Sex-Based Differences in the Association between Serum Copper and Kidney Function: Evidence from NHANES 2011–2016
Abstract
1. Introduction
2. Materials and Methods
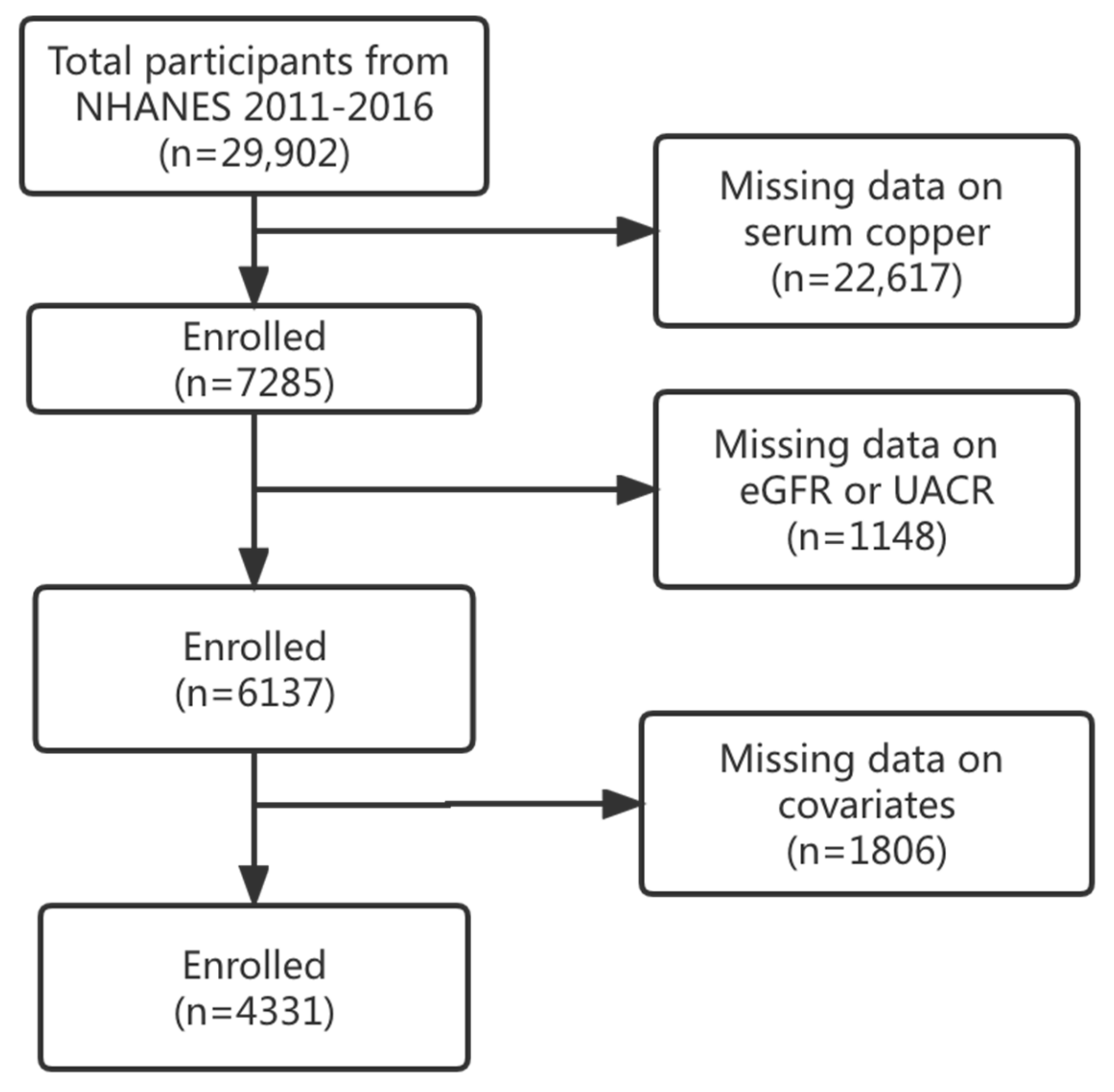
2.1. Study Population
2.2. Measurement of Serum Cu
2.3. Outcome Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Overall and Sex-Specific Associations between Serum Cu and Kidney Function
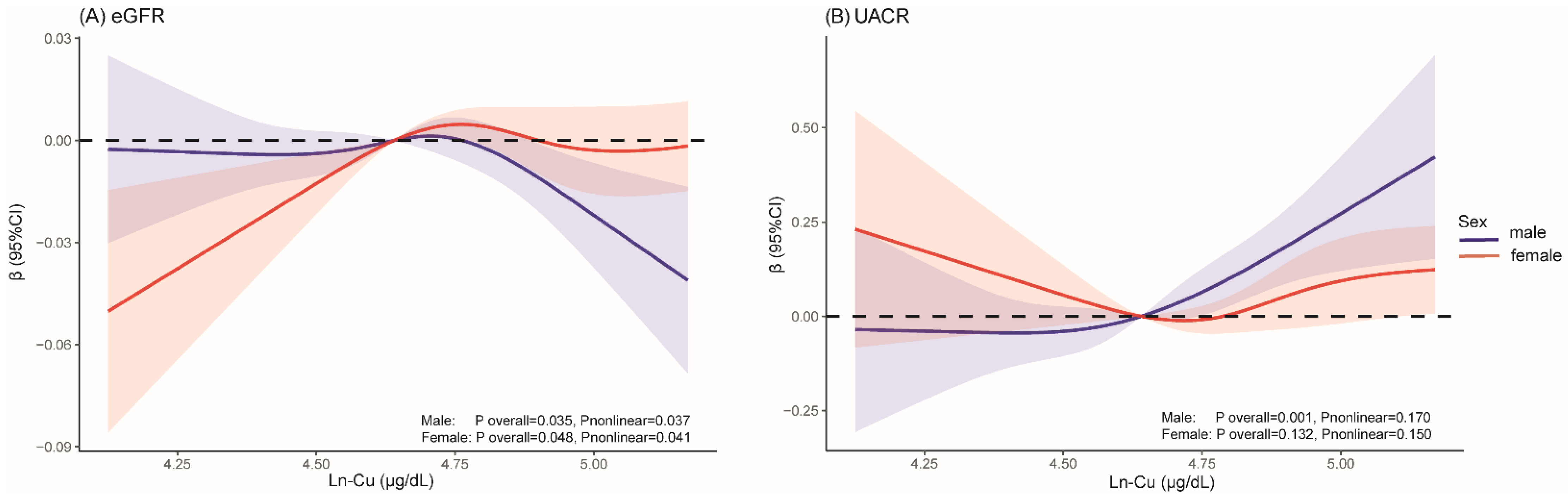
3.3. Dose–Response Associations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
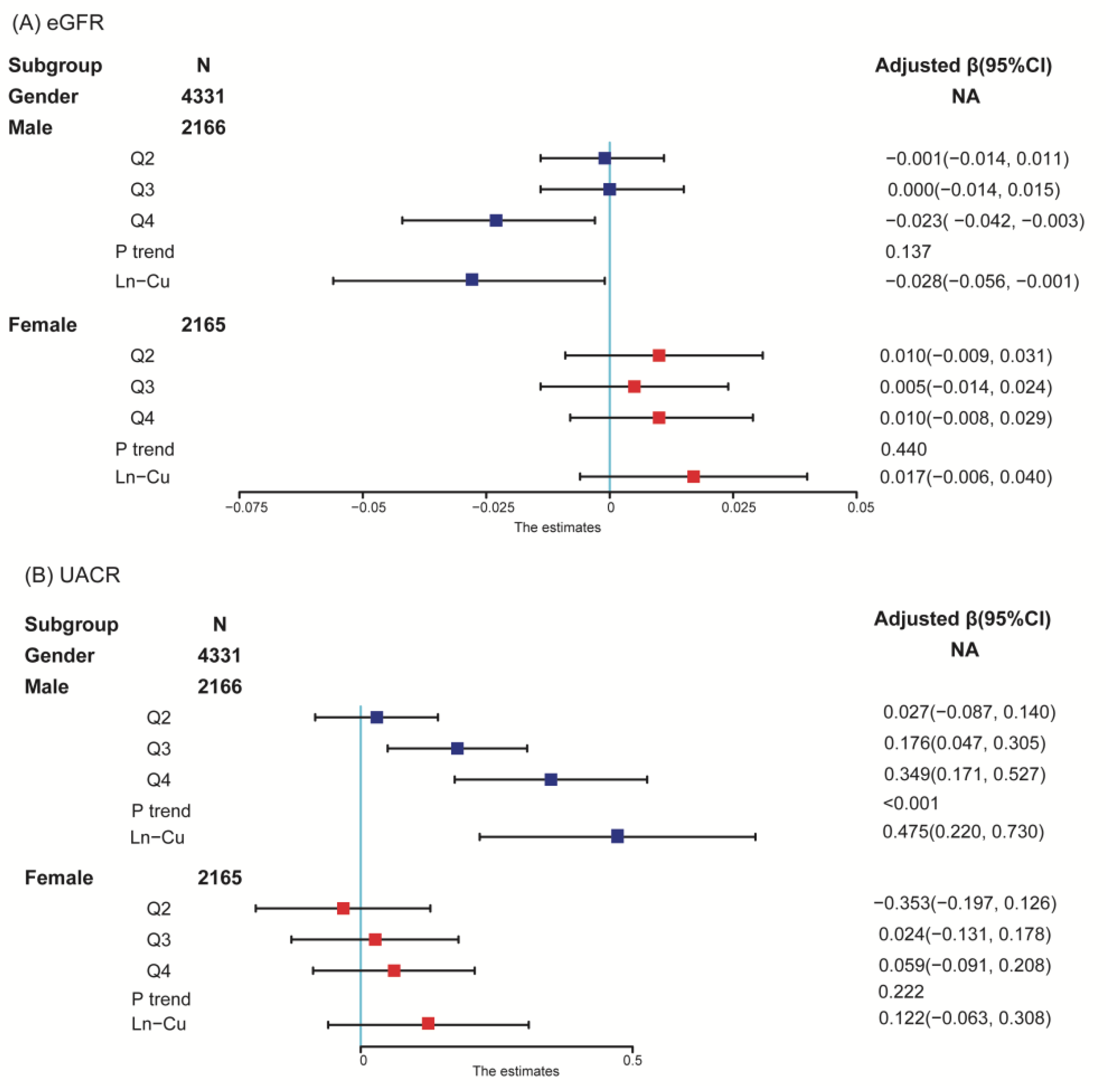
| Serum Cu (μg/dL) | eGFR (Ln Transformed) | UACR (Ln Transformed) | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Overall | ||||
| Q1 | 0.00(Reference) | 0.00(Reference) | ||
| Q2 | 0.002(−0.008, 0.013) | 0.649 | 0.051(−0.040, 0.142) | 0.269 |
| Q3 | 0.000(−0.011, 0.011) | 0.969 | 0.149(0.054, 0.245) | 0.002 |
| Q4 | −0.001(−0.012, 0.011) | 0.925 | 0.209(0.106, 0.312) | <0.001 |
| p trend | 0.839 | <0.001 | ||
| Ln-Cu | 0.003(−0.014, 0.021) | 0.720 | 0.303(0.151, 0.455) | <0.001 |
| Male | ||||
| Q1 | 0.00(Reference) | 0.00(Reference) | ||
| Q2 | −0.002 (−0.015, 0.010) | 0.698 | 0.028 | 0.628 |
| Q3 | −0.002(−0.016, 0.012) | 0.799 | 0.179 | 0.007 |
| Q4 | −0.003(−0.045, 0.006) | 0.011 | 0.352 | <0.001 |
| p trend | 0.066 | <0.001 | ||
| Ln-Cu | −0.034(−0.062, −0.006) | 0.017 | 0.483(0.226, 0.739) | <0.001 |
| Female | ||||
| Q1 | 0.00(Reference) | 0.00(Reference) | ||
| Q2 | 0.010(−0.010, 0.030) | 0.332 | −0.034(−0.195, 0.128) | 0.682 |
| Q3 | 0.003(−0.015, 0.022) | 0.718 | 0.026(−0.129, 0.181) | 0.742 |
| Q4 | 0.008(−0.011, 0.026) | 0.408 | 0.063(−0.087, 0.213) | 0.413 |
| p trend | 0.628 | 0.158 | ||
| Ln-Cu | 0.015(−0.008, 0.038) | 0.201 | 0.125(−0.060, 0.311) | 0.185 |
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Funes, D.R.; Blanco, D.G.; Hong, L.; Lo Menzo, E.; Szomstein, S.; Rosenthal, R.J. Prevalence of Chronic Kidney Disease and End-Stage Renal Disease in a Bariatric versus Nonbariatric Population: A Retrospective Analysis of the U.S. National Inpatient Sample Database. Surg. Obes. Relat. Dis. 2022, 18, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Vart, P.; Grams, M.E. Measuring and Assessing Kidney Function. In Proceedings of the Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 36, pp. 262–272. [Google Scholar]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Tsai, H.J.; Hung, C.H.; Wang, C.W.; Tu, H.P.; Li, C.H.; Tsai, C.C.; Lin, W.Y.; Chen, S.C.; Kuo, C.H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics 2021, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Moody, E.C.; Coca, S.G.; Sanders, A.P. Toxic Metals and Chronic Kidney Disease: A Systematic Review of Recent Literature. Curr. Environ. Health Rep. 2018, 5, 453–463. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between Exposure to Heavy Metals and the Risk of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Altarelli, M.; Ben-Hamouda, N.; Schneider, A.; Berger, M.M. Copper Deficiency: Causes, Manifestations, and Treatment. Nutr. Clin. Pract. 2019, 34, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F.B. Copper: Effects of Deficiency and Overload BT—Interrelations between Essential Metal Ions and Human Diseases. Interrelat. Between Essent. Met. Ions Hum. Dis. 2013, 13, 359–387. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef]
- Dai, C.; Liu, Q.; Li, D.; Sharma, G.; Xiong, J.; Xiao, X. Molecular Insights of Copper Sulfate Exposure-Induced Nephrotoxicity: Involvement of Oxidative and Endoplasmic Reticulum Stress Pathways. Biomolecules 2020, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhong, G.; Ning, Z.; Liao, J.; Yu, W.; Wang, C.; Han, Q.; Li, Y.; Pan, J.; Tang, Z.; et al. Long-Term Exposure to Copper Induces Autophagy and Apoptosis through Oxidative Stress in Rat Kidneys. Ecotoxicol. Environ. Saf. 2020, 190, 110158. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Q.; Yang, R.; Wu, Z.; Yuan, H.; Zhang, N.; Zhi, M.; Zhang, Y.; Ni, X.; Wang, Z.; et al. Copper Exposure Association with Prevalence of Non-Alcoholic Fatty Liver Disease and Insulin Resistance among US Adults (NHANES 2011–2014). Ecotoxicol. Environ. Saf. 2021, 218, 112295. [Google Scholar] [CrossRef]
- Peng, X.; Dai, C.; Zhang, M.; Das Gupta, S. Molecular Mechanisms Underlying Protective Role of Quercetin on Copper Sulfate-Induced Nephrotoxicity in Mice. Front. Vet. Sci. 2021, 7, 586033. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of Plasma and Urine Metals Levels with Kidney Function: A Population-Based Cross-Sectional Study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. Health and Nutrition Examination Survey Plan and Operations, 1999–2010. Natl. Cent. Heal. Stat. Vital Heal. Stat. 1 2013, 1–37. [Google Scholar]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M. New Creatinine-and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- CDC (Centers for Disease Control and Prevention Chronic Kidney Disease). 2015. Available online: https://www.dc.gov/kidneydisease/pdf/CKD-common-serious-costly-h.pdf (accessed on 3 May 2022).
- Kibria, G.M.; Al; Crispen, R. Prevalence and Trends of Chronic Kidney Disease and Its Risk Factors among US Adults: An Analysis of NHANES 2003-18. Prev. Med. Rep. 2020, 20, 101193. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.P.; Choi, K. Exposure to Phthalates and Environmental Phenols in Association with Chronic Kidney Disease (CKD) among the General US Population Participating in Multi-Cycle NHANES (2005–2016). Sci. Total Environ. 2021, 791, 148343. [Google Scholar] [CrossRef]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of Renal Function with Urinary Excretion of Metals: Evidence from NHANES 2003–2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.; Yang, J.L.; Chen, C.L.; Liu, L.; Huang, Y.Q.; Feng, Y.Q.; Yang, A.M. Associations between Blood and Urinary Manganese with Metabolic Syndrome and Its Components: Cross-Sectional Analysis of National Health and Nutrition Examination Survey 2011–2016. Sci. Total Environ. 2021, 780, 146527. [Google Scholar] [CrossRef]
- Eom, S.Y.; Yim, D.H.; Huang, M.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; Kim, Y.D.; Kim, H. Copper–Zinc Imbalance Induces Kidney Tubule Damage and Oxidative Stress in a Population Exposed to Chronic Environmental Cadmium. Int. Arch. Occup. Environ. Health 2020, 93, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Thiamin, R. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline1; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar] [CrossRef]
- Dabbaghmanesh, M.H.; Salehi, N.M.; Siadatan, J.; Omrani, G.R. Copper Concentration in a Healthy Urban Adult Population of Southern Iran. Biol. Trace Elem. Res. 2011, 144, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kouremenou-Dona, E.; Dona, A.; Papoutsis, J.; Spiliopoulou, C. Copper and Zinc Concentrations in Serum of Healthy Greek Adults. Sci. Total Environ. 2006, 359, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Núñez, H.; López, G.; Pizarro, F.; Ayala, M.; Araya, M. Influence of Estrogens on Copper Indicators: In Vivo and in Vitro Studies. Biol. Trace Elem. Res. 2010, 134, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Garmizo, G.; Frauens, B.J. Corneal Copper Deposition Secondary to Oral Contraceptives. Optom. Vis. Sci. 2008, 85, E802–E807. [Google Scholar] [CrossRef]
- Johnson, P.E.; Milne, D.B.; Lykken, G.I. Effects of Age and Sex on Copper Absorption, Biological Half-Life, and Status in Humans. Am. J. Clin. Nutr. 1992, 56, 917–925. [Google Scholar] [CrossRef]
- Toscano, C.M.; Filetti, F.M.; Almenara, C.C.P.; Fioresi, M.; Vassallo, D.V. Copper Exposure for 30 Days at a Daily Dose Twice the Recommended Increases Blood Pressure and Cardiac Contractility. Life Sci. 2022, 300, 120579. [Google Scholar] [CrossRef]
- Stern, B.R. Essentiality and Toxicity in Copper Health Risk Assessment: Overview, Update and Regulatory Considerations. J. Toxicol. Environ. Health Part A Curr. Issues 2010, 73, 114–127. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and Gender Disparities in the Epidemiology and Outcomes of Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Brar, A.; Markell, M. Impact of Gender and Gender Disparities in Patients with Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of Gender on the Progression of Nondiabetic Renal Disease: A Meta-Analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, C.; Dellê, H.; Cavaglieri, R.C.; Dominguez, W.V.; Noronha, I.L. Gender Differences in the Progression of Experimental Chronic Kidney Disease Induced by Chronic Nitric Oxide Inhibition. BioMed Res. Int. 2017, 2017, 2159739. [Google Scholar] [CrossRef] [PubMed]
| Overall | Males | Females | ||
|---|---|---|---|---|
| Variable a | N = 4331 | N = 2166 | N = 2165 | p-Value b |
| Age groups, years | 0.394 | |||
| <20 | 147 (3.39%) | 67 (3.09%) | 80 (3.70%) | |
| 20–39 | 1476 (34.1%) | 756 (34.9%) | 720 (33.3%) | |
| 40–59 | 1358 (31.4%) | 662 (30.6%) | 696 (32.1%) | |
| ≥60 | 1350 (31.2%) | 681 (31.4%) | 669 (30.9%) | |
| Race/ethnicity | 0.038 | |||
| Non-Hispanic White | 1739 (40.2%) | 886 (40.9%) | 853 (39.4%) | |
| Non-Hispanic Black | 891 (20.6%) | 462 (21.3%) | 429 (19.8%) | |
| Other Hispanic | 467 (10.8%) | 206 (9.51%) | 261 (12.1%) | |
| Other race | 1234 (28.5%) | 612 (28.3%) | 622 (28.7%) | |
| PIR | 0.371 | |||
| Low | 1429 (33.0%) | 694 (32.0%) | 735 (33.9%) | |
| Middle | 1591 (36.7%) | 801 (37.0%) | 790 (36.5%) | |
| High | 1311 (30.3%) | 671 (31.0%) | 640 (29.6%) | |
| Educational attainment | 0.015 | |||
| Less than high school | 932 (21.5%) | 486 (22.4%) | 446 (20.6%) | |
| High school or equivalent | 1001 (23.1%) | 528 (24.4%) | 473 (21.8%) | |
| College or above | 2398 (55.4%) | 1152 (53.2%) | 1246 (57.6%) | |
| BMI, kg/m2 | <0.001 | |||
| Underweight | 66 (1.54%) | 23 (1.07%) | 43 (2.01%) | |
| Normal weight | 1227 (28.6%) | 607 (28.2%) | 620 (28.9%) | |
| Overweight | 1391 (32.4%) | 806 (37.4%) | 585 (27.3%) | |
| Obese | 1613 (37.5%) | 718 (33.3%) | 895 (41.8%) | |
| Smoking status | <0.001 | |||
| Never | 2485 (57.4%) | 1053 (48.6%) | 1432 (66.1%) | |
| Ever | 1014 (23.4%) | 631 (29.1%) | 383 (17.7%) | |
| Current | 832 (19.2%) | 482 (22.3%) | 350 (16.2%) | |
| Alcohol drinking status | <0.001 | |||
| No | 1225 (28.3%) | 378 (17.5%) | 847 (39.1%) | |
| Yes | 3103 (71.6%) | 1787 (82.5%) | 1316 (60.8%) | |
| Diabetes | 0.030 | |||
| No | 3712 (85.7%) | 1831 (84.5%) | 1881 (86.9%) | |
| Yes | 619 (14.3%) | 335 (15.5%) | 284 (13.1%) | |
| Hypertension | 0.260 | |||
| No | 2756 (63.6%) | 1360 (62.8%) | 1396 (64.5%) | |
| Yes | 1575 (36.4%) | 806 (37.2%) | 769 (35.5%) | |
| Serum Cu, μg/dL | 114 [98.7;133] | 104 [91.8;117] | 127 [110;147] | <0.001 |
| eGFR, ml/min per 1.73 m2 | 103 [90.5;115] | 102 [90.2;113] | 104 [90.9;117] | <0.001 |
| UACR, mg/g | 7.08 [4.69;13.3] | 6.16 [4.12;11.9] | 8.00 [5.40;14.7] | <0.001 |
| Serum Cu (μg/dL) | eGFR (Ln Transformed) | UACR (Ln Transformed) | ||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Overall | ||||
| Q1 | 0.00(Reference) | 0.00(Reference) | ||
| Q2 | 0.003(−0.007, 0.014) | 0.537 | 0.049(−0.042, 0.134) | 0.293 |
| Q3 | 0.002(−0.009, 0.013) | 0.702 | 0.145(0.050, 0.240) | 0.003 |
| Q4 | 0.001(−0.010, 0.013) | 0.805 | 0.203(0.100, 0.306) | <0.001 |
| p trend | 0.882 | <0.001 | ||
| Ln-Cu | 0.006(−0.012, 0.022) | 0.533 | 0.297(0.145, 0.448) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, Y.; Bai, Y. Sex-Based Differences in the Association between Serum Copper and Kidney Function: Evidence from NHANES 2011–2016. Int. J. Environ. Res. Public Health 2022, 19, 14086. https://doi.org/10.3390/ijerph192114086
Nan Y, Bai Y. Sex-Based Differences in the Association between Serum Copper and Kidney Function: Evidence from NHANES 2011–2016. International Journal of Environmental Research and Public Health. 2022; 19(21):14086. https://doi.org/10.3390/ijerph192114086
Chicago/Turabian StyleNan, Yaxing, and Yana Bai. 2022. "Sex-Based Differences in the Association between Serum Copper and Kidney Function: Evidence from NHANES 2011–2016" International Journal of Environmental Research and Public Health 19, no. 21: 14086. https://doi.org/10.3390/ijerph192114086
APA StyleNan, Y., & Bai, Y. (2022). Sex-Based Differences in the Association between Serum Copper and Kidney Function: Evidence from NHANES 2011–2016. International Journal of Environmental Research and Public Health, 19(21), 14086. https://doi.org/10.3390/ijerph192114086






