Accelerated Digitalization of the Epidemiological Measures: Overcoming the Technological and Process Complexities of Establishing the EU Digital COVID Certificate in Slovenia
Abstract
1. Introduction
2. Methods
2.1. Research Design
2.2. Sample
2.3. Data Collection
2.4. Data Analysis
3. Results
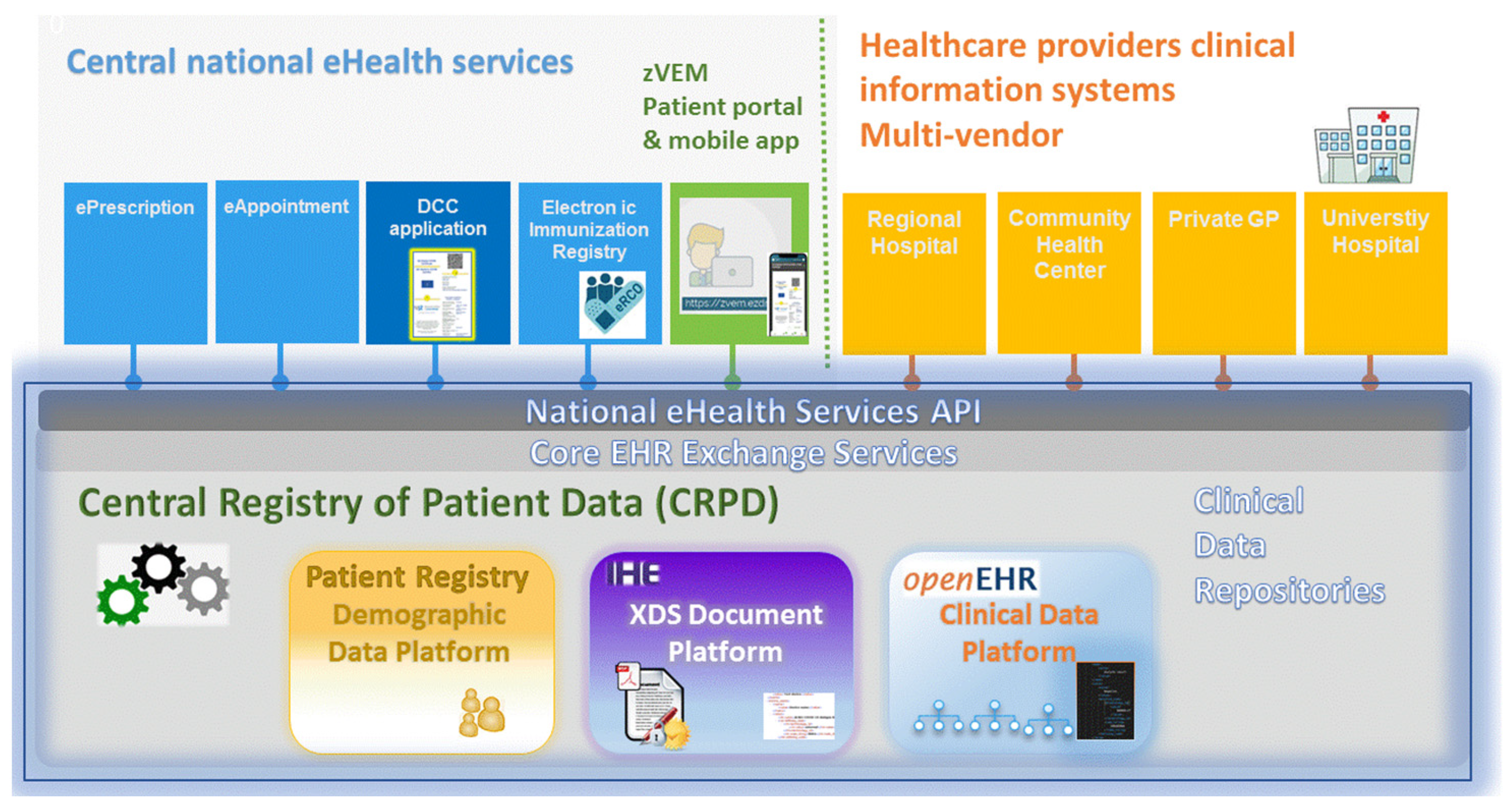
3.1. Technological Complexities—The Slovenian eHealth Architecture and the CRPD
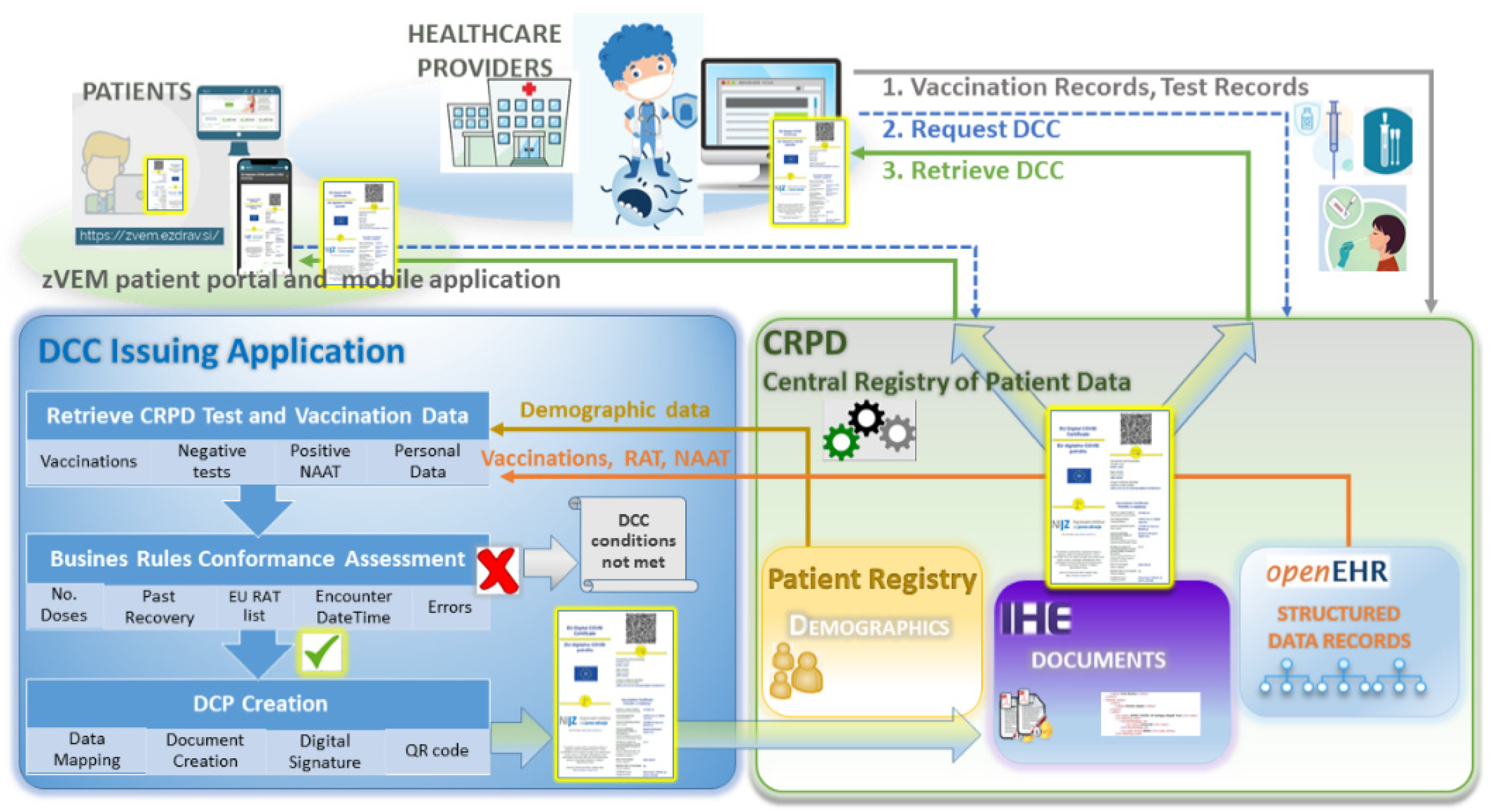
3.2. The DCC Application
3.3. Verifier Application
3.4. Patient Access to the DCC
Process Complexities—Operational and Organizational Aspects of the DCC Introduction in Slovenia
3.5. The National Legislation, the GDPR, and the European Health Data Space
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU Digital COVID Certificate. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-COVID-19-vaccines-europeans/eu-digital-COVID-certificate_en (accessed on 6 March 2022).
- Stanimirovic, D.; Tepej Jocic, L. Introduction of the EU Digital COVID Certificate in Slovenia: Technological and Process Aspects. Stud. Health Technol. Inform. 2022, 289, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2021/953 of the European Parliament and of the Council of 14 June 2021 on a Framework for the Issuance, Verification and Acceptance of Interoperable COVID-19 Vaccination, Test and Recovery Certificates (EU Digital COVID Certificate) to Facilitate Free Movement during the COVID-19 Pandemic; European Commission: Brussels, Belgium, 2021.
- Technical specifications for EU Digital COVID Certificates. Available online: https://ec.europa.eu/health/publications/technical-specifications-eu-digital-COVID-certificates-volumes-1-5_en (accessed on 9 April 2022).
- Pavli, A.; Maltezou, H.C. COVID-19 vaccine passport for safe resumption of travel. J. Travel Med. 2021, 28, taab079. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Botlero, R.; Hoque, N.; Alif, S.M.; Nazmul Karim, M.; Islam, S.M.S. A pragmatic approach to COVID-19 vaccine passport. BMJ Glob. Health 2021, 6, e006956. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, N.C. Advancing strategic policy on European Union digital COVID-19 certificate. J. Public Health 2022, 44, e313–e314. [Google Scholar] [CrossRef] [PubMed]
- Montanari Vergallo, G.; Zaami, S.; Negro, F.; Brunetti, P.; Del Rio, A.; Marinelli, E. Does the EU COVID Digital Certificate Strike a Reasonable Balance between Mobility Needs and Public Health? Medicina 2021, 57, 1077. [Google Scholar] [CrossRef]
- Elgersma, I.H.; Svarstad, E.; Kløvstad, H.; Nygård, K.M.; Kristoffersen, A.B. No evidence for added value of introducing mandatory COVID-19 testing for international travellers entering Norway with a valid EU digital COVID certificate. Infect. Dis. 2022, 54, 934–939. [Google Scholar] [CrossRef]
- Wilf-Miron, R.; Myers, V.; Saban, M. Incentivizing Vaccination Uptake: The “Green Pass” Proposal in Israel. JAMA 2021, 325, 1503–1504. [Google Scholar] [CrossRef]
- Priesemann, V. Epidemic dynamics after the DCC introduction: How to best shape COVID-19 evidence-informed policies. Eur. J. Public Health 2021, 31, ckab164.829. [Google Scholar] [CrossRef]
- De Giorgio, A.; Kuvačić, G.; Maleš, D.; Vecchio, I.; Tornali, C.; Ishac, W.; Ramaci, T.; Barattucci, M.; Milavić, B. Willingness to Receive COVID-19 Booster Vaccine: Associations between Green-Pass, Social Media Information, Anti-Vax Beliefs, and Emotional Balance. Vaccines 2022, 10, 481. [Google Scholar] [CrossRef]
- Prainsack, B. Assessing policies for the implementation of new technological interventions to combat COVID-19. Eur. J. Public Health 2021, 31, ckab164.827. [Google Scholar] [CrossRef]
- Wang, B.; Ping, Y. A comparative analysis of COVID-19 vaccination certificates in 12 countries/regions around the world: Rationalising health policies for international travel and domestic social activities during the pandemic. Health Policy 2022, 126, 755–762. [Google Scholar] [CrossRef]
- Lingri, D.; Petelos, E.; Zeegers, D.; Valaris, S.; Favaretti, C. COVID-19 passes and public health law: Considerations for surveillance and social justice. Eur. J. Public Health 2021, 31, ckab164.826. [Google Scholar] [CrossRef]
- Falkenbach, M.; Willison, C. Resources or trust: What matters more in the vaccination strategies of high-income liberal democracies? Health Policy Technol. 2022, 11, 100618. [Google Scholar] [CrossRef]
- Alemanno, A.; Bialasiewicz, L. Certifying Health: The Unequal Legal Geographies of COVID-19 Certificates. Eur. J. Risk Regul. 2021, 12, 273–286. [Google Scholar] [CrossRef]
- Ussai, S.; Pistis, M.; Missoni, E.; Formenti, B.; Armocida, B.; Pedrazzi, T.; Castelli, F.; Monasta, L.; Lauria, B.; Mariani, I. “Immuni” and the National Health System: Lessons Learnt from the COVID-19 Digital Contact Tracing in Italy. Int. J. Environ. Res. Public Health 2022, 19, 7529. [Google Scholar] [CrossRef] [PubMed]
- Pericàs-Gornals, R.; Mut-Puigserver, M.; Payeras-Capellà, M.M. Highly private blockchain-based management system for digital COVID-19 certificates. Int. J. Inf. Secur. 2022, 21, 1069–1090. [Google Scholar] [CrossRef] [PubMed]
- Karopoulos, G.; Hernandez-Ramos, J.L.; Kouliaridis, V.; Kambourakis, G. A survey on digital certificates approaches for the COVID-19 pandemic. IEEE Access 2021, 9, 138003–138025. [Google Scholar] [CrossRef]
- Wilford, S.; Mcbride, N.; Brooks, L.; Eke, D.O.; Akintoye, S.; Owoseni, A.; Leach, T.; Flick, C.; Fisk, M.; Stacey, M. The Digital Network of Networks: Regulatory Risk and Policy Challenges of Vaccine Passports. Eur. J. Risk Regul. 2021, 12, 393–403. [Google Scholar] [CrossRef]
- Mithani, S.S.; Bota, A.B.; Zhu, D.T.; Wilson, K. A scoping review of global vaccine certificate solutions for COVID-19. Hum. Vaccin. Immunother. 2022, 18, 1–12. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Dhama, K.; Rabaan, A.A.; Alhumaid, S. COVID-19 vaccination passport: Prospects, scientific feasibility, and ethical concerns. Hum. Vaccin. Immunother. 2021, 17, 4108–4111. [Google Scholar] [CrossRef]
- Nehme, M.; Kaiser, L.; Gillet, P.; Thevoz, P.; Stringhini, S.; Guessous, I. Digital COVID Credentials: An Implementation Process. Front. Digit. Health 2021, 3, 594124. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.K. Case Study Research and Applications: Design and Methods; Sage Publications: Los Angeles, CA, USA, 2017. [Google Scholar]
- Thomas, G. How to do Your Case Study? Sage Publications: Thousand Oaks, CA, USA, 2021. [Google Scholar]
- Mohajan, H.K. Qualitative research methodology in social sciences and related subjects. J. Econ. Dev. Environ. People 2018, 7, 23–48. [Google Scholar] [CrossRef]
- Ahmad, S.; Hussain, S.; Aamir, M.; Yasmeen, U.; Shabbir, J.; Ahmad, Z. Dual use of auxiliary information for estimating the finite population mean under the stratified random sampling scheme. J. Math. 2021, 2021, 3860122. [Google Scholar] [CrossRef]
- Yasmeen, U.; Noor-ul-Amin, M. The Estimation of Finite Population Variance Under Stratified Sampling Technique. J. Reliab. Stat. Stud. 2021, 14, 565–584. [Google Scholar] [CrossRef]
- Hennink, M.; Kaiser, B.N. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc. Sci. Med. 2022, 292, 114523. [Google Scholar] [CrossRef] [PubMed]
- Koro-Ljungberg, M.; Bussing, R.; Williamson, P.; M’Cormack-Hale, F. Reflecting on the experience sampling method in the qualitative research context: Focus on knowledge production and power during the data-collection process. Field Method 2008, 20, 338–355. [Google Scholar] [CrossRef]
- Winter, G. A Comparative Discussion of the Notion of ‘Validity’ in Qualitative and Quantitative Research. Qual. Rep. 2000, 4, 1–14. [Google Scholar] [CrossRef]
- Queirós, A.; Faria, D.; Almeida, F. Strengths and limitations of qualitative and quantitative research methods. Eur. J. Educ. Stud. 2017, 3, 369–387. [Google Scholar] [CrossRef]
- Lindgren, B.M.; Lundman, B.; Graneheim, U.H. Abstraction and interpretation during the qualitative content analysis process. Int. J. Nurs. Stud. 2020, 108, 103632. [Google Scholar] [CrossRef]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology; Sage Publications: Los Angeles, CA, USA, 2018. [Google Scholar]
- Integrated Healthcare Enterprise Cross Document Sharing. Available online: https://www.ihe.net/ (accessed on 5 February 2022).
- OpenEHR. Available online: https://www.openehr.org/ (accessed on 12 February 2022).
- IHE Profiles. Cross-Enterprise Document Sharing (XDS.b). Available online: https://profiles.ihe.net/ITI/TF/Volume1/ch-10.html (accessed on 16 February 2022).
- Government of the Republic of Slovenia. Decree on the Implementation of Screening Programs for the Early Detection of SARS-CoV-2 Virus Infection. Official Gazette of the Republic of Slovenia, no. 204/2020, 20/2021, 59/2021, 64/2021, 103/2021, 118/2021. Available online: http://pisrs.si/Pis.web/pregledPredpisa?id=URED8241 (accessed on 3 March 2022).
- Government of the Republic of Slovenia. Ordinance on the Temporary Measures for the Prevention and Control of Infectious Disease COVID-19. Official Gazette of the Republic of Slovenia, no. 22/2022, 29/2022, 37/2022, 51/2022. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=ODLO2661 (accessed on 5 March 2022).
- Stanimirovic, D. eHealth Patient Portal—Becoming an Indispensable Public Health Tool in the Time of Covid-19. Stud. Health Technol. Inform. 2021, 281, 880–884. [Google Scholar] [CrossRef] [PubMed]
- SI-PASS Authentication. Available online: https://www.si-trust.gov.si/en/si-pass/ (accessed on 3 May 2022).
- Stanimirovic, D.; Matetic, V. Can the COVID-19 pandemic boost the global adoption and usage of eHealth solutions? J. Glob. Health 2020, 10, 0203101. [Google Scholar] [CrossRef] [PubMed]
- SI-TRUST. Trust Service Authority of Slovenia. Available online: https://www.si-trust.gov.si/en/ (accessed on 5 May 2022).
- El Benny, M.; Kabakian-Khasholian, T.; El-Jardali, F.; Bardus, M. Application of the eHealth Literacy Model in Digital Health Interventions: Scoping Review. J. Med. Internet Res. 2021, 23, e23473. [Google Scholar] [CrossRef] [PubMed]
- Götz, N.A.; Hannemann, N.; Schmidt, L.; Babitsch, B. Digital Skills and Skills to Deal with COVID-19 Information: Sociodemographic Differences in a Cross-Sectional Study. Stud. Health Technol. Inform. 2021, 281, 824–825. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Chen, X.; Wang, L.; Gao, B.; Zhu, Q. Health information privacy concerns, antecedents, and information disclosure intention in online health communities. Inf. Manag. 2018, 55, 482–493. [Google Scholar] [CrossRef]
- Gstrein, O.J. The EU digital COVID certificate: A preliminary data protection impact assessment. Eur. J. Risk Regul. 2021, 12, 370–381. [Google Scholar] [CrossRef]
- McWay, D.C. Legal and Ethical Aspects of Health Information Management; Cengage Learning: New York, NY, USA, 2020. [Google Scholar]
- Personal Data Protection Act (ZVOP-1); no. 94/2007, 177/2020; Official Gazette of the Republic of Slovenia: Ljubljana, Slovenia, 2022.
- General Data Protection Regulation. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC; European Commission: Brussels, Belgium, 2016.
- European Commission. eHealth Network. Statistics and Reporting; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Healthcare Databases Act; no. 65/2000, 47/2015, 31/2018; Official Gazette of the Republic of Slovenia: Ljubljana, Slovenia, 2022.
- Directive (EU) 2016/1148 of the European Parliament and of the Council of 6 July 2016 Concerning Measures for a High Common Level of Security of Network and Information Systems across the Union; European Commission: Brussels, Belgium, 2016.
- Proposal for a Regulation of the European Parliament and of the Council on the European Health Data Space. COM(2022) 197 final. 2022/0140(COD); European Commission: Brussels, Belgium, 2022.
- Karahanna, E.; Chen, A.; Liu, Q.B.; Serrano, C. Capitalizing on health information technology to enable digital advantage in US hospitals. MIS Quarterly 2019, 43, 113–140. [Google Scholar] [CrossRef]
- Voigt, I.; Benedict, M.; Susky, M.; Scheplitz, T.; Frankowitz, S.; Kern, R.; Müller, O.; Schlieter, H.; Ziemssen, T. A digital patient portal for patients with multiple sclerosis. Front. Neurol. 2020, 11, 400. [Google Scholar] [CrossRef]
- Ghai, S. Teledentistry during COVID-19 pandemic. Diabetes Metab. Syndr. 2020, 14, 933–935. [Google Scholar] [CrossRef]
- Auener, S.; Kroon, D.; Wackers, E.; Dulmen, S.V.; Jeurissen, P. COVID-19: A Window of Opportunity for Positive Healthcare Reforms. Int. J. Health Policy Manag. 2020, 9, 419–422. [Google Scholar] [CrossRef]
- Chen, L.H.; Petersen, E.; Blumberg, L.; Piyaphanee, W.; Steffen, R. COVID-19 health passes: Current status and prospects for a global approach. J. Travel Med. 2021, 28, taab118. [Google Scholar] [CrossRef] [PubMed]
- Mbunge, E.; Dzinamarira, T.; Fashoto, S.G.; Batani, J. Emerging technologies and COVID-19 digital vaccination certificates and passports. Public Health Pract. 2021, 2, 100136. [Google Scholar] [CrossRef]
- Marques, J.G. The EU Digital COVID Certificate: Should We Differentiate Between Previously Infected and Fully Vaccinated People? Acta Med. Port. 2021, 34, 803–804. [Google Scholar] [CrossRef] [PubMed]
- European Commission. eHealth Network. Available online: https://ec.europa.eu/health/ehealth-digital-health-and-care/eu-cooperation/ehealth-network_en (accessed on 10 May 2022).
- Corpuz, J.C.G. COVID-19 vaccination certificate (CVC) for ASEAN: The way forward? J. Public Health 2022, 44, e289–e290. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Deng, C.; Gu, F. Vaccinations, Mobility and COVID-19 Transmission. Int. J. Environ. Res. Public Health 2021, 19, 97. [Google Scholar] [CrossRef]
- Roder-DeWan, S. Health system quality in the time of COVID-19. Lancet Glob. Health 2020, 8, e738–e739. [Google Scholar] [CrossRef]
- Lauriola, P.; Martín-Olmedo, P.; Leonardi, G.S.; Bouland, C.; Verheij, R.; Dückers, M.L.A.; Tongeren, M.V.; Laghi, F.; van den Hazel, P.; Gokdemir, O.; et al. On the importance of primary and community healthcare in relation to global health and environmental threats: Lessons from the COVID-19 crisis. BMJ Glob. Health 2021, 6, e004111. [Google Scholar] [CrossRef]
- Sim, J.X.Y.; Conceicao, E.P.; Wee, L.E.; Aung, M.K.; Seow, S.Y.W.; Teo, R.C.Y.; Goh, J.Q.; Yeo, D.W.T.; Kuo, B.J.; Lim, J.W.; et al. Utilizing the electronic health records to create a syndromic staff surveillance system during the COVID-19 outbreak. Am. J. Infect. Control 2021, 49, 685–689. [Google Scholar] [CrossRef]
- Sudat, S.E.K.; Robinson, S.C.; Mudiganti, S.; Mani, A.; Pressman, A.R. Mind the clinical-analytic gap: Electronic health records and COVID-19 pandemic response. J. Biomed. Inform. 2021, 116, 103715. [Google Scholar] [CrossRef]
- Maggu, G.; Sharma, S.; Jaishy, R.; Jangid, S. COVID-19 moral dilemmas viewed through Eastern and Western philosophy. Ind. Psychiatry J. 2021, 30, S273–S276. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Ali Reza, S.M.; Reza, M.N.; Chowdhury, M.S. Thank you, COVID-19: Positive social psychology towards the new normal. J. Public Aff. 2021, e2766. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, M.; Kagialis, A.; Karekla, M. COVID-19 Scientific Facts vs. Conspiracy Theories: Is Science Failing to Pass Its Message? Int. J. Environ. Res. Public Health 2021, 18, 6343. [Google Scholar] [CrossRef] [PubMed]
- Choukou, M.A.; Sanchez-Ramirez, D.C.; Pol, M.; Uddin, M.; Monnin, C.; Syed-Abdul, S. COVID-19 infodemic and digital health literacy in vulnerable populations: A scoping review. Digit. Health 2022, 8, 20552076221076927. [Google Scholar] [CrossRef] [PubMed]
- Freckelton, I. COVID-19 Denialism, Vaccine Scepticism and the Regulation of Health Practitioners. J. Law Med. 2021, 28, 613–619. [Google Scholar] [PubMed]
- Brogan, J.; Goodier, H.; Nijjar, M.; Rose, C. Trial by fire: How physicians responding to the COVID-19 pandemic illuminated the need for digital credentials. Digit Health 2022, 8, 20552076221084462. [Google Scholar] [CrossRef]
- Støme, L.N.; Wilhelmsen, C.R.; Kværner, K.J. Enabling Guidelines for the Adoption of eHealth Solutions: Scoping Review. JMIR Form Res. 2021, 5, e21357. [Google Scholar] [CrossRef]
- Mehtar, S.; Preiser, W.; Lakhe, N.A.; Bousso, A.; TamFum, J.J.M.; Kallay, O.; Seydi, M.; Zumla, A.; Nachega, J.B. Limiting the spread of COVID-19 in Africa: One size mitigation strategies do not fit all countries. Lancet Glob. Health 2020, 8, e881–e883. [Google Scholar] [CrossRef]
- Fiorini, M.; LA Gioia, A. COVID-19: Black Swan or clumsy use? J. Prev. Med. Hyg. 2021, 62, E7–E9. [Google Scholar] [CrossRef]
- Roncati, L.; Roncati, M. COVID-19 “Green Pass”: A Lesson on the Proportionality Principle from Galicia. Eur. J. Health Law 2021, 28, 525–532. [Google Scholar] [CrossRef]
- Zoumpourlis, V.; Goulielmaki, M.; Rizos, E.; Baliou, S.; Spandidos, D.A. The COVID-19 pandemic as a scientific and social challenge in the 21st century. Mol. Med. Rep. 2020, 22, 3035–3048. [Google Scholar] [CrossRef]
- Whitelaw, S.; Mamas, M.A.; Topol, E.; Van Spall, H.G.C. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit. Health 2020, 2, e435–e440. [Google Scholar] [CrossRef]
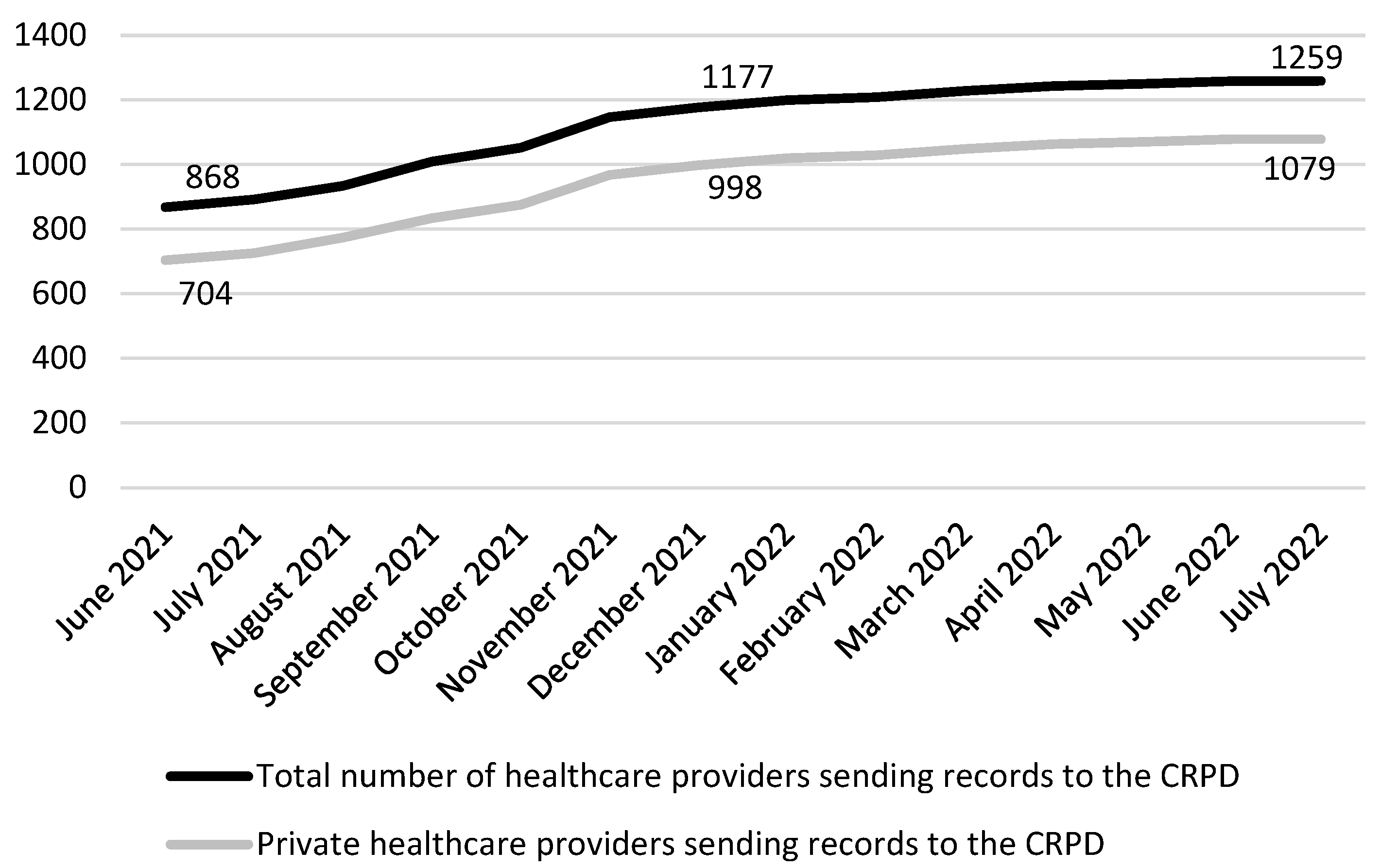
| DCCs Issued Monthly | zVEM Patient Portal Visits Monthly | zVEM Registered Users (Total End of Month) | |
|---|---|---|---|
| May 2021 | 2,592,507 | 133,405 | |
| June 2021 | 1,841,263 | 3,806,710 | 249,958 |
| July 2021 | 1,709,241 | 1,152,201 | 313,643 |
| August 2021 | 1,019,596 | 2,264,642 | 336,770 |
| September 2021 | 1,760,842 | 3,191,890 | 363,864 |
| October 2021 | 1,247,155 | 2,101,672 | 375,928 |
| November 2021 | 2,307,858 | 3,000,222 | 393,558 |
| December 2021 | 2,926,062 | 2,460,899 | 406,892 |
| January 2022 | 3,188,018 | 3,737,162 | 420,870 |
| February 2022 | 2,167,248 | 2,943,059 | 431,383 |
| March 2022 | 432,426 | 1,353,052 | 435,603 |
| April 2022 | 322,236 | 1,065,575 | 439,020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanimirovic, D.; Tepej Jocic, L. Accelerated Digitalization of the Epidemiological Measures: Overcoming the Technological and Process Complexities of Establishing the EU Digital COVID Certificate in Slovenia. Int. J. Environ. Res. Public Health 2022, 19, 14322. https://doi.org/10.3390/ijerph192114322
Stanimirovic D, Tepej Jocic L. Accelerated Digitalization of the Epidemiological Measures: Overcoming the Technological and Process Complexities of Establishing the EU Digital COVID Certificate in Slovenia. International Journal of Environmental Research and Public Health. 2022; 19(21):14322. https://doi.org/10.3390/ijerph192114322
Chicago/Turabian StyleStanimirovic, Dalibor, and Lucija Tepej Jocic. 2022. "Accelerated Digitalization of the Epidemiological Measures: Overcoming the Technological and Process Complexities of Establishing the EU Digital COVID Certificate in Slovenia" International Journal of Environmental Research and Public Health 19, no. 21: 14322. https://doi.org/10.3390/ijerph192114322
APA StyleStanimirovic, D., & Tepej Jocic, L. (2022). Accelerated Digitalization of the Epidemiological Measures: Overcoming the Technological and Process Complexities of Establishing the EU Digital COVID Certificate in Slovenia. International Journal of Environmental Research and Public Health, 19(21), 14322. https://doi.org/10.3390/ijerph192114322







