Ten-Year Trend in Emergency Department Visits for Sexually Transmitted Infections among Adolescents: A Retrospective Cross-Sectional Study in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Participants
2.2. Ethics
2.3. Statistical Analyses
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Sexually Transmitted Infections (STIs). Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 3 October 2022).
- WHO. Consolidated Guidelines on HIV, Viral Hepatitis and STI Prevention, Diagnosis, Treatment and Care for Key Populations; WHO: Geneva, Switzerland, 2022; p. 144. [Google Scholar]
- European Centre for Disease Prevention and Control. Syphilis—Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control. Chlamydia Infection—Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control. Lymphogranuloma Venereum—Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control. Gonorrhoea—Annual Epidemiological Report for 2018. In Annual Epidemiological Report for 2018; Surveillance Report; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Miller, M.K.; Dowd, M.D.; Harrison, C.J.; Mollen, C.J.; Selvarangan, R.; Humiston, S.G. Prevalence of 3 Sexually Transmitted Infections in a Pediatric Emergency Department. Pediatr. Emerg. Care 2015, 31, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.S.; Gaydos, C.A.; Witter, F. Trichomonas Vaginalis Vaginitis in Obstetrics and Gynecology Practice: New Concepts and Controversies. Obstet. Gynecol. Surv. 2013, 68, 43–50. [Google Scholar] [CrossRef]
- De Carvalho, N.S.; Palú, G.; Witkin, S.S. Mycoplasma Genitalium, a Stealth Female Reproductive Tract. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Hafner, L.M. Pathogenesis of Fallopian Tube Damage Caused by Chlamydia Trachomatis Infections. Contraception 2015, 92, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Tamarelle, J.; Thiébaut, A.C.M.; de Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The Vaginal Microbiota and Its Association with Human Papillomavirus, Chlamydia Trachomatis, Neisseria Gonorrhoeae and Mycoplasma Genitalium Infections: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2019, 25, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually Transmitted Diseases and Infertility. Am. J. Obstet. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef]
- Davies, B.; Turner, K.M.E.; Frølund, M.; Ward, H.; May, M.T.; Rasmussen, S.; Benfield, T.; Westh, H. Risk of Reproductive Complications Following Chlamydia Testing: A Population-Based Retrospective Cohort Study in Denmark. Lancet Infect. Dis. 2016, 16, 1057–1064. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Notiziario Dell’ISS (Volume 35, n. 6, 2022)—Le Infezioni Sessualmente Trasmesse: Aggiornamento Dei Dati Dei Due Sistemi Di Sorveglianza Sentinella Attivi in Italia al 31 Dicembre 2020. Available online: https://www.epicentro.iss.it/ist/NotiziarioIssCoa (accessed on 4 August 2022).
- CDC. Women Who Have Sex with Women (WSW) and Women Who Have Sex with Women and Men (WSWM). Available online: https://www.cdc.gov/std/treatment-guidelines/wsw.htm (accessed on 8 August 2022).
- Francis, S.C.; Parajuli, A.; Mardh, O.; Falconer, J.; Andreasen, A.; Harding-Esch, E.; on behalf of NASSTI. Technologies, Strategies and Approaches for Testing Populations at Risk of Sexually Transmitted Infections: A Systematic Review Protocol to Inform Prevention and Control in EU/EEA Countries. Syst. Rev. 2020, 9, 64. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030; WHO: Geneva, Switzerland, 2022; p. 134. [Google Scholar]
- Bergquist, E.P.; Trolard, A.; Zhao, Y.; Kuhlmann, A.S.; Loux, T.; Liang, S.Y.; Stoner, B.P.; Reno, H. Single and Repeated Use of the Emergency Department for Chlamydia and Gonorrhea Care. Sex. Transm. Dis. 2020, 47, 14–18. [Google Scholar] [CrossRef]
- Ware, C.E.; Ajabnoor, Y.; Mullins, P.M.; Mazer-Amirshahi, M.; Pines, J.M.; May, L. A Retrospective Cross-Sectional Study of Patients Treated in US EDs and Ambulatory Care Clinics with Sexually Transmitted Infections from 2001 to 2010. Am. J. Emerg. Med. 2016, 34, 1808–1811. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, E.P.; Trolard, A.; Fox, B.; Sebert Kuhlmann, A.; Loux, T.; Liang, S.Y.; Stoner, B.P.; Reno, H. Presenting to the Emergency Department Versus Clinic-Based Sexually Transmitted Disease Care Locations for Testing for Chlamydia and Gonorrhea: A Spatial Exploration. Sex. Transm. Dis. 2019, 46, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Felsen, U.R.; Torian, L.V.; Futterman, D.C.; Stafford, S.; Xia, Q.; Allan, D.; Esses, D.; Cunningham, C.O.; Weiss, J.M.; Zingman, B.S. An Expanded HIV Screening Strategy in the Emergency Department Fails to Identify Most Patients with Undiagnosed Infection: Insights from a Blinded Serosurvey. AIDS Care 2020, 32, 202–208. [Google Scholar] [CrossRef]
- Klein, P.W.; Martin, I.B.K.; Quinlivan, E.B.; Gay, C.L.; Leone, P.A. Missed Opportunities for Concurrent HIV-STD Testing in an Academic Emergency Department. Public Health Rep. 2014, 129 (Suppl. 1), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.; Chase, A.; Badolato, G.M.; Teach, S.J.; Trent, M.; Chamberlain, J.; Goyal, M. Adolescent Sexual Behavior and Emergency Department Use. Pediatr. Emerg. Care 2020, 36. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Hayes, K.; Mollen, C. Sexually Transmitted Infection Prevalence in Symptomatic Adolescent Emergency Department Patients. Pediatr. Emerg. Care 2012, 28, 1277–1280. [Google Scholar] [CrossRef]
- Breslin, K.; Tuchman, L.; Hayes, K.L.; Badolato, G.; Goyal, M.K. Sensitivity and Specificity of Empiric Treatment for Sexually Transmitted Infections in a Pediatric Emergency Department. J. Pediatr. 2017, 189, 48–53. [Google Scholar] [CrossRef]
- Sheele, J.M.; Niforatos, J.D.; Elkins, J.M.; Campos, S.C.; Thompson, C.L. Prediction Model for Gonorrhea, Chlamydia, and Trichomoniasis in the Emergency Department. Am. J. Emerg. Med. 2022, 51, 313–319. [Google Scholar] [CrossRef]
- Territo, H.M.; Wrotniak, B.H.; Verni, C.; Burstein, G.R. Trichomonas Infection Rates in Males Presenting to the Emergency Department for Sexually Transmitted Infections. J. Emerg. Med. 2022, 62, 1–8. [Google Scholar] [CrossRef]
- Baldo, V.; Cocchio, S.; Buja, A.; Baldovin, T.; Furlan, P.; Bertoncello, C.; Saia, M. Hospitalization for Diseases Attributable to Human Papillomavirus in the Veneto Region (North-East Italy). BMC Infect. Dis. 2013, 13, 462. [Google Scholar] [CrossRef][Green Version]
- Cocchio, S.; Prandi, G.M.; Furlan, P.; Bertoncello, C.; Fonzo, M.; Saia, M.; Baldovin, T.; Baldo, V. Time-Trend of Hospitalizations for Anogenital Warts in Veneto Region in the HPV Vaccination Era: A Cross Sectional Study (2007–2018). BMC Infect. Dis. 2020, 20, 857. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.D.; Zahnd, W.; Kovach, R.; Kissinger, P. Chlamydia and Gonorrhea Screening in United States Emergency Departments. J. Emerg. Med. 2013, 44, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Carella, M.; García Pereiro, T.; Pace, R.; Paterno, A. The “Dating Game”: Age Differences at First Sex of College Students in Italy. Genus 2020, 76, 23. [Google Scholar] [CrossRef]
- Minello, A.; Caltabiano, M.; Dalla-Zuanna, G.; Vignoli, D. Catching up! The Sexual Behaviour and Opinions of Italian Students (2000–2017). Genus 2020, 76, 16. [Google Scholar] [CrossRef]
- Smorti, M.; Milone, A.; Gonzalez Gonzalez, J.; Vitali Rosati, G. Adolescent Selfie: An Italian Society of Paediatrics Survey of the Lifestyle of Teenagers. Ital. J. Pediatr. 2019, 45, 62. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, L.; Windlin, B.; Reis, M.; Gabhainn, S.N.; Jovic, S.; Matos, M.G.; Magnusson, J.; Godeau, E. Gendered Trends in Early and Very Early Sex and Condom Use in 20 European Countries from 2002 to 2010. Eur. J. Public Health 2015, 25 (Suppl. 2), 65–68. [Google Scholar] [CrossRef]
- United Nations Educational, Scientific and Cultural Organization (UNESCO); Joint United Nations Programme on HIV/AIDS (UN-AIDS); United Nations Entity for Gender Equality and the Empowerment of Women (UN Women); United Nations Children’s Fund (UNICEF); United Nations Population Fund (UNFPA); World Health Organization (WHO). International Technical Guidance on Sexuality Education 2018; UNESCO: Paris, France, 2018. [Google Scholar]
- Salfa, M.C.; Chinelli, A.; Cellini, A.; Ubbiali, M.; Ceccarelli, L.; Farinella, M.; Rancilio, L.; Caraglia, A.; Palamara, A.T.; Mortari, L.; et al. Infezioni sessualmente trasmesse e salute sessuale: Indroduzione di attività educative integrate nel contesto scolastico italiano. Not Ist Super Sanità 2021, 34, 13–17. [Google Scholar]
- Di Xia, F.; Fuhlbrigge, M.; Dommasch, E.; Joyce, C.; Mostaghimi, A. Cost of Routine Herpes Simplex Virus Infection Visits to U.S. Emergency Departments 2006–2013. West. J. Emerg. Med. 2018, 19, 689–692. [Google Scholar] [CrossRef]
- Durukan, D.; Fairley, C.K.; Bradshaw, C.S.; Read, T.R.H.; Druce, J.; Catton, M.; Caly, L.; Chow, E.P.F. Increasing Proportion of Herpes Simplex Virus Type 1 among Women and Men Diagnosed with First-Episode Anogenital Herpes: A Retrospective Observational Study over 14 Years in Melbourne, Australia. Sex. Transm. Infect. 2019, 95, 307–313. [Google Scholar] [CrossRef]
- Clark, M.; Jembere, N.; Kupets, R. The Impact of a Universal Human Papilloma Virus (HPV) Vaccination Program on Lower Genital Tract Dysplasia and Genital Warts. Prev. Med. 2021, 150, 106641. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Boily, M.-C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.L.; Cummings, T.; et al. Population-Level Impact and Herd Effects Following Human Papillomavirus Vaccination Programmes: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef]
- Herweijer, E.; Ploner, A.; Sparén, P. Substantially Reduced Incidence of Genital Warts in Women and Men Six Years after HPV Vaccine Availability in Sweden. Vaccine 2018, 36, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Goldman, D.P.; Seabury, S.A. Incidence of Sexually Transmitted Infections After Human Papillomavirus Vaccination Among Adolescent Females. JAMA Intern. Med. 2015, 175, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Lukács, A.; Máté, Z.; Farkas, N.; Mikó, A.; Tenk, J.; Hegyi, P.; Németh, B.; Czumbel, L.M.; Wuttapon, S.; Kiss, I.; et al. The Quadrivalent HPV Vaccine Is Protective against Genital Warts: A Meta-Analysis. BMC Public Health 2020, 20, 691. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, P.; Tanton, C.; Mesher, D.; King, E.; Beddows, S.; Field, N.; Mercer, C.H.; Soldan, K.; Johnson, A.M. Epidemiology of Genital Warts in the British Population: Implications for HPV Vaccination Programmes. Sex. Transm. Infect. 2019, 95, 386–390. [Google Scholar] [CrossRef]
- Ministero della Salute. Vaccinazione contro il papilloma virus (HPV)—Coperture vaccinali 2015–2020. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=27 (accessed on 11 October 2021).
- European Centre for Disease Prevention and Control. The European Surveillance System (TESSy). Available online: https://www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy (accessed on 25 July 2022).
- Tsuboi, M.; Evans, J.; Davies, E.P.; Rowley, J.; Korenromp, E.L.; Clayton, T.; Taylor, M.M.; Mabey, D.; Chico, R.M. Prevalence of Syphilis among Men Who Have Sex with Men: A Global Systematic Review and Meta-Analysis from 2000–20. Lancet Glob. Health 2021, 9, e1110–e1118. [Google Scholar] [CrossRef]
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; Geneva, Switzerland, 2021; ISBN 978-92-4-002707-7. [Google Scholar]
- Goyal, M.K.; Hayes, K.L.; Mollen, C.J. Racial Disparities in Testing for Sexually Transmitted Infections in the Emergency Department. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2012, 19, 604–607. [Google Scholar] [CrossRef]
- Timm, N.; Bouvay, K.; Scheid, B.; Defoor, W.R. Evaluation and Management of Sexually Transmitted Infections in Adolescent Males Presenting to a Pediatric Emergency Department: Is the Chief Complaint Diagnostic? Pediatr. Emerg. Care 2011, 27, 1042–1044. [Google Scholar] [CrossRef]
- Dixon, B.E.; Rahurkar, S.; Ho, Y.; Arno, J.N. Reliability of Administrative Data to Identify Sexually Transmitted Infections for Population Health: A Systematic Review. BMJ Health Care Inform. 2019, 26. [Google Scholar] [CrossRef]
- Ho, Y.A.; Rahurkar, S.; Tao, G.; Patel, C.G.; Arno, J.N.; Wang, J.; Broyles, A.A.; Dixon, B.E. Validation of International Classification of Diseases, Tenth Revision, Clinical Modification Codes for Identifying Cases of Chlamydia and Gonorrhea. Sex. Transm. Dis. 2021, 48, 335–340. [Google Scholar] [CrossRef]
- Smith, M.; Lix, L.M.; Azimaee, M.; Enns, J.E.; Orr, J.; Hong, S.; Roos, L.L. Assessing the Quality of Administrative Data for Research: A Framework from the Manitoba Centre for Health Policy. J. Am. Med. Inform. Assoc. JAMIA 2018, 25, 224–229. [Google Scholar] [CrossRef] [PubMed]
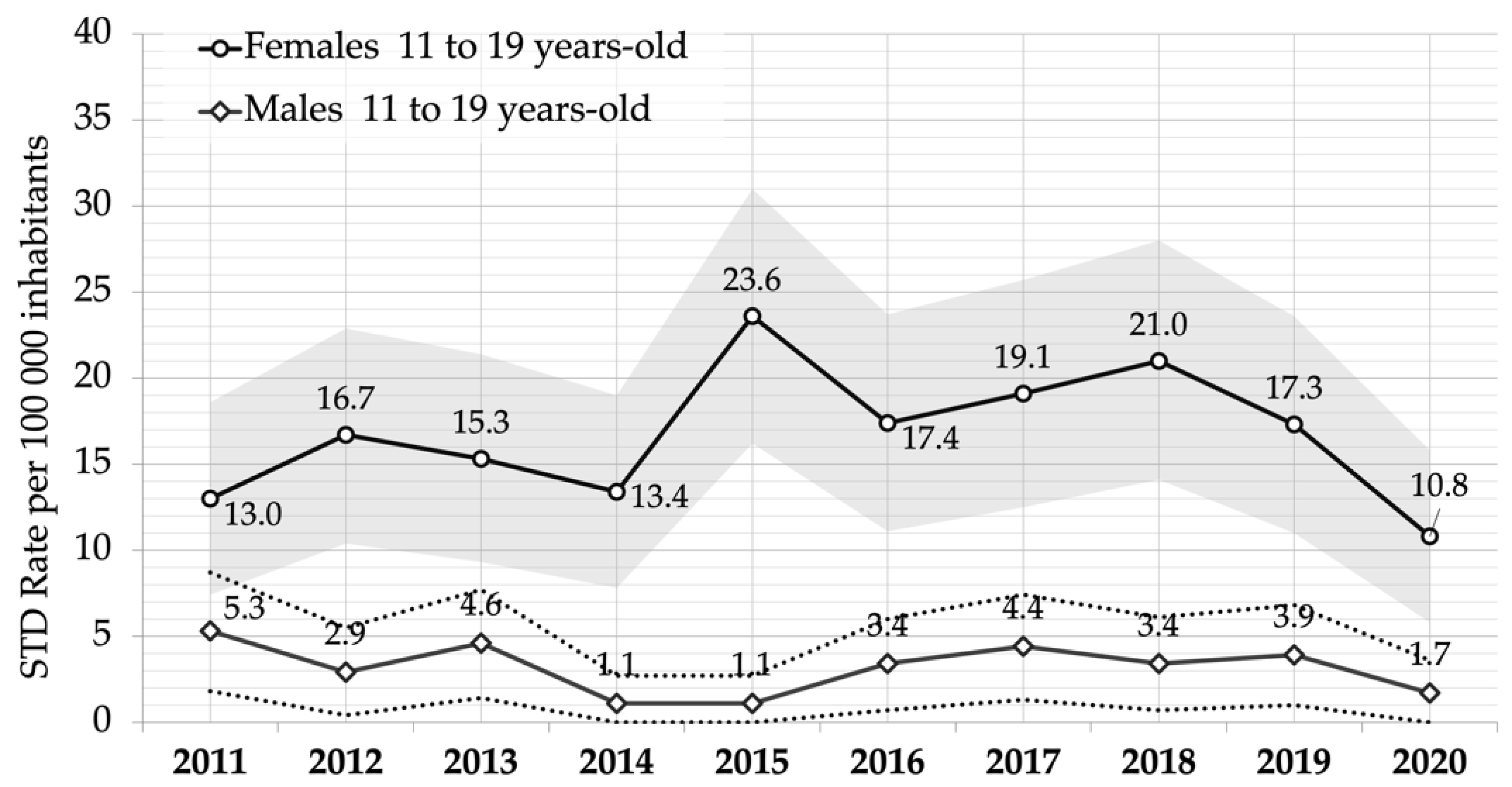
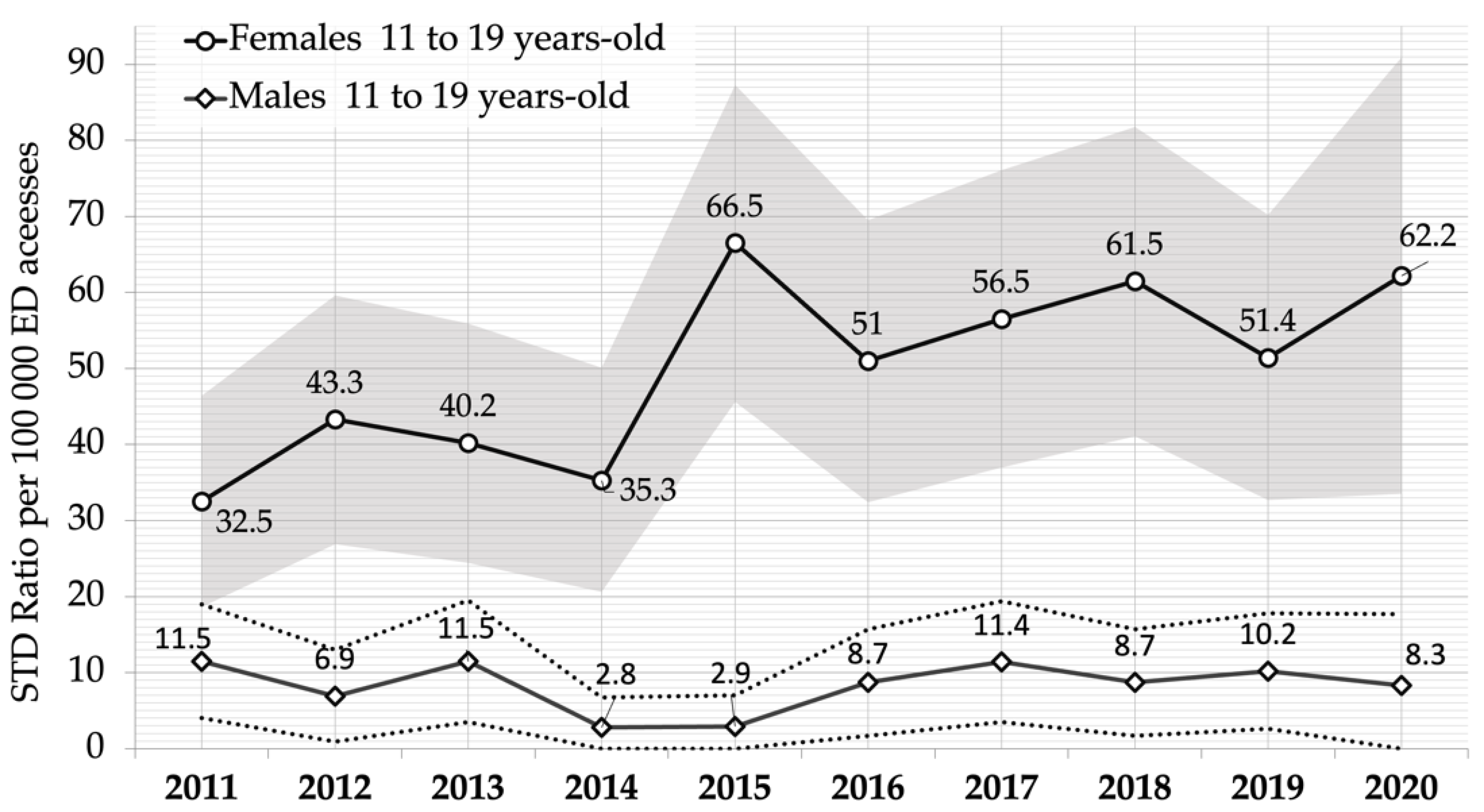
| Gender | Year | 11 to 13-Year-Olds | 14 to 16-Year-Olds | 17 to 19-Year-Olds | |||
|---|---|---|---|---|---|---|---|
| STI Visits | Rate (95% CI) | STI Visits | Rate (95% CI) | STI Visits | Rate (95% CI) | ||
| Females | 2011 | 1 | 1.83 (0.3–10.4) | 6 | 11.29 (5.2–24.6) | 14 | 25.99 (15.5–43.6) |
| 2012 | - | - | 9 | 16.70 (8.8–31.7) | 18 | 33.62 (21.3–53.1) | |
| 2013 | 3 | 5.37 (1.8–15.8) | 2 | 3.64 (1.0–13.3) | 20 | 36.99 (23.9–57.1) | |
| 2014 | 1 | 1.80 (0.3–10.2) | 4 | 7.22 (2.8–18.6) | 17 | 31.27 (19.5–50.1) | |
| 2015 | 1 | 1.79 (0.3–10.2) | 6 | 10.82 (5.0–23.6) | 32 | 58.29 (41.3–82.3) | |
| 2016 | 1 | 1.80 (0.3–10.2) | 9 | 16.12 (8.5–30.6) | 25 | 45.25 (30.7–66.8) | |
| 2017 | 3 | 5.37 (1.8–15.8) | 7 | 12.61 (6.1–26.0) | 22 | 39.44 (26.1–59.7) | |
| 2018 | 2 | 3.57 (1.0–13.0) | 7 | 12.67 (6.1–26.2) | 26 | 46.88 (32.0–68.7) | |
| 2019 | 1 | 1.78 (0.3–10.1) | 4 | 7.23 (2.8–18.6) | 24 | 42.98 (28.9–64.0) | |
| 2020 | 1 | 1.77 (0.3–10.0) | 5 | 9.01 (3.9–21.1) | 12 | 21.64 (12.4–37.8) | |
| Males | 2011 | - | - | - | - | 9 | 15.82 (8.3–30.1) |
| 2012 | - | - | - | - | 5 | 8.83 (3.8–20.7) | |
| 2013 | 2 | 3.40 (0.9–12.4) | 3 | 5.14 (1.8–15.1) | 3 | 5.20 (1.8–15.3) | |
| 2014 | - | - | 1 | 1.68 (0.3–9.5) | 1 | 1.72 (0.3–9.8) | |
| 2015 | - | - | - | - | 2 | 3.39 (0.9–12.4) | |
| 2016 | 1 | 1.68 (0.3–9.5) | 2 | 3.39 (0.9–12.4) | 3 | 5.01 (1.7–14.7) | |
| 2017 | - | - | 3 | 5.12 (1.7–15.0) | 5 | 8.10 (3.5–19.0) | |
| 2018 | 2 | 3.37 (0.9–12.3) | 3 | 5.11 (1.7–15.0) | 1 | 1.66 (0.3–9.4) | |
| 2019 | 2 | 3.34 (0.9–12.2) | 2 | 3.38 (0.9–12.3) | 3 | 5.04 (1.7–14.8) | |
| 2020 | 1 | 1.67 (0.3–9.5) | 2 | 3.38 (0.9–12.3) | - | - | |
| Gender | Year | 11 to 13-Year-Olds | 14 to 16-Year-Olds | 17 to 19-Year-Olds | |||
|---|---|---|---|---|---|---|---|
| ED Visits | Ratio (95% CI) | ED Visits | Ratio (95% CI) | ED Visits | Ratio (95% CI) | ||
| Females | 2011 | 18,326 | 5.46 (1.0–30.9) | 18,915 | 31.72 (14.5–69.2) | 24,421 | 57.33 (34.2–96.2) |
| 2012 | 18,360 | - | 18,790 | 47.90 (25.2–91.0) | 23,099 | 77.93 (49.3–123.2) | |
| 2013 | 18,644 | 16.09 (5.5–47.3) | 18,945 | 10.56 (2.9–38.5) | 22,814 | 87.67 (56.8–135.4) | |
| 2014 | 19,018 | 5.26 (0.9–29.8) | 19,551 | 20.46 (8.0–52.6) | 22,595 | 75.24 (47.0–120.5) | |
| 2015 | 17,963 | 5.57 (1.0–31.5) | 18,971 | 31.63 (14.5–69.0) | 21,126 | 151.47 (107.3–213.8) | |
| 2016 | 17,486 | 5.72 (1.0–32.4) | 18,040 | 49.89 (26.3–94.8) | 20,557 | 121.61 (82.4–179.5) | |
| 2017 | 17,600 | 17.05 (5.8–50.1) | 18,388 | 38.07 (18.4–78.6) | 20,355 | 108.08 (71.4–163.6) | |
| 2018 | 18,087 | 11.06 (3.0–40.3) | 17,850 | 39.22 (19.0–80.9) | 20,614 | 126.13 (86.1–184.8) | |
| 2019 | 18,184 | 5.50 (1.0–31.2) | 17,854 | 22.40 (8.7–57.6) | 20,337 | 118.01 (79.3–175.5) | |
| 2020 | 8637 | 11.58 (2.0–65.6) | 9057 | 55.21 (23.6–129.2) | 10,569 | 113.54 (65.0–198.4) | |
| Males | 2011 | 26,447 | - | 22,434 | - | 24,738 | 36.38 (19.1–69.1) |
| 2012 | 26,031 | - | 21,637 | - | 22,741 | 21.99 (9.4–51.5) | |
| 2013 | 25,908 | 7.72 (2.1–28.2) | 21,690 | 13.83 (4.7–40.7) | 21,775 | 13.78 (4.7–40.5) | |
| 2014 | 26,519 | - | 22,275 | 4.49 (0.8–25.4) | 21,903 | 4.57 (0.8–25.9) | |
| 2015 | 25,134 | - | 21,314 | - | 21,433 | 9.33 (2.6–34.0) | |
| 2016 | 25,332 | 3.95 (0.7–22.4) | 21,388 | 9.35 (2.6–34.1) | 21,737 | 13.80 (4.7–40.6) | |
| 2017 | 25,907 | - | 21,487 | 13.96 (4.8–41.1) | 22,118 | 22.61 (9.7–52.9) | |
| 2018 | 25,858 | 7.73 (2.1–28.2) | 21,042 | 14.26 (4.9–41.9) | 21,927 | 4.56 (0.8–25.8) | |
| 2019 | 25,787 | 7.76 (2.1–28.3) | 21,201 | 9.43 (2.6–34.4) | 21,650 | 13.86 (4.7–40.7) | |
| 2020 | 12,869 | 7.77 (1.4–44.0) | 11,488 | 17.41 (4.8–63.5) | 12,152 | - | |
| STI/Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | N (Ratio) | ||
| Females | Chlamydia | - | - | - | - | - | - | - | - | - | - |
| Genital Herpes | 17 (27.57) | 20 (33.2) | 15 (24.83) | 20 (32.7) | 28 (48.23) | 28 (49.93) | 28 (49.7) | 30 (53.05) | 28 (49.67) | 18 (63.69) | |
| Genital Warts | 3 (4.87) | 6 (9.96) | 8 (13.24) | 1 (1.63) | 8 (13.78) | 4 (7.13) | 1 (1.77) | 4 (7.07) | 1 (1.77) | - | |
| Gonorrhea | 1 (1.62) | 1 (1.66) | 1 (1.66) | 1 (1.63) | 1 (1.72) | 1 (1.78) | 2 (3.55) | 1 (1.77) | - | - | |
| Inguinal granuloma | - | - | 1 (1.66) | - | 1 (1.72) | 2 (3.57) | - | - | - | - | |
| HIV | - | - | - | - | 1 (1.72) | - | 1 (1.77) | - | - | - | |
| Ulcer venereal | - | - | - | - | - | - | - | - | - | - | |
| Males | Chlamydia | - | 1 (1.61) | - | - | - | - | - | - | - | - |
| Genital Herpes | 2 (3.14) | 1 (1.61) | 1 (1.60) | 1 (1.58) | 1 (1.66) | 1 (1.72) | 2 (3.43) | 1 (1.71) | 1 (1.71) | - | |
| Genital Warts | 2 (3.14) | 2 (3.21) | 1 (1.60) | 1 (1.58) | - | - | - | 1 (1.71) | 1 (1.71) | 1 (3.3) | |
| Gonorrhea | 2 (3.14) | 1 (1.61) | 5 (8.60) | - | 1 (1.66) | 5 (8.61) | 5 (8.57) | 4 (6.83) | 4 (6.85) | 2 (6.6) | |
| Inguinal granuloma | 2 (3.14) | - | - | - | - | - | 1 (1.71) | - | 1 (1.71) | - | |
| HIV | - | - | - | - | - | - | - | - | - | - | |
| Ulcer venereal | 1 (1.57) | - | 1 (1.60) | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viottini, E.; Albanesi, B.; Casabona, E.; Onorati, R.; Campagna, S.; Borraccino, A. Ten-Year Trend in Emergency Department Visits for Sexually Transmitted Infections among Adolescents: A Retrospective Cross-Sectional Study in Italy. Int. J. Environ. Res. Public Health 2022, 19, 14207. https://doi.org/10.3390/ijerph192114207
Viottini E, Albanesi B, Casabona E, Onorati R, Campagna S, Borraccino A. Ten-Year Trend in Emergency Department Visits for Sexually Transmitted Infections among Adolescents: A Retrospective Cross-Sectional Study in Italy. International Journal of Environmental Research and Public Health. 2022; 19(21):14207. https://doi.org/10.3390/ijerph192114207
Chicago/Turabian StyleViottini, Elena, Beatrice Albanesi, Elena Casabona, Roberta Onorati, Sara Campagna, and Alberto Borraccino. 2022. "Ten-Year Trend in Emergency Department Visits for Sexually Transmitted Infections among Adolescents: A Retrospective Cross-Sectional Study in Italy" International Journal of Environmental Research and Public Health 19, no. 21: 14207. https://doi.org/10.3390/ijerph192114207
APA StyleViottini, E., Albanesi, B., Casabona, E., Onorati, R., Campagna, S., & Borraccino, A. (2022). Ten-Year Trend in Emergency Department Visits for Sexually Transmitted Infections among Adolescents: A Retrospective Cross-Sectional Study in Italy. International Journal of Environmental Research and Public Health, 19(21), 14207. https://doi.org/10.3390/ijerph192114207









