.; Karhula, H.; Saloniemi, H.; Sukura, A. Persistence of Trichinella spiralis in rat carcasses experimentally mixed in different feed. Acta Vet. Scand. 2002, 43, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Murrell, K.D.; Djordjevic, M.; Cuperlovic, K.; Sofronic, L.; Savic, M.; Djordjevic, M.; Damjanovic, S. Epidemiology of Trichinella infection in the horse: The risk from animal product feeding practices. Vet. Parasitol. 2004, 123, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Olsen, O.; Robinson, H. Role of rats and mice in transmitting Trichinella spiralis through their feces. J. Parasitol. 1958, 44, 35. [Google Scholar]
- Liciardi, M.; Marucci, G.; Addis, G.; Ludovisi, A.; Morales, M.A.G.; Deiana, B.; Cabaj, W.; Pozio, E. Trichinella britovi and Trichinella spiralis mixed infection in a horse from Poland. Vet. Parasitol. 2009, 161, 345–348. [Google Scholar] [CrossRef]
- Pomares, C.; Ajzenberg, D.; Bornard, L.; Bernardin, G.; Hasseine, L.; Darde, M.-L.; Marty, P. Toxoplasmosis and horse meat, France. Emerg. Infect. Dis. 2011, 17, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- Papo, M.; Valeyrie-Allanore, L.; Razazi, K.; Carteaux, G.; Wolkenstein, P.; Chosidow, O.; Brun-Buisson, C.; Mekontso Dessap, A.; de Prost, N. Renal replacement therapy during Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective observational study of 238 patients. Br. J. Dermatol. 2017, 176, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Directive 77/96/EEC of 21 December 1976 on the examination for trichinae (Trichinella spiralis) upon importation from third countries of fresh meat derived from domestic swine. Off. J. L 026 1977, 31, 67–77. [Google Scholar]
- Noeckler, K.; Pozio, E.; van der Giessen, J.; Hill, D.E.; Gamble, H.R. International Commission on Trichinellosis: Recommendations on post-harvest control of Trichinella in food animals. Food Waterborne Parasitol. 2019, 14, e00041. [Google Scholar] [CrossRef]
- Honsa, J.D.; McIntyre, D.A. ISO 17025: Practical Benefits of Implementing a Quality System. J. AOAC Int. 2019, 86, 1038–1044. [Google Scholar] [CrossRef]
- ISO 18743:2015; Microbiology of the Food Chain—Detection of Trichinella Larvae in Meat by Artificial Digestion Method. ISO: Geneva, Switzerland, 2015.
- In Vitro—Wykaz Testów do Diagnostyki In Vitro. Available online: www.wetgiw.gov.pl/nadzor-weterynaryjny/diagnostyka-in-vitro (accessed on 28 July 2022).
- Wykaz zakładów Zatwierdzonych Zgodnie z Rozporządzeniem (WE) nr 853/2004. Available online: zywnosc.wetgiw.gov.pl/spi/zatw/index.php?sekcja=2 (accessed on 25 October 2022).
- Rozycki, M.; Korpysa-Dzirba, W.; Belcik, A.; Bilska-Zajac, E.; Kochanowski, M.; Karamon, J.; Sroka, J.; Cencek, T. Validation of the magnetic stirrer method for the detection of Trichinella larvae in muscle samples based on proficiency tests results. Foods 2022, 11, 525. [Google Scholar] [CrossRef]
- Marucci, G.; Tonanzi, D.; Cherchi, S.; Galati, F.; Bella, A.; Interisano, M.; Ludovisi, A.; Amati, A.; Pozio, E. Proficiency testing to detect Trichinella larvae in meat in the European Union. Vet. Parasitol. 2016, 231, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Gajadhar, A.A.; Noeckler, K.; Boireau, P.; Rossi, P.; Scandrett, B.; Gamble, H.R. International Commission on Trichinelosis: Recommendations for quality assurance in digestion testing programs for Trichinella. Food Waterborne Dis. 2019, 16, e00059. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.B.; Hill, D.E.; Parker, S.; Tessaro, S.V.; Gamble, H.R.; Gajadhar, A.A. Complete validation of a unique digestion assay to detect Trichinella larvae in horse meat demonstrates the reliability of this assay for meeting food safety and trade requirements. J. Food Prot. 2008, 71, 558–563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ISO 16140-3:2021; Microbiology of the Food Chain—Method Validation—Part 3: Protocol for the Verification of Reference Methods and Validated Alternative Methods in a Single Laboratory. ISO: Geneva, Switzerland, 2021.
- ISO/IEC GUIDE 98-3:2008; Uncertainty of Measurement—Part 3: Guide to the Expression of Uncertainty in Measurement (GUM:1995). ISO: Geneva, Switzerland, 2008.
- Prost, E.K.; Nowakowski, Z. Detectability of Trichinella spiralis in muscles by pooled-sample-digestion-method. Fleischwirtschaft 1990, 70, 593–595. [Google Scholar]
- Rossi, P.; Pozio, E. Guidelines for the detection of Trichinella larvae at the slaughterhouse in a quality assurance system. Ann. Ist. Super. Sanita 2008, 44, 195–199. [Google Scholar]
- Forbes, L.B.; Rajic, A.; Gajadhar, A.A. Proficiency samples for quality assurance in Trichinella digestion tests. J. Food Prot. 1998, 61, 1396–1399. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Reckinger, S.; Nockler, K. German national proficiency testing for the detection of Trichinella in meat (2012). Fleischwirtschaft 2013, 93, 86–89. [Google Scholar]
- Riehn, K.; Hasenclever, D.; Petroff, D.; Nockler, K.; Mayer-Scholl, A.; Makrutzki, G.; Lucker, E. Trichinella detection: Identification and statistical evaluation of sources of error in the magnetic stirrer method for pooled sample digestion. Vet. Parasitol. 2013, 194, 106–109. [Google Scholar] [CrossRef]
- Glawischnig, W.; Schleicher, C.; Griesbacher, A.; Stadlmuller, L.; Dablander, K. Results of the proficiency tests for Austrian laboratories performing Trichinella digestion assays from 2008 to 2013. Wien. Tierarztl. Mon. 2014, 101, 221–227. [Google Scholar]
- Johne, A.; Bahn, P.; Thaben, N.; Nockler, K.; Mayer-Scholl, A. German national proficiency testing for the detection of Trichinella in meat (2016). Fleischwirtschaft 2018, 98, 92–96. [Google Scholar]
- Petroff, D.; Hasenclever, D.; Makrutzki, G.; Riehn, K.; Lucker, E. Assessing laboratory performance in Trichinella ring trials. Parasitol. Res. 2014, 113, 2837–2843. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, E.; Rozycki, M.; Bilska-Zajac, E.; Karamon, J.; Cencek, T. Results of proficiency testing (PT) for Trichinella in 2014. Med. Weter 2016, 72, 312–316. [Google Scholar]
- Schirone, M.; Visciano, P.; Aldo Olivastri, A.M.; Sgalippa, M.P.; Paparella, A. Accreditation procedure for Trichinella spp. detection in slaughterhouses: The experience of an internal laboratory in Italy. Foods 2019, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Robertson, L.J.; Dorny, P.; Jordan, S.; Kärssin, A.; Katzer, F.; La Carbona, S.; Lalle, M.; Lassen, B.; Mladineo, I.; et al. Parasite detection in food: Current status and future needs for validation. Trends Food Sci. Technol. 2020, 99, 337–350. [Google Scholar] [CrossRef]
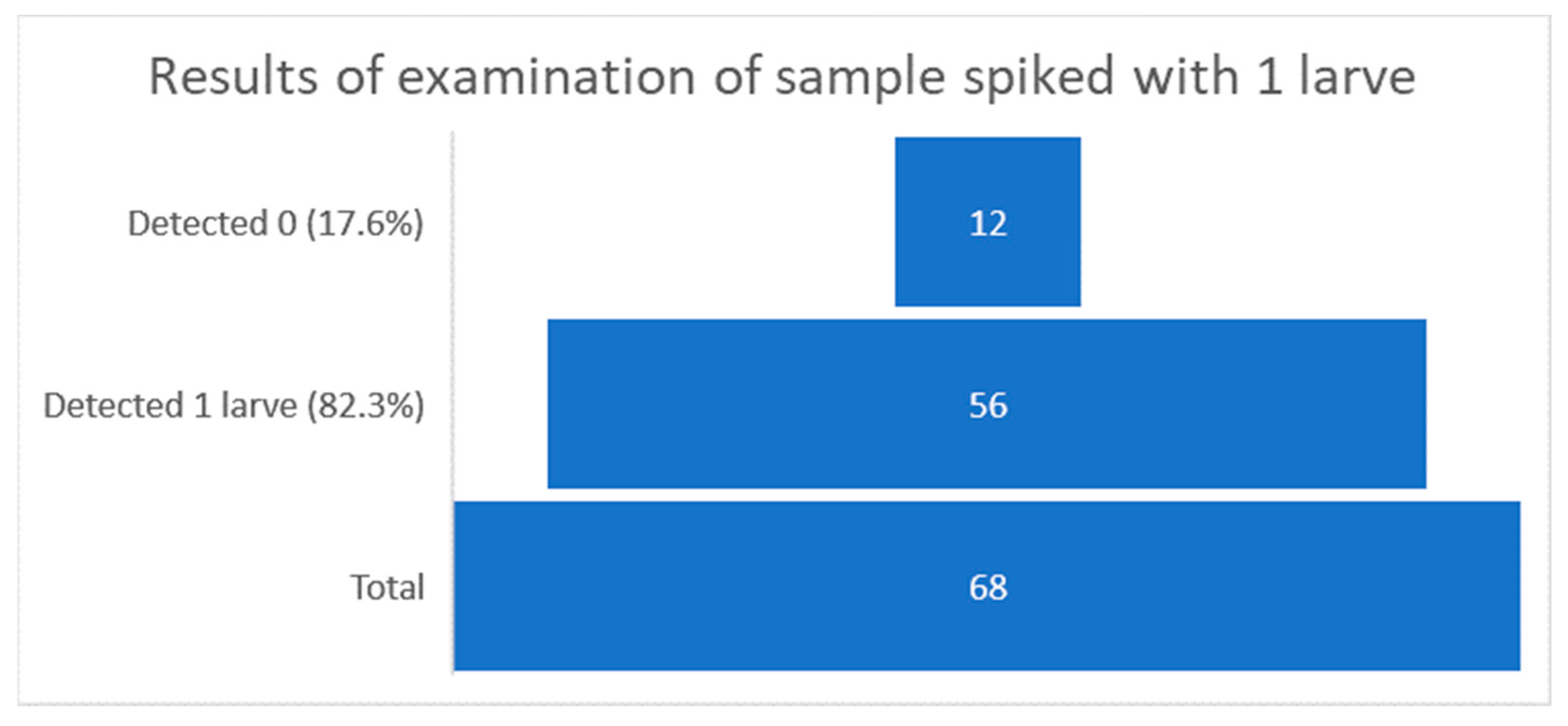

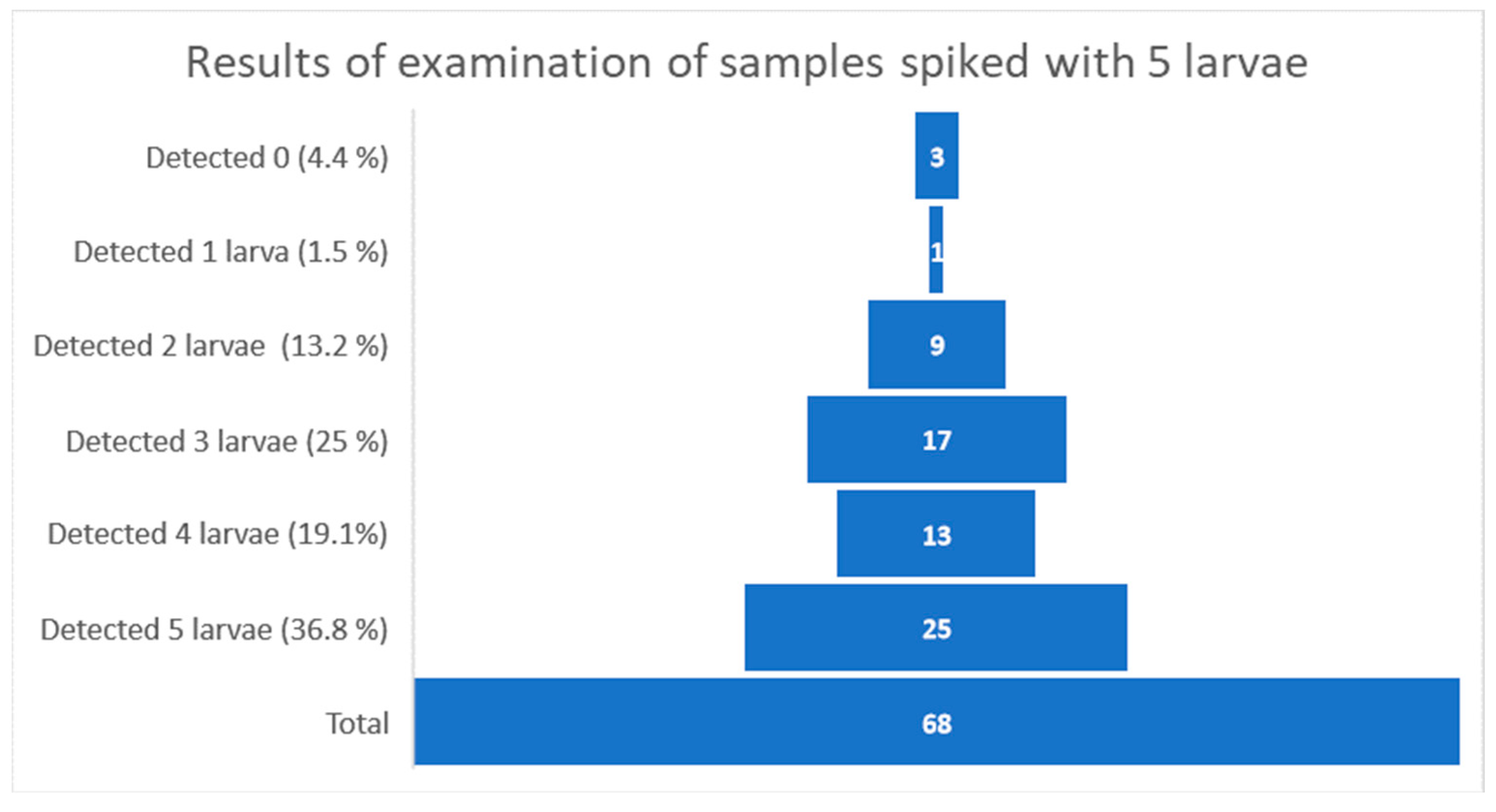
| Year | Number of Laboratories Participating in the Study | Total Number of Sent Samples (Contamination Levels 0, 3, 5) | Total Number of Samples Correctly Assessed | Total Number of Samples Incorrectly Assessed | % of Samples Correctly Assessed |
|---|---|---|---|---|---|
| 2014 | 14 | 42 | 42 | 0 | 100 |
| 2015 | 3 | 9 | 9 | 0 | 100 |
| 2016 | 13 | 39 | 37 | 2 | 95 |
| 2017 | 13 | 39 | 37 | 2 | 95 |
| 2018 | 8 | 24 | 24 | 0 | 100 |
| 2019 | 17 | 51 | 49 | 2 | 96 |
| Number of Larvae per Sample | Year/Number of Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014/14 | 2015/3 | 2016/13 | 2017/13 | 2018/8 | 2019/17 | |||||||
| C | I | C | I | C | I | C | I | C | I | C | I | |
| 0 | 14 | 0 | 3 | 0 | 13 | 0 | 13 | 0 | 8 | 0 | 17 | 0 |
| 1 | 10 | 4 | 3 | 0 | 8 | 5 | 11 | 2 | 7 | 1 | 17 | 0 |
| 3 | 14 | 0 | 3 | 0 | 11 | 2 | 12 | 1 | 8 | 0 | 17 | 0 |
| 5 | 14 | 0 | 3 | 0 | 13 | 0 | 12 | 1 | 8 | 0 | 15 | 2 |
| Year | Number of Laboratories Participating in the Study | Total Number of Laboratories with Correct Results | Total Number of Laboratories Reporting Incorrect Results | % of Laboratories That Passed PT Comparisons |
|---|---|---|---|---|
| 2014 | 14 | 14 | 0 | 100 |
| 2015 | 3 | 3 | 0 | 100 |
| 2016 | 13 | 11 | 2 | 84.6 |
| 2017 | 13 | 12 | 1 | 92.3 |
| 2018 | 8 | 8 | 0 | 100 |
| 2019 | 17 | 15 | 2 | 88.2 |
| Parameters | Level 0 | Level 1 | Level 3 | Level 5 |
|---|---|---|---|---|
| Mean | 0.00 | 0.82 | 2.35 | 3.63 |
| Standard error | 0.00 | 0.05 | 0.10 | 0.17 |
| Median | 0.00 | 1.00 | 3.00 | 4.00 |
| Mode | 0.00 | 1.00 | 3.00 | 5.00 |
| Standard deviation | 0.00 | 0.38 | 0.86 | 1.37 |
| Sample variance | 0.00 | 0.15 | 0.74 | 1.88 |
| Kurtosis | Nd | 1.04 | 0.62 | 0.22 |
| Skewness | Nd | −1.74 | −1.20 | −0.84 |
| Range | 0.00 | 1.00 | 3.00 | 5.00 |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 0.00 | 1.00 | 3.00 | 5.00 |
| Sum of detected larvae | 0.00 | 56.00 | 160.00 | 247.00 |
| number | 68.00 | 68.00 | 68.00 | 68.00 |
| Confidence level (95%) | 0.00 | 0.09 | 0.21 | 0.33 |
| Year | Number of Samples per Level | Number of Samples in Quantitative Assessment (n (%)) | |||||
|---|---|---|---|---|---|---|---|
| Level 0 | Level 3 | Level 3 | Level 5 | Level 5 | Level 5 | ||
| |Δ| = 0 Correct | |Δ| ≤ 2 Correct | |Δ| > 3 Incorrect | |Δ| ≤ 2 Correct | |Δ| = 3 Doubtful * | |Δ| > 3 Incorrect | ||
| 2014 | 14 | 14 (100) | 14 (100) | 0 | 11 (78.6) | 3 (21.4) | 0 |
| 2015 | 3 | 3 (100) | 3 (100) | 0 | 3 (100) | 0 | 0 |
| 2016 | 13 | 13 (100) | 11 (84.6) | 2 (15.4) | 11 (84.6) | 2 (15.4) | 0 |
| 2017 | 13 | 13 (100) | 12 (92.3) | 1 (7.7) | 12 (92.3) | 0 | 1 (7.7) |
| 2018 | 8 | 8 (100) | 8 (100) | 0 | 6 (75) | 2 (25) | 0 |
| 2019 | 17 | 17 (100) | 17 (100) | 0 | 14 (82.3) | 2 (11.7) | 3 (17.6) |
| Year | Number of Laboratories Participating in the Study | Number Negatively Evaluated Laboratories | Number Positively Evaluated Laboratories |
|---|---|---|---|
| 2014 | 14 | 0 | 14 |
| 2015 | 3 | 0 | 3 |
| 2016 | 13 | 2 | 11 |
| 2017 | 13 | 1 | 12 |
| 2018 | 8 | 0 | 8 |
| 2019 | 17 | 2 | 15 |
| Reference Value | Level 0 | Level 1 | Level 3 | Level 5 |
|---|---|---|---|---|
| s | 0.00 | 0.38 | 0.86 | 1.37 |
| X mean | 0.00 | 0.82 | 2.35 | 3.63 |
| CV | nd | 0.46 | 0.36 | 0.37 |
| Contamination Level | Species of Trichinella ssp. | Number of Examined Samples | Number of Samples Positively Assessed | Number of Samples Negatively Assessed |
|---|---|---|---|---|
| Negative: 0 | T. spiralis | 68 | 68 | 0 |
| Low level: 3 | T. spiralis | 68 | 65 | 3 |
| High level: 5 | T. spiralis | 68 | 65 | 3 |
| Validation Parameters (%) | Value | Upper Confidence Interval (UCL) | Lower Confidence Interval (LCL) |
|---|---|---|---|
| Specificity | 100 | 100 | 100 |
| Sensitivity | 95.6 | 93.8 | 97.3 |
| Accuracy | 97.1 | 95.9 | 98.2 |
| Sample Matrix | Horse Meat | Pork Meat | ||
|---|---|---|---|---|
| Number of Larvae Added to Samples | 3 | 5 | 3 | 5 |
| Mean | 2.35 | 3.63 | 2.14 | 3.51 |
| Standard error | 0.10 | 0.17 | 0.02 | 0.03 |
| Median | 3.00 | 4.00 | 2.00 | 4.00 |
| Mode | 3.00 | 5.00 | 3.00 | 5.00 |
| Standard deviation | 0.86 | 1.37 | 1.01 | 1.39 |
| Sample variance | 0.74 | 1.88 | 1.02 | 1.94 |
| Kurtosis | 0.62 | 0.22 | 0.87 | −0.22 |
| Skewness | −1.20 | −0.84 | −0.39 | −0.66 |
| Range | 3.00 | 5.00 | 9.00 | 7.00 |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 3.00 | 5.00 | 9.00 | 7.00 |
| Sum | 68.00 | 68.00 | 4057.00 | 6646.00 |
| Confidence level (95.0%) | 0.21 | 0.33 | 0.05 | 0.06 |
| Validation Parameters | Horse Meat | Pork Meat |
|---|---|---|
| Specificity (%) | 100 | 97.3 |
| Sensitivity (%) | 95.6 | 86.5 |
| Accuracy (%) | 97.1 | 89.2 |
| LOD | 1.14 | 1.08 |
| LOQ | 3.42 | 3.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różycki, M.; Korpysa-Dzirba, W.; Bełcik, A.; Bilska-Zając, E.; Gontarczyk, A.; Kochanowski, M.; Samorek-Pieróg, M.; Karamon, J.; Rubiola, S.; Chiesa, F.; et al. Validation Parameters of the Magnetic Stirrer Method for Pooled Sample Digestion for Trichinella spp. in Horse Meat Based on Proficiency Tests Results. Int. J. Environ. Res. Public Health 2022, 19, 14356. https://doi.org/10.3390/ijerph192114356
Różycki M, Korpysa-Dzirba W, Bełcik A, Bilska-Zając E, Gontarczyk A, Kochanowski M, Samorek-Pieróg M, Karamon J, Rubiola S, Chiesa F, et al. Validation Parameters of the Magnetic Stirrer Method for Pooled Sample Digestion for Trichinella spp. in Horse Meat Based on Proficiency Tests Results. International Journal of Environmental Research and Public Health. 2022; 19(21):14356. https://doi.org/10.3390/ijerph192114356
Chicago/Turabian StyleRóżycki, Mirosław, Weronika Korpysa-Dzirba, Aneta Bełcik, Ewa Bilska-Zając, Aneta Gontarczyk, Maciej Kochanowski, Małgorzata Samorek-Pieróg, Jacek Karamon, Selene Rubiola, Francesco Chiesa, and et al. 2022. "Validation Parameters of the Magnetic Stirrer Method for Pooled Sample Digestion for Trichinella spp. in Horse Meat Based on Proficiency Tests Results" International Journal of Environmental Research and Public Health 19, no. 21: 14356. https://doi.org/10.3390/ijerph192114356
APA StyleRóżycki, M., Korpysa-Dzirba, W., Bełcik, A., Bilska-Zając, E., Gontarczyk, A., Kochanowski, M., Samorek-Pieróg, M., Karamon, J., Rubiola, S., Chiesa, F., & Cencek, T. (2022). Validation Parameters of the Magnetic Stirrer Method for Pooled Sample Digestion for Trichinella spp. in Horse Meat Based on Proficiency Tests Results. International Journal of Environmental Research and Public Health, 19(21), 14356. https://doi.org/10.3390/ijerph192114356







