The Relationship between Health-Related Fitness and Quality of Life in Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.3.1. NAFLD Definition and Classification
2.3.2. Assessment of Quality of Life
2.3.3. Assessment of Health-Related Fitness
2.3.4. Assessments and Definitions of Other Variables
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Characteristics of Health-Related Fitness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| BP | bodily pain |
| CAP | continuous attenuation parameter |
| CRF | cardiorespiratory fitness |
| FBS | fast glucose sugar |
| GH | general health |
| GS | grip strength |
| HC | hip circumference |
| HDL-C | high-density lipoprotein cholesterol |
| HT | health transition |
| LDL-C | low-density lipoprotein cholesterol |
| LSM | liver stiffness measurement |
| MCS | mental component summary score |
| MH | mental health |
| PBF | percent body fat |
| PCS | physical component summary score |
| PF | physical functioning |
| PSM | percent skeletal muscle mass |
| RE | role limitation due to emotional problem |
| RGS | relative grip strength |
| RP | role limitation due to physical problems |
| SF | social functioning |
| SFF | sitting forward flexion |
| SF-36 | 36-item Short Form Health Survey Questionnaire |
| TC | total cholesterol |
| TG | triglyceride |
| VFA | visceral fat area |
| VO2max | maximal oxygen uptake |
| VT | vitality |
| WC | waist circumference |
| WHR | waist-to-hip ratio |
| γ-GT | γ-glutamyl transferase |
References
- Fan, J.G.; Wei, L.; Zhuang, H.; National Workshop on Fatty Liver and Alcoholic Liver Disease; Chinese Society of Hepatology; Chinese Medical Association; Fatty Liver Disease Expert Committee; Chinese Medical Doctor Association; Cai, W.; Feng Chen, D.; et al. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J. Dig. Dis. 2019, 20, 163–173. [Google Scholar] [CrossRef] [PubMed]
- David, K.; Kowdley, K.V.; Unalp, A.; Kanwal, F.; Brunt, E.M.; Schwimmer, J.B.; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: Baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009, 49, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.; Damery, S.; Combes, G. The effectiveness of integrated care interventions in improving patient quality of life (QoL) for patients with chronic conditions. An overview of the systematic review evidence. Health Qual. Life Outcomes 2017, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Afendy, A.; Kallman, J.B.; Stepanova, M.; Younoszai, Z.; Aquino, R.D.; Bianchi, G.; Marchesini, G.; Younossi, Z.M. Predictors of health-related quality of life in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2009, 30, 469–476. [Google Scholar] [CrossRef]
- Kim, H.J.; Chu, H.; Lee, S. Factors influencing on health-related quality of life in South Korean with chronic liver disease. Health Qual. Life Outcomes 2018, 16, 142. [Google Scholar] [CrossRef]
- Huang, R.; Fan, J.G.; Shi, J.P.; Mao, Y.M.; Wang, B.Y.; Zhao, J.M.; Lu, L.G.; Zhong, B.H.; Zou, Z.S.; Xu, Y.Q.; et al. Health-related quality of life in Chinese population with non-alcoholic fatty liver disease: A national multicenter survey. Health Qual. Life Outcomes 2021, 19, 140. [Google Scholar] [CrossRef]
- Golabi, P.; Otgonsuren, M.; Cable, R.; Felix, S.; Koenig, A.; Sayiner, M.; Younossi, Z.M. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual. Life Outcomes 2016, 14, 18. [Google Scholar] [CrossRef]
- Ozawa, N.; Sato, K.; Sugimura, A.; Maki, S.; Tanaka, T.; Yamamoto, K.; Ito, T.; Ishizu, Y.; Kuzuya, T.; Honda, T.; et al. Quality of Life in patients with nonalcoholic fatty liver disease: Structure and related factors focusing on illness uncertainty. Jpn. J. Nurs. Sci. 2021, 18, e12415. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Castro-Pinero, J.; Artero, E.G.; Ortega, F.B.; Sjostrom, M.; Suni, J.; Castillo, M.J. Predictive validity of health-related fitness in youth: A systematic review. Br. J. Sports Med. 2009, 43, 909–923. [Google Scholar] [CrossRef]
- Appelqvist-Schmidlechner, K.; Vaara, J.P.; Vasankari, T.; Hakkinen, A.; Mantysaari, M.; Kyrolainen, H. Muscular and cardiorespiratory fitness are associated with health-related quality of life among young adult men. BMC Public Health 2020, 20, 842. [Google Scholar] [CrossRef]
- Hao, L.; Wang, Z.; Wang, Y.; Wang, J.; Zeng, Z. Association between Cardiorespiratory Fitness, Relative Grip Strength with Non-Alcoholic Fatty Liver Disease. Med. Sci. Monit. 2020, 26, e923015. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Li, L.; Wang, H.M.; Shen, Y. Chinese SF-36 Health Survey: Translation, cultural adaptation, validation, and normalisation. J. Epidemiol. Community Health 2003, 57, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urbistondo, D.; San Cristobal, R.; Villares, P.; Martinez-Gonzalez, M.A.; Babio, N.; Corella, D.; Del Val, J.L.; Ordovas, J.M.; Alonso-Gomez, A.M.; Warnberg, J.; et al. Role of NAFLD on the Health Related QoL Response to Lifestyle in Patients With Metabolic Syndrome: The PREDIMED Plus Cohort. Front. Endocrinol. Lausanne 2022, 13, 868795. [Google Scholar] [CrossRef] [PubMed]
- Alrasheed, M.; Guo, J.J.; Lin, A.C.; Wigle, P.R.; Hardee, A.; Hincapie, A.L. The effect of polypharmacy on quality of life in adult patients with nonalcoholic fatty liver disease in the United States. Qual. Life Res. 2022, 31, 2481–2491. [Google Scholar] [CrossRef]
- Kennedy-Martin, T.; Bae, J.P.; Paczkowski, R.; Freeman, E. Health-related quality of life burden of nonalcoholic steatohepatitis: A robust pragmatic literature review. J. Patient Rep. Outcomes 2017, 2, 28. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- General Administration of Sport of China. Handbook of National Physical Fitness Standards; People’s Sports Publishing House: Beijing, China, 2003. [Google Scholar]
- Cho, J.; Lee, I.; Park, D.H.; Kwak, H.B.; Min, K. Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. Int. J. Environ. Res. Public Health 2021, 18, 1892. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Ofusa, Y.; Golding, L.A. YMCA Fitness Testing and Assessment Manual; Human Kinetics: Champaign, IL, USA, 2000. [Google Scholar]
- Kaminsky, L.A.; Arena, R.; Myers, J. Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data From the Fitness Registry and the Importance of Exercise National Database. Mayo Clin. Proc. 2015, 90, 1515–1523. [Google Scholar] [CrossRef]
- Xue, R.; Li, Q.; Geng, Y.; Wang, H.; Wang, F.; Zhang, S. Abdominal obesity and risk of CVD: A dose-response meta-analysis of thirty-one prospective studies. Br. J. Nutr. 2021, 126, 1420–1430. [Google Scholar] [CrossRef]
- Sharma, M.; Kulkarni, A.; Kumar, P.; Nori, V.B.; Jagtap, N.; Gupta, R.; Reddy, D.N.; Rao, P.N. Difference in lifestyle and metabolic profile of non-alcoholic fatty liver disease with raised alanine amino-transferases between obese and non-overweight subjects. Sci. Rep. 2020, 10, 15232. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; Group, I.D.F.E.T.F.C. The metabolic syndrome--a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Joint Committee for Guideline Revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J. Geriatr. Cardiol. 2018, 15, 1–29. [Google Scholar] [CrossRef]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Krasnoff, J.B.; Painter, P.L.; Wallace, J.P.; Bass, N.M.; Merriman, R.B. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008, 47, 1158–1166. [Google Scholar] [CrossRef]
- Afolabi, P.R.; Scorletti, E.; Calder, P.C.; Byrne, C.D. Factors independently associated with cardiorespiratory fitness in patients with non-alcoholic fatty liver disease. Liver Int. 2020, 40, 2998–3007. [Google Scholar] [CrossRef]
- Shin, H.S. Association between periodontal status and non-alcoholic fatty liver disease in a Korean adult population: A nationwide cross-sectional study. J. Periodontol. 2020, 91, 524–532. [Google Scholar] [CrossRef]
- Pratesi, A.; Tarantini, F.; Di Bari, M. Skeletal muscle: An endocrine organ. Clin. Cases Miner. Bone Metab. 2013, 10, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, S.E.; Lee, Y.B.; Jun, J.E.; Ahn, J.; Bae, J.C.; Jin, S.M.; Hur, K.Y.; Jee, J.H.; Lee, M.K.; et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018, 68, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Gray, S.R.; Forrest, E.; Welsh, P.; Sattar, N.; Celis-Morales, C.; Ho, F.K.; Pell, J.P. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 2022, 76, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Ermolao, A.; van Baak, M.A.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraca, E.V.; Encantado, J.; Dicker, D.; Farpour-Lambert, N.; et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat-A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. S4), e13269. [Google Scholar] [CrossRef]
- Nuzzo, J.L. The Case for Retiring Flexibility as a Major Component of Physical Fitness. Sports Med. 2020, 50, 853–870. [Google Scholar] [CrossRef]
- The World Health Organization. Quality of Life Assessment (WHOQOL): Development and general psychometric properties. Soc. Sci. Med. 1998, 46, 1569–1585. [Google Scholar] [CrossRef]
- Samala, N.; Desai, A.; Vilar-Gomez, E.; Smith, E.R.; Gawrieh, S.; Kettler, C.D.; Pike, F.; Chalasani, N. Decreased Quality of Life Is Significantly Associated With Body Composition in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2980–2988.e2984. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Lawitz, E.J.; Reddy, K.R.; Wai-Sun Wong, V.; Mangia, A.; Muir, A.J.; Jacobson, I.; Djedjos, C.S.; Gaggar, A.; et al. Patients With Nonalcoholic Steatohepatitis Experience Severe Impairment of Health-Related Quality of Life. Am. J. Gastroenterol. 2019, 114, 1636–1641. [Google Scholar] [CrossRef]
- Gerber, L.H.; Weinstein, A.A.; Mehta, R.; Younossi, Z.M. Importance of fatigue and its measurement in chronic liver disease. World J. Gastroenterol. 2019, 25, 3669–3683. [Google Scholar] [CrossRef]

| Variables | All (N = 104) | Gender | Degree of Liver Steatosis | ||
|---|---|---|---|---|---|
| Male (N = 82) | Female (N = 22) | Mild to Moderate (N = 19) | Severe (N = 85) | ||
| Demographic data | |||||
| Age (years) | 37.0 ± 9.7 | 34.5 ± 7.4 | 46.4 ± 11.5 ** | 38.0 ± 11.2 | 36.8 ± 9.4 |
| Height (cm) | 173.4 ± 8.3 | 176.5 ± 6.2 | 162.1 ± 4.5 ** | 172.7 ± 9.2 | 173.6 ± 8.2 |
| Weight (kg) | 86.1 ± 15.8 | 90.4 ± 13.9 | 70.3 ± 11.9 ** | 84.8 ± 16.2 | 86.4 ± 15.8 |
| BMI (kg/m2) | 28.6 ± 3.8 | 29.1 ± 3.6 | 26.7 ± 3.9 ** | 28.2 ± 3.7 | 28.6 ± 3.8 |
| Obesity, n (%) | 88 (84.6%) | 74 (90.2%) | 14 (63.6%) ** | 18 (94.7%) | 70 (82.4%) |
| Central obesity, n (%) | 99 (95.2%) | 79 (96.3%) | 20 (90.9%) | 18 (94.7%) | 81 (95.3%) |
| Comorbidities | |||||
| T2DM, n (%) | 9 (8.7%) | 7 (8.5%) | 2 (9.1%) | 1 (5.30%) | 8 (9.40%) |
| Dyslipidemia, n (%) | 78 (75.0%) | 62 (75.6%) | 16 (72.7%) | 14 (73.70%) | 64 (75.30%) |
| Hypertension, n (%) | 7 (6.7%) | 4 (4.90%) | 3 (13.6%) | 3 (15.80%) | 4 (4.70%) |
| Hyperuricemia, n (%) | 24 (23.1%) | 23 (28.0%) | 1 (4.5%) ** | 3 (15.80%) | 21 (24.70%) |
| Laboratory data | |||||
| ALT (U/L) | 74.5 ± 54.5 | 83.4 ± 56.4 | 41.5 ± 28.9 ** | 54.3 ± 40.4 | 79.1 ± 56.3 |
| AST (U/L) | 43.0 ± 23.8 | 46.1 ± 25.0 | 31.4 ± 13.7 ** | 40.2 ± 26.7 | 43.6 ± 23.2 |
| γ-GT (U/L) | 64.2 ± 65.0 | 64.1 ± 69.8 | 64.3 ± 43.7 | 49.7 ± 42.2 | 67.4 ± 68.8 |
| ALP (U/L) | 81.8 ± 20.4 | 82.3 ± 20.1 | 79.7 ± 22.0 | 79.3 ± 17.4 | 82.3 ± 21.1 |
| FBS (mmol/L) | 5.4 ± 0.7 | 5.4 ± 0.7 | 5.5 ± 0.6 | 5.3 ± 0.4 | 5.5 ± 0.7 |
| TG (mmol/L) | 2.2 ± 2.4 | 2.3 ± 2.7 | 1.6 ± 0.6 | 1.6 ± 0.6 | 2.3 ± 2.6 |
| TC (mmol/L) | 5.3 ± 1.1 | 5.3 ± 1.2 | 5.4 ± 0.9 | 5.3 ± 0.8 | 5.3 ± 1.2 |
| LDL-C (mmol/L) | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.3 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.3 |
| HDL-C (mmol/L) | 3.4 ± 2.1 | 3.4 ± 2.3 | 3.3 ± 0.9 ** | 3.2 ± 0.8 | 3.4 ± 2.3 |
| Uric acid (μmol/L) | 425.8 ± 88.5 | 449.8 ± 81.4 | 336.4 ± 47.6 ** | 431.7 ± 87.2 | 424.4 ± 89.3 |
| CAP (dB/m) | 329.8 ± 37.4 | 331.5 ± 36.9 | 323.7 ± 39.4 | 271.3 ± 16.4 | 342.9 ± 26.5 ## |
| LSM (kPa) | 6.4 ± 2.3 | 6.5 ± 2.4 | 6.0 ± 1.7 | 6.3 ± 2.4 | 6.4 ± 2.3 |
| Health-related fitness | |||||
| PBF (%) | 31.9 ± 5.7 | 30.4 ± 4.9 | 37.5 ± 5.0 ** | 31.3 ± 4.6 | 32.0 ± 5.9 |
| VFA (cm2) | 124.6 ± 39.0 | 122.5 ± 38.9 | 132.2 ± 39.5 | 116.9 ± 27.3 | 126.3 ± 41.1 |
| PSM (%) | 38.2 ± 3.6 | 39.3 ± 2.9 | 34.0 ± 2.8 ** | 38.4 ± 3.1 | 38.1 ± 3.7 |
| WC (cm) | 99.4 ± 10.7 | 101.5 ± 9.5 | 91.2 ± 11.3 ** | 97.7 ± 11.2 | 99.7 ± 10.7 |
| HC (cm) | 105.1 ± 7.1 | 106.2 ± 6.6 | 101.2 ± 7.7 ** | 104.6 ± 7.2 | 105.2 ± 7.2 |
| WHR | 0.9 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.1 ** | 0.9 ± 0.1 | 0.9 ± 0.1 |
| VO2max (mL/kg/min) | 31.8 ± 5.9 | 32.1 ± 6.1 | 30.7 ± 5.1 | 33.3 ± 7.8 | 31.4 ± 5.4 |
| RGS | 1.2 ± 0.5 | 1.3 ± 0.5 | 0.8 ± 0.3 ** | 1.3 ± 0.4 | 1.2 ± 0.5 |
| GS (kg) | 40.6 ± 10.1 | 44.3 ± 7.5 | 26.8 ± 5.4 ** | 39.6 ± 11.1 | 40.8 ± 9.9 |
| SFF (cm) | -2.9 ± 8.7 | -3.7 ± 8.5 | 0.0 ± 9.0 | 0.5 ± 8.4 | −3.7 ± 8.7 |
| Quality of life | |||||
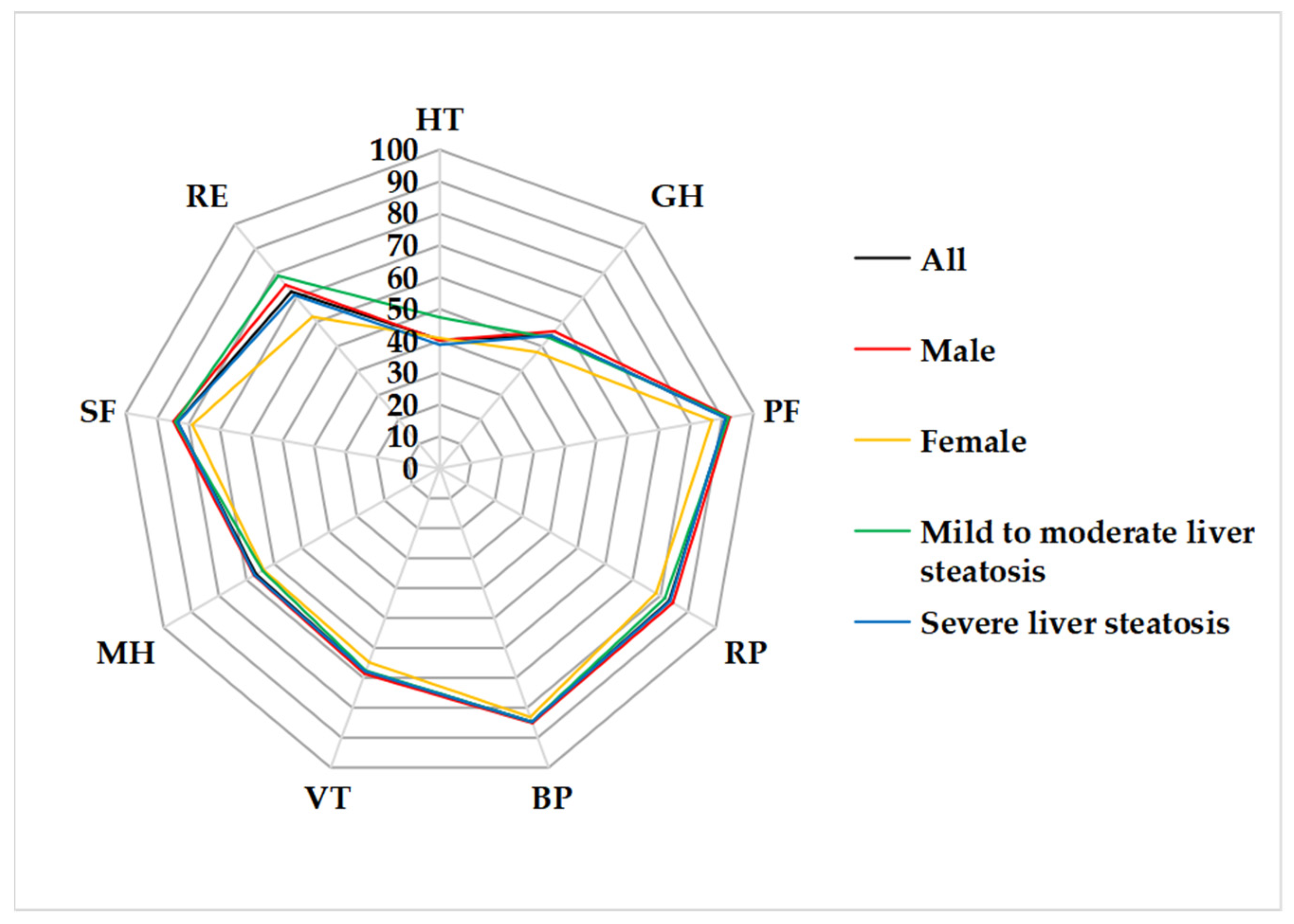
| HT | 40.4 ± 27.3 | 40.2 ± 25.4 | 40.9 ± 34.1 | 47.4 ± 24.9 | 38.8 ± 27.7 |
| PCS | 78.4 ± 12.8 | 79.5 ± 12.8 | 74.0 ± 12.2 | 78.0 ± 11.2 | 78.4 ± 13.2 |
| GH | 54.3 ± 24.6 | 56.1 ± 26.3 | 47.6 ± 14.9 | 53.5 ± 19.8 | 54.4 ± 25.6 |
| PF | 91.3 ± 7.8 | 92.5 ± 7.2 | 86.8 ± 8.2 ** | 92.1 ± 5.8 | 91.1 ± 8.1 |
| RP | 83.2 ± 29.4 | 84.5 ± 28.5 | 78.4 ± 33.0 | 81.6 ± 31.0 | 83.5 ± 29.3 |
| BP | 84.7 ± 16.4 | 85.1 ± 17.2 | 83.1 ± 13.4 | 84.7 ± 16.4 | 85.1 ± 17.2 |
| MCS | 72.6 ± 16.6 | 74.0 ± 15.7 | 67.4 ± 19.1 | 73.8 ± 16.7 | 72.3 ± 16.7 |
| MH | 66.5 ± 15.7 | 67.2 ± 14.7 | 63.8 ± 18.9 | 64.2 ± 20.1 | 67.0 ± 14.6 |
| VT | 67.9 ± 15.9 | 68.7 ± 15.3 | 64.8 ± 17.9 | 67.6 ± 21.4 | 67.9 ± 14.5 |
| SF | 83.5 ± 15.6 | 84.8 ± 15.4 | 78.8 ± 16.0 | 84.2 ± 15.4 | 83.4 ± 15.8 |
| RE | 72.4 ± 36.7 | 75.2 ± 35.1 | 62.1 ± 41.5 | 78.9 ± 33.7 | 71.0 ± 37.4 |
| Variables | Proportion (%) | χ2 | P | ||||
|---|---|---|---|---|---|---|---|
| VO2max | |||||||
| Gender | Poor | Fair | Average | Good | Excellent | 6.456 | 0.168 |
| Male, n (%) | 57 (69.5%) | 18 (22.0%) | 5 (6.1%) | 0 (0.0%) | 2 (2.4%) | ||
| Female, n (%) | 12 (60.0%) | 4 (20.0%) | 3 (15.0%) | 1 (5.0%) | 0 (0.0%) | ||
| All, n (%) | 69 (67.6%) | 22 (21.6%) | 8 (7.8%) | 1 (1.0%) | 2 (2.0%) | ||
| Grip strength | |||||||
| Gender | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | 2.455 | 0.783 |
| Male, n (%) | 16 (19.5%) | 22 (26.8%) | 28 (34.1%) | 9 (11.0%) | 5 (6.1%) | ||
| Female, n (%) | 3 (13.6%) | 8 (36.4%) | 6 (27.3%) | 4 (18.2%) | 1 (4.5%) | ||
| All, n (%) | 19 (18.3%) | 30 (28.8%) | 34 (32.7%) | 13 (12.5%) | 6 (5.8%) | ||
| Sitting forward flexion | |||||||
| Gender | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | 13.015 | 0.011 |
| Male, n (%) | 22 (26.8%) | 23 (28.0%) | 6 (7.3%) | 5 (6.1%) | 0 (0.0%) | ||
| Female, n (%) | 4 (18.2%) | 3 (13.6%) | 8 (36.4%) | 1 (4.5%) | 0 (0.0%) | ||
| All, n (%) | 26 (25.0%) | 26 (25.0%) | 14 (13.5%) | 6 (5.8%) | 0 (0.0%) | ||
| Variables | HT | PCS | GH | PF | RP | BP | MCS | MH | VT | SF | RE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | −0.193 * | 0.000 | −0.187 | −0.102 | 0.211 * | −0.051 | 0.009 | 0.035 | −0.054 | −0.048 | 0.045 |
| PBF | −0.199 * | −0.197 * | −0.337 ** | −0.380 ** | 0.054 | −0.029 | −0.079 | −0.015 | −0.190 | −0.083 | −0.019 |
| VFA | −0.245 * | −0.109 | −0.313 ** | −0.298 ** | 0.149 | 0.004 | −0.046 | −0.014 | −0.200 * | −0.088 | 0.046 |
| PSM | 0.171 | 0.215 * | 0.315 ** | 0.392 ** | −0.016 | 0.043 | 0.100 | 0.033 | 0.192 | 0.095 | 0.044 |
| WC | −0.278 ** | −0.032 | −0.199 * | −0.111 | 0.146 | −0.013 | −0.013 | 0.022 | −0.140 | −0.053 | 0.051 |
| HC | −0.202 * | 0.057 | −0.184 | −0.079 | 0.230 * | 0.078 | 0.050 | −0.005 | −0.134 | −0.014 | 0.157 |
| WHR | −0.274 ** | −0.113 | −0.144 | −0.093 | 0.006 | −0.102 | −0.055 | 0.050 | −0.084 | −0.053 | −0.063 |
| VO2max | 0.119 | 0.134 | 0.242 * | 0.318 ** | −0.006 | −0.083 | 0.013 | −0.002 | 0.112 | 0.099 | −0.066 |
| RGS | 0.035 | 0.126 | 0.032 | 0.050 | 0.105 | 0.133 | 0.173 | 0.085 | 0.145 | 0.122 | 0.162 |
| SFF | 0.034 | 0.003 | 0.070 | 0.044 | −0.074 | 0.014 | 0.131 | 0.093 | 0.198 * | 0.017 | 0.105 |
| GS | −0.132 | 0.141 | 0.042 | 0.150 | 0.147 | 0.042 | 0.115 | 0.059 | 0.065 | 0.111 | 0.107 |
| Dependent Variable | Unstandardized Coefficients | Standardized Coefficients | 95% CI | P | |||
|---|---|---|---|---|---|---|---|
| B | Standard Error | β | Lower | Upper | |||
| HT | Intercept | 110.498 | 24.161 | 62.575 | 158.422 | 0.004 | |
| WC | −0.706 | 0.242 | −0.278 | −1.185 | −0.226 | ||
| GH | Intercept | 100.442 | 12.977 | 74.703 | 126.181 | 0.004 | |
| PBF | −1.449 | 0.401 | −0.337 | −2.243 | −0.654 | ||
| PF | Intercept | 56.104 | 7.493 | 33.438 | 68.821 | <0.001 | |
| PSM | 0.690 | 0.204 | 0.321 | 0.286 | 1.094 | ||
| VO2max | 0.278 | 0.125 | 0.211 | 0.030 | 0.526 | ||
| RP | Intercept | −16.492 | 41.884 | −99.568 | 66.584 | 0.019 | |
| HC | 0.948 | 0.398 | 0.230 | 0.160 | 1.737 | ||
| PCS | Intercept | 49.247 | 13.142 | 23.180 | 75.314 | 0.028 | |
| PSM | 0.762 | 0.343 | 0.215 | 0.083 | 1.441 | ||
| VT | Intercept | 78.018 | 5.149 | 67.805 | 88.232 | 0.003 | |
| VFA | −0.081 | 0.039 | −0.200 | −0.160 | −0.003 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, J.; Liu, Y.; Zhou, H.; Yan, W.; Ren, H. The Relationship between Health-Related Fitness and Quality of Life in Nonalcoholic Fatty Liver Disease. Int. J. Environ. Res. Public Health 2022, 19, 14215. https://doi.org/10.3390/ijerph192114215
Wang L, Zhang J, Liu Y, Zhou H, Yan W, Ren H. The Relationship between Health-Related Fitness and Quality of Life in Nonalcoholic Fatty Liver Disease. International Journal of Environmental Research and Public Health. 2022; 19(21):14215. https://doi.org/10.3390/ijerph192114215
Chicago/Turabian StyleWang, Lina, Jing Zhang, Yali Liu, Huixuan Zhou, Wenjing Yan, and Hong Ren. 2022. "The Relationship between Health-Related Fitness and Quality of Life in Nonalcoholic Fatty Liver Disease" International Journal of Environmental Research and Public Health 19, no. 21: 14215. https://doi.org/10.3390/ijerph192114215
APA StyleWang, L., Zhang, J., Liu, Y., Zhou, H., Yan, W., & Ren, H. (2022). The Relationship between Health-Related Fitness and Quality of Life in Nonalcoholic Fatty Liver Disease. International Journal of Environmental Research and Public Health, 19(21), 14215. https://doi.org/10.3390/ijerph192114215






