Abstract
While dietary polyphenols supplements can improve endothelial function and blood flow to exercise, the effects of chronic supplementation with grape seed extract (GSE) containing a high dose of polyphenols on endurance performance are not known. Accordingly, in 12 elite athletes, we compared the effects of both GSE and placebo (PL) on submaximal VO2, time to exhaustion performance, and endothelial function during progressive cycling exercise for 14 days. Endothelial function was evaluated from the brachial artery via flow-mediated dilation (FMD). Compared to PL, GSE decreased submaximal VO2 at 80% and 120% of VO2peak and increased the time to exhaustion (p < 0.05). GSE also resulted in FMD-induced increase in brachial artery diameter (14.4 ± 5.2% vs. 17.6 ± 4.5%, p = 0.035). We demonstrated that chronic supplementation with GSE improved endurance performance and these effects may partially be due to vasodilation in active skeletal muscle mediated by enhanced endothelial function. Thus, our results suggest that GSE appears to be an ergogenic nutraceutical that can improve exercise performance in elite athletes.
1. Introduction
Dietary supplementation to improve exercise and athletic performance has received widespread attention in sports. These supplements referred to as ergogenic aids have been known to enhance strength or endurance, exercise efficiency, recovery from exercise, and increase exercise tolerance to high-intensity exercise [1,2,3,4,5]. Proper supplementation strategies can help elite athletes endure more intensive training and competition. Accordingly, in the sports field, many athletes increasingly rely on dietary supplements, believing that they can promote recovery between training sessions and enhance competitive performance (e.g., cycling, running, rowing, and triathlon) [6,7].
When endurance athletes participate in a repetitive and high-intensity competition, their performance is limited by various physiological factors such as muscle fatigue, energy efficiency, and maximum oxygen consumption [8]. For the past several years, dietary nitrate (NO3−) supplementation with beetroot juice has become a popular nutraceutical among athletes due to an improvement in blood flow, oxygen efficiency, and exercise tolerance [2,3,9]. The main production of nitrate metabolism is nitric oxide (NO) which has a major role in blood pressure and vascular smooth muscle relaxation. NO contributes to an increase in organ blood flow [10]. NO is endogenously produced by the oxidation of L-arginine through NO synthases [11] and exogenously from dietary nitrate via the NO3−-NO2− -NO pathway [12].
Using nutritional polyphenols supplements is of considerable interest to elite athletes for improving sports performance as well as use in human health. Grapes have been known to be rich in polyphenols which are found mostly in the seeds. Grape seed extract (GSE) primarily accounts for flavanols (flavan-3-ol derivatives) that are present in both monomeric and polymeric forms and are the principal active compounds [13]. These active compounds contain monomers of (+)-catechin, (−)-epicatechin, and their gallic acid esters [13]. GSE acts as an antioxidant and has been known to be effective in counteracting exercise-induced oxidative stress [14] which has been known to induce endothelial dysfunction and limit blood flow to exercising muscles [15,16]
Recently, an extract of grape seed has been developed via hydrolysis of specific enzymes [17] that is highly purified and rich in polyphenolic compounds. It has been demonstrated that this extract lowers blood pressure (BP) in human subjects [17,18]. The mechanism underlying the antihypertensive effects of this extract involves the activation of endothelial nitric oxide synthase (eNOS) and in turn production of nitric oxide NO via the improved function of the endothelium [19,20]. NO is generally known to play an important role in increasing blood flow to active skeletal muscles. However, the potential beneficial effects of this highly purified form of GSE on endurance exercise performance have not been examined.
The sport of basketball demands specific skills that are able to be performed under dynamic conditions. For success in basketball performance, players are required to possess high strength, power, and agility. Accordingly, coaches often take less attention to the contribution of the aerobic capacity to improve sports performance in basketball; however, endurance is related to the successful performance of high-intensity work for a longer period of time before the onset of fatigue [21].
Research related to the knowledge of dietary GSE supplementation is not known in elite athletes. Thus, this study hypothesized that chronic dietary GSE supplementation reduces oxygen levels during submaximal exercise and increases time to exhaustion performance due to an improvement in endothelial function in elite athletes.
2. Methods
2.1. Participants
This study was a randomized double-blind placebo-controlled experimental study that involved 12 division I collegiate basketball players from Kyung Hee University. The Exclusion criteria for this study involved: renal, or cardiovascular disorders, those who consumed antibiotics or supplemental vitamins and minerals, and those who consumed drugs that impact metabolic profiles. Prior to testing, all subjects gave written informed consent. The body composition, including height, weight, body mass index, fat mass, and percent body fat was measured using a body composition analyzer (MC190-EM; Tanita, Tokyo, Japan) (Table 1). All procedures were reviewed and approved by the Kyung Hee University Institutional Review Board (KHU 2014-G21).

Table 1.
Physical characteristics of subjects.
2.2. Exercise Test Protocol
Resting BP was measured while the participant was in a seated position. At least two measurements were obtained, 2 min. apart, using a sphygmomanometer and pressure cuff. To determine the relative exercise intensities for the three workloads used in this study, a maximal exercise test was conducted using a cycle ergometer. Pulmonary gases were measured on a breath-by-breath basis using an Ultima CPX Metabolic Cart (Medgraphic, St. Paul, MN, USA). The incremental exercise protocol started with 2 min. of unloaded baseline cycling followed by increases in the workload of 30 Watts per minute until they could no longer maintain a pedal cadence of 60 rpm. Rating of perceived exertion (RPE) and heart rate were measured continuously and recorded every minute.
All participants then completed two bouts of cycling exercise at constant submaximal workloads corresponding to 50% and 80% of their predetermined VO2peak on the same day. The duration of each workload was 5 min. To avoid muscle fatigue, they completed a supramaximal bout of cycling exercise corresponding to 120% of VO2peak on a different day. During the time-to-exhaustion trial, participants were instructed to cycle for as long as possible at their targeted 60 rpm. The trial is commonly used to assess endurance performance [22]. There was a cessation of exercise when a given workload was not maintained. All participants exercised at the same workloads after PL and GSE supplementation (Figure 1).
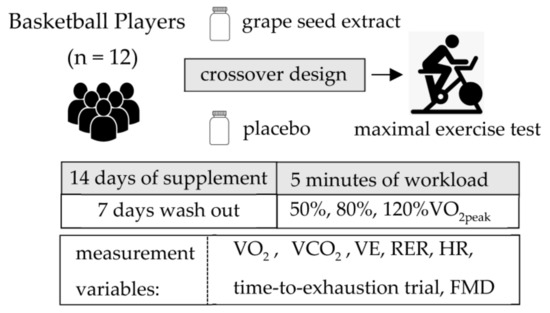
Figure 1.
Study design.
2.3. Supplementation
Following twenty-four hours after the VO2peak exercise test, a randomized double-blind crossover study design was initiated to determine the effects of 14 days of GSE supplementation (administrated as a single dose of 300 mg in capsule form; MegaNaturalbβ®-BP, Polyphenolics Inc., Madera, CA, USA) compared with PL treatment (300 mg of starch). This GSE product is a highly concentrated purified source of polyphenolic flavan-3-ols. The total phenol content determined by the Folin–Ciocalteu method was 95.3% (grams gallic acid equivalent/100 g), contained 2.8% gallic acid, 5.1% catechin plus epicatechin, and 87.4% total oligomers as determined by high-performance liquid chromatography. It was manufactured via hydrolysis using specific proprietary enzymes [17] to produce a product rich in polyphenolic compounds. All participants were instructed to take the GSE around the same time every morning. Neither GSE nor PL supplements were taken for 24 h prior to exercise testing. A one-week washout period separated the supplementation period. Participants were asked to maintain their normal daily activities and food intake during the entire study period. They received a list of vegetables that influenced the production of NO and were asked to record it in a food diary during the duration of the study. When the participants made any major changes, their data were excluded from the data analysis. The investigator administering the exercise tests was blinded to the type of supplementation being consumed by the participants. The main researcher monitored the participants via text message to remind them to follow these guidelines.
2.4. Flow-Mediated Dilation (FMD)
The FMD technique was used to determine endothelial function in the brachial artery in the morning after each supplementation. This non-invasive technique is a well-known bioassay of peripheral endothelial function that involves the release of a temporary occlusion of the arm vasculature to induce an acute increase in shear stress [23]. Brachial artery diameter and velocity were measured by an ultrasound 12-MHz linear-array vascular probe (ClearVue 550, Philips, Cambridge, MA, USA). The probe was placed 3–5 cm proximal to the bifurcation of the antecubital fossa. When the images were obtained, the width of the artery was insonated at an angle of 60°. Blood velocity was acquired simultaneously using a pulsed-wave Doppler. The images were obtained and analyzed by the same examiner in a blinded manner. Ten cardiac cycles were evaluated to calculate baseline arterial diameter. To produce reactive hyperemia, the pressure cuff was placed on the upper arm and inflated by 200 mmHg for 5 min followed by rapid deflation. The brachial artery was imaged and recorded for 2 min. The peak diameter was determined as the average of five cardiac cycles. Brachial artery images were selected when they occurred near the end of diastole. The absolute change in diameter was determined, and FMD was expressed as a percent change in diameter from baseline (%FMD).
2.5. Statistical Analysis
Statistical analyses were performed with SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). All variables assessed by the metabolic cart were averaged over the last 30-s periods during a steady-state submaximal exercise. The peak VO2 was measured during the time to exertion trial. The total time from beginning to exhaustion was measured. To compare the effects of GSE between groups, a paired t-test was used. Statistical significance was accepted at p < 0.05. A power analysis (power = 0.80) revealed the necessity for 12 participants to achieve statistically significant results of VO2 between PL and GSE supplementations.
3. Results
Table 2 shows maximum oxygen consumption after PL and GSE Supplementation. There was no difference in VO2peak between GSE and PL supplementation. Table 3, Table 4 and Table 5 illustrate cardiorespiratory responses during submaximal and supramaximal exercise after PL and GSE supplementation. There was no difference in VO2 at 50% workload between the two groups, but VO2 significantly decreased at 80% and 120% workloads after GSE supplementation compared to PL (p = 0.041 and p = 0.031, respectively). The time to exhaustion trial had a significant increase after GSE (p = 0.035). There were no significant differences in the other variables across workloads. Figure 2 shows flow-mediated dilatation after PL and GSE supplementation. FMD was significantly increased after GSE compared to PL (22%, p = 0.035).

Table 2.
Maximum oxygen consumption after PL and GSE supplementation.

Table 3.
Cardiorespiratory responses during the submaximal exercise of 50% of VO2peak after PL and GSE supplementation.

Table 4.
Cardiorespiratory responses during the submaximal exercise of 80% of VO2peak after PL and GSE supplementation.

Table 5.
Cardiorespiratory responses during the submaximal exercise of 120% of VO2peak after PL and GSE supplementation.
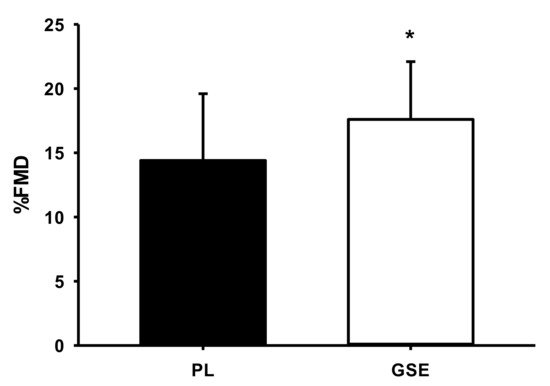
Figure 2.
Flow-mediated dilatation after PL and GSE supplementation. FMD: flow-mediated dilation. Values are expressed as means ± SE. * p < 0.05. vs. PL supplementation.
4. Discussion
To my knowledge, this study was to investigate the effects of chronic supplementation with GSE on VO2, endurance performance, and endothelial function in division 1 collegiate basketball athletes. GSE substantially reduced the O2 cost of cycling at a fixed submaximal work rate and the time to exhaustion trial was significantly increased during supramaximal exercise compared to PL. Furthermore, endothelial function was improved after GSE. Our study demonstrated that an improvement in endurance performance was likely due to an increase in NO bioavailability.
The ergogenic effect of GSE may be mediated by the effect of NO in the improvement of mitochondrial oxygen efficiency. It has been reported that increasing NO levels after the beetroot juice (BRJ) supplementation with NO donors induced benefits in exercise performance because the supplement can decrease the cost of ATP production which enhances mitochondrial ATP production efficiency [2,24]. Although the mechanism utilized by the nitrates found in vegetables such as beetroot, lettuce, and spinach to improve the efficiency of submaximal exercise is still unclear, it has been known to be due to an enhanced oxidative phosphorylation efficiency as assessed by the amount of oxygen consumption per ATP production (P/O ratio) [25]. A previous study demonstrated that the increase in mitochondrial P/O ratio was highly correlated with the reduction in oxygen cost during exercise after nitrate supplementation [25]. Thus, a reduction in submaximal oxygen consumption appears to be a good index of an improvement in mitochondrial efficiency. To demonstrate this hypothesis, Bailey et al. and others investigated the effects of BRJ supplementation in a time trial performance in humans and found a significant improvement compared to PL [3,26,27]. These findings were attributed to a significant increase in the level of nitrite (NO2−) following the supplement. This study suggested that dietary nitrate can be an ergogenic aid that can improve sports performance. Our study also found that GSE supplementation lowered VO2 during 80% and 120% of VO2peak workloads and increased the time to exhaustion performance compared to PL. Thus, exercise can be likely performed at high intensity for a longer period of time before the onset of fatigue with the same energy production following dietary supplementation with GSE.
The endothelium regulates vascular tone, mainly by stimulating NO production in response to various stimuli [28]. NO is a potent vasodilator that mediates the control of vascular function and blood flow during exercise [29,30]. Despite the fact that dietary nitrate supplementation can act as an ergogenic nutraceutical to improve exercise performance [30,31], high intakes of dietary nitrate may cause harmful effects on health (i.e., cancer). In this regard, there should be some regulations on nitrate concentration in food to minimize safety issues. Considering another alternative source, a recent study investigated the effect of acute GSE supplementation on peripheral vasoconstriction and endothelial function in prehypertensive men [19]. It demonstrated that GSE improved endothelial function and increased peripheral vasodilation. Another study showed that dietary nitrate supplementation increased the production of NO and markedly enhanced FMD, indicating that endothelial function was profoundly improved in college students [30], Similarly, this current study found that endurance performance enhancement, following GSE supplementation, was attributed to improved endothelial function (i.e., increased FMD) and oxygen delivery as a result of increased vasodilation in contracting skeletal muscle. These findings suggest that GSE supplements can increase blood flow to skeletal muscles due to an improvement in NO bioavailability and exercise performance before the development of muscle fatigue.
Our study was limited by not measuring the indices of NO bioavailability (e.g., nitrates and nitrite concentrations). Assessment of these substances would have confirmed our conclusions concerning the contribution of NO bioavailability to GSE-induced improvements in endothelial function and endurance performance. Secondly, each of the pre-treatment conditions was not measured to compare to those of post-treatments. Therefore, our results need to be carefully considered with these facts since the effects of PL or GSE supplementations on endothelial function cardiorespiratory responses were not quantified. This is an important distinction because the change from pre-treatment to post-treatment is necessary to reveal physiological effects.
5. Conclusions
In conclusion, although some studies demonstrated that dietary supplementation with NO donors is effective to improve exercise performance, others found no consistent effects. In our study, dietary supplementation with GSE enhanced exercise performance and endothelial function in collegiate basketball players. In consequence, the onset of muscle fatigue may be delayed in athletes performing exercise for longer periods of time. Findings suggest that a small dosage of GSE supplementation can act as an ergogenic aid capable of enhancing O2 delivery and endurance performance. Due to the findings from this study, future research should be examined where dietary GSE supplementation improves endurance performance (i.e., time trial performance).
Author Contributions
H.N.: conception and design; H.N. and K.-A.K.: performed experiments; H.N.: interpreted results of experiments and edited and revised manuscript; H.N. and K.-A.K.: approved final version of manuscripts. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by by the Kyung Hee University Institutional Review Board (KHU 2014-G21).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Lanhers, C.; Pereira, B.; Naughton, G.; Trousselard, M.; Lesage, F.X.; Dutheil, F. Creatine Supplementation and Upper Limb Strength Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar]
- Maughan, R.J.; King, D.S.; Lea, T. Dietary supplements. J. Sports Sci. 2004, 22, 95–113. [Google Scholar] [CrossRef]
- Dominguez, R.; Cuenca, E.; Mate-Munoz, J.L.; Garcia-Fernandez, P.; Serra-Paya, N.; Estevan, M.C.; Herreros, P.V.; Garnacho-Castano, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Richards, J.C.; Racine, M.L.; Hearon, C.M., Jr.; Kunkel, M.; Luckasen, G.J.; Larson, D.G.; Allen, J.D.; Dinenno, F.A. Acute ingestion of dietary nitrate increases muscle blood flow via local vasodilation during handgrip exercise in young adults. Physiol. Rep. 2018, 6, e13572. [Google Scholar] [CrossRef]
- Doshi, S.N.; Naka, K.K.; Payne, N.; Jones, C.J.; Ashton, M.; Lewis, M.J.; Goodfellow, J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: The contribution of nitric oxide. Clin. Sci. (Lond.) 2001, 101, 629–635. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Gokbel, H.; Okudan, N.; Basarali, K. Effects of grape seed extract supplementation on exercise-induced oxidative stress in rats. Br. J. Nutr. 2012, 108, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.K.; Proctor, D.N.; Dietz, N.M.; Joyner, M.J. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J. Physiol. 1995, 488 Pt 1, 259–265. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Shrikhande, A.J.; Wang, H.; Kupina, S.A. Grape Extract, Dietary Supplement Thereof, and Processes Therefor. U.S. Patent B2:8075929, 13 December 2011. [Google Scholar]
- Robinson, M.; Lu, B.; Edirisinghe, I.; Kappagoda, C.T. Effect of grape seed extract on blood pressure in subjects with pre-hypertension. J. Pharm. Nutri. Sci. 2012, 2, 155–159. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, K.A.; Choi, H.M.; Park, S.K.; Stebbins, C.L. Grape Seed Extract Supplementation Attenuates the Blood Pressure Response to Exercise in Prehypertensive Men. J. Med. Food 2018, 21, 445–453. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Fernández-Vallinas, S.; Pons, Z.; Arola, L.; Aleixandre, A.; Muguerza, B. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J. Funct. Foods 2014, 6, 419–427. [Google Scholar] [CrossRef]
- Meckel, Y.; Gottlieb, R.; Eliakim, A. Repeated sprint tests in young basketball players at different game stages. Eur. J. Appl. Physiol. 2009, 107, 273–279. [Google Scholar] [CrossRef]
- Laursen, P.B.; Francis, G.T.; Abbiss, C.R.; Newton, M.J.; Nosaka, K. Reliability of time-to-exhaustion versus time-trial running tests in runners. Med. Sci. Sports Exerc. 2007, 39, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Bach, C.W.; Baur, D.A. Pre-exercise nutrition: The role of macronutrients, modified starches and supplements on metabolism and endurance performance. Nutrients 2014, 6, 1782–1808. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
- Cermak, N.M.; Gibala, M.J.; van Loon, L.J. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rokkedal-Lausch, T.; Franch, J.; Poulsen, M.K.; Thomsen, L.P.; Weitzberg, E.; Kamavuako, E.N.; Karbing, D.S.; Larsen, R.G. Chronic high-dose beetroot juice supplementation improves time trial performance of well-trained cyclists in normoxia and hypoxia. Nitric Oxide 2019, 85, 44–52. [Google Scholar] [CrossRef]
- Murad, F. The 1996 Albert Lasker Medical Research Awards. Signal transduction using nitric oxide and cyclic guanosine monophosphate. JAMA 1996, 276, 1189–1192. [Google Scholar] [CrossRef]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591 Pt 2, 547–557. [Google Scholar] [CrossRef]
- Lee, J.S.; Stebbins, C.L.; Jung, E.; Nho, H.; Kim, J.K.; Chang, M.J.; Choi, H.M. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R459–R466. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).