Biomarker Expression of Peri-Implantitis Lesions before and after Treatment: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- A.
- Population: Studies including:
- Patients aged ≥18 years;
- Diagnosis of peri-implantitis;
- Reporting on baseline (untreated) and post-treatment PICF biomarker concentration.
- B.
- Intervention: Non-surgical and surgical treatment of peri-implantitis.
- C.
- Comparison: Participants with healthy peri-implant tissues (diagnosis of peri-implant health).
- D.
- Outcomes:
- Primary Outcome: Reported biomarker concentrations in PICF before and after treatment of peri-implantitis.
- Secondary Outcomes: Report of systemic biomarkers, and biomarker concentration in saliva and/or other tissues as reported by the authors (in participants diagnosed with peri-implant health, peri-implant mucositis, and peri-implantitis), and PICF volume. Bacterial (microbiome) reports of composition of peri-implantitis lesions before and after treatment, and peri-implant health. Clinical parameter changes, e.g., Probing Pocket Depth (PD), Clinical Attachment Loss (CAL), recession, BOP, suppuration, Plaque Index (PI), phenotype of keratinised tissue, vertical/horizontal components of keratinised tissue. Radiographic parameters, e.g., bone levels/loss.
2.2. Search Strategy
- A.
- Eligibility Criteria
- B.
- Selection of Studies
2.3. Data Extraction
2.4. Quality Assessment of Included Studies
2.5. Data Synthesis
3. Results
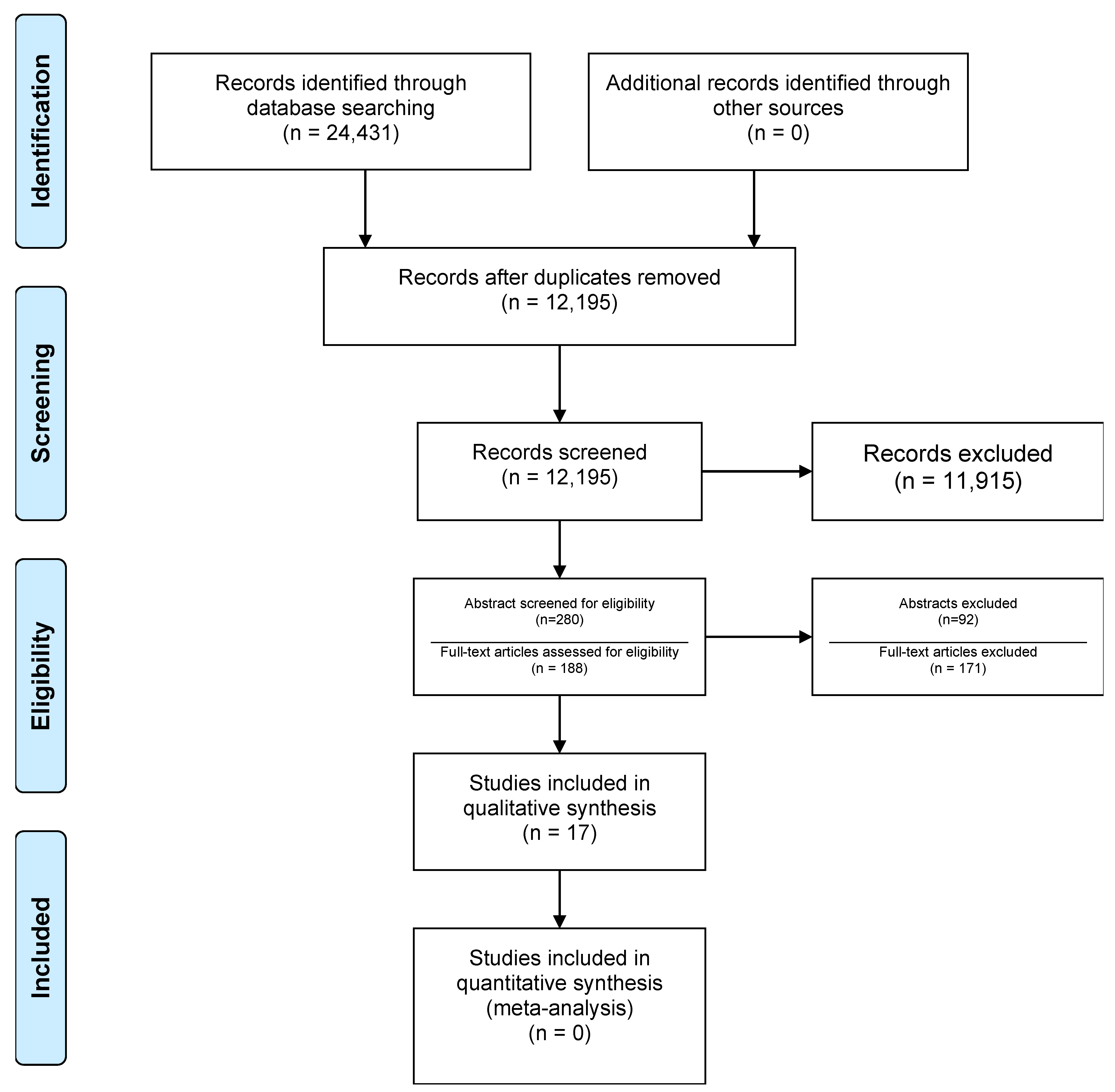
3.1. Study Selection
3.2. Study and Patient Characteristics
3.3. Primary Outcome Results
3.3.1. Non-Surgical Treatment
3.3.2. Surgical Treatment Outcomes
3.4. Secondary Outcomes
3.4.1. Non-Surgical Treatment Alone
- A.
- Probing Pocket Depth:
- B.
- Radiographic Bone Level:
- C.
- Bleeding on Probing:
- D.
- Suppuration:
- E.
- Plaque Index:
- F.
- Peri-Implant Crevicular Fluid Volume:
3.4.2. Non-Surgical Treatment with Adjunctive Use of Laser
- A.
- Probing Pocket Depth:
- B.
- Clinical Attachment Levels:
- C.
- Recession:
- D.
- Bleeding on Probing:
- E.
- Suppuration:
- F.
- Plaque Index:
- G.
- Microbiome Analysis:
3.4.3. Non-Surgical Treatment with Use of Adjunctive Antimicrobials
- A.
- Probing Pocket Depth:
- B.
- Clinical Attachment Levels:
- C.
- Recession:
- D.
- Radiographic Bone Levels:
- E.
- Bleeding on Probing:
- F.
- Suppuration:
- G.
- Plaque Index:
- H.
- Microbiome Analysis:
3.4.4. Access Flap Surgery Alone
- A.
- Probing Pocket Depth:
- B.
- Clinical Attachment Levels:
- C.
- Bleeding on Probing:
- D.
- Suppuration:
- E.
- Plaque Index:
3.4.5. Access Flap Surgery with the Use of Adjunctive Antimicrobials
3.5. Risk of Bias within Studies Results
4. Discussion
4.1. Non-Surgical Treatment
4.1.1. Non-Surgical Treatment Alone
4.1.2. Non-Surgical Treatment with Adjunctive Use of Lasers
4.1.3. Non-Surgical Treatment with Adjunctive Use of Antimicrobials
4.2. Surgical Intervention
4.2.1. Access Flap Debridement Only
4.2.2. Access Flap Debridement with Adjunctive Use of Lasers
4.2.3. Adjunctive Use of Antibiotics to Access Flap Debridement
4.2.4. Regenerative Surgical Debridement with Enamel Matrix Derivative
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Barone, S.; Vocaturo, C.; Nucci, L.; Antonelli, A.; Giudice, A. Usefulness of Magnetic Mallet in Oral Surgery and Implantology: A Systematic Review. J. Pers. Med. 2022, 12, 108. [Google Scholar] [CrossRef]
- Cardoso, J.M.; Duarte, S.; Ribeiro, A.C.; Mascarenhas, P.; Noronha, S.; Alves, R.C. Association between IL-1A, IL-1B and IL-1RN Polymorphisms and Peri-Implantitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 6958. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, H.-L. Biomarkers associated with periimplant diseases. Implant Dent. 2014, 23, 607–611. [Google Scholar] [CrossRef]
- Ghallab, N.A. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: Review of the current evidence. Arch. Oral Biol. 2018, 87, 115–124. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; the QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, J.; Whiting, P.; Vlassov, V. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; Version 1.0.0; The Cochrane Collaboration: London, UK, 2009. [Google Scholar]
- Bassetti, M.; Schär, D.; Wicki, B.; Eick, S.; Ramseier, C.A.; Arweiler, N.B.; Sculean, A.; Salvi, G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Ebersole, J.L.; Dawson, D.R.; Thompson, K.L.; Al-Sabbagh, M. Biological response to peri-implantitis treatment. J. Periodontal Res. 2019, 54, 720–728. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, A.C.; Santos, V.R.; César-Neto, J.B.; Duarte, P.M. Tumor necrosis factor-alpha levels after surgical anti-infective mechanical therapy for peri-implantitis: A 12-month follow-up. J. Periodontol. 2009, 80, 693–699. [Google Scholar] [CrossRef]
- Duarte, P.M.; de Mendonça, A.C.; Máximo, M.B.B.; Santos, V.R.; Bastos, M.F.; Nociti, F.H. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J. Periodontol. 2009, 80, 234–243. [Google Scholar] [CrossRef]
- Esberg, A.; Isehed, C.; Holmlund, A.; Lundberg, P. Peri-implant crevicular fluid proteome before and after adjunctive enamel matrix derivative treatment of peri-implantitis. J. Clin. Periodontol. 2019, 46, 669–677. [Google Scholar] [CrossRef]
- Gershenfeld, L.; Kalos, A.; Whittle, T.; Yeung, S. Randomized clinical trial of the effects of azithromycin use in the treatment of peri-implantitis. Aust. Dent. J. 2018, 63, 374–381. [Google Scholar] [CrossRef]
- Granfeldt, F.R. Surgical Treatment of Peri-Implantitis and It’s Effects on Matrix Metallo Proteinase 8 (MMP-8) Levels. 2010. Available online: https://www.duo.uio.no/handle/10852/33005 (accessed on 7 June 2021).
- Hallström, H.; Lindgren, S.; Widén, C.; Renvert, S.; Twetman, S. Probiotic supplements and debridement of peri-implant mucositis: A randomized controlled trial. Acta Odontol. Scand. 2016, 74, 60–66. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; de Waal, Y.C.M.; Vissink, A.; jan van Winkelhoff, A.; Meijer, H.J.A.; Liefers, S.C.; Kroese, F.G.M.; Raghoebar, G.M. Biomarker levels in peri-implant crevicular fluid of healthy implants, untreated and non-surgically treated implants with peri-implantitis. J. Clin. Periodontol. 2021, 48, 590–601. [Google Scholar] [CrossRef]
- Kalos, A.P. The Effect of Azithromycin on the Non-Surgical Treatment of Peri-Implantitis. A Prospective Double Blind Placebo Controlled Randomised Clinical Trial. A Pilot Study. Ph.D. Thesis, The University of Sydney, Sydney, NSW, Australia, 2015. Available online: https://ses.library.usyd.edu.au/handle/2123/13447 (accessed on 7 June 2021).
- Komatsu, Y.; Kubota, T.; Yasuda, T.; Takahashi, T.; Yamamoto, A.; Kono, T.; Tabata, H.; Nohno, K.; Shibutani, T.; Umeda, M.; et al. Effectiveness of an erbium-doped: Yttrium, aluminum and garnet laser for treatment of peri-implant disease: Clinical, microbiological, and biochemical marker analyses. J. Clin. Exp. Dent. 2018, 10, e970–e978. [Google Scholar] [CrossRef]
- Malik, N.; Naik, D.; Uppoor, A. Levels of Myeloperoxidase and Alkaline Phosphatase in Periimplant Sulcus Fluid in Health and Disease and After Nonsurgical Therapy. Implant Dent. 2015, 24, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Peres Pimentel, S.; Vieira Ribeiro, F.; Correa Casarin, R.; Ribeiro Cirano, F.; Haguihara Luchesi, V.; Gallego Arias Pecorari, V.; Zaffalon Casati, M. Triclosan-containing fluoride toothpaste on clinical parameters and osteo-inflammatory mediators when applied in a stent during experimental peri-implant mucositis in smokers. Clin Oral Impl Res. 2019, 30, 187–195. [Google Scholar] [CrossRef]
- Renvert, S.; Widén, C.; Persson, R.G. Cytokine and microbial profiles in relation to the clinical outcome following treatment of peri-implantitis. Clin. Oral Implants Res. 2017, 28, 1127–1132. [Google Scholar] [CrossRef]
- Ribeiro, F.V.; Casati, M.Z.; Casarin, R.C. Impact of a triclosan-containing toothpaste during the progression of experimental peri-implant mucositis: Clinical parameters and local pattern of osteo-immunoinflammatory mediators in peri-implant fluid. J. Periodontol. 2018, 89, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Thierbach, R.; Maier, K.; Sorsa, T.; Mäntylä, P. Peri-Implant Sulcus Fluid (PISF) Matrix Metalloproteinase (MMP)—8 Levels in Peri-Implantitis. J. Clin. Diagn. Res. JCDR 2016, 10, ZC34–ZC38. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, J.C.; Aass, A.M.; Granfeldt, F.; Lyngstadaas, S.P.; Reseland, J.E. Sulcus fluid bone marker levels and the outcome of surgical treatment of peri-implantitis. J. Clin. Periodontol. 2014, 41, 424–431. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.-M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennström, J.; Berglundh, T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-implantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef]
- Isehed, C.; Svenson, B.; Lundberg, P.; Holmlund, A. Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: 3- and 5-year follow-up. J. Clin. Periodontol. 2018, 45, 744–753. [Google Scholar] [CrossRef]
| Investigator, Year (Country) | 1. Methods 2. Study Type 3. Arm 4. Sample Size Calculation | Participants 1. Number; M/F 2. Distribution between Groups 3. Age (Mean) 4. Source of Recruitment 5.History of Periodontitis a. in total n (%) b. in Test Group n (%) c. in Control Group n (%) | Study Outcomes 1. Primary Outcome 2. Secondary Outcome 3. Other | 1.Definition for Peri-implantitis 2. Implant Position 3. Type of Prosthesis | Type of Procedure/Clinical Details | Laboratory Technique |
|---|---|---|---|---|---|---|
| Bassetti et al., 2014 [13] (Switzerland) | 1. RCT, 1 blind examiner 2. Parallel arm 3. 20 per group, power of 68%, standard deviation of 1.3 | 1. 40; 20 M/20 F 2. Equal Distribution between groups (20/20) 3. 58 4. Private practice and university 5.a. In total: 26 (65%) 5.b. In test group: 18 (45%) 5.c. In control Group: 8 (20%) | 1. Number of BOP + sites 2. PPD, REC, CAL, microbiological (Porphyromonas gingivalis, T. f, T. d, A. a, P.i, Campylobacter rectus, F. n, Capnocytophaga gingivalis, Parvimonas micra, Eubacterium nodatum, Eikenella corrodens) and IL-1b, IL-8, IL-10, MMP-1 and MMP-8) change in PICF. | 1. PPD of 4–6 mm with concomitant BOP at ≥1 peri-implant site and - Radiographic marginal bone loss ranging from 0.5 to 2 mm between delivery of the supra structure and pre-screening appointment 2. NR3.NR | Adjunctive LDD vs. adjunct PDT to Mechanical debridement with titanium curettes and a glycine-based powder air polishing for submucosal biofilm removal | 1. Microbial analysis by Real-time PCR 2. Biomarker assessment by ELISA |
| Bhavsar et al., 2019 [14] (USA) | 1. Case control 2. Study has 2 arms 3. NR | 1. 48; 21/27 2. Sex-matched controls and cases 3. 66.37 4. University of Kentucky College of Dentistry 5.a. In total: 50% 5.b. In test group: 24 (100%) 5.c. In control Group: 0 (0%) | 1. IL-1β, MMP-8, MIP-1α in PICF before and after surgical and anti- microbial therapy. 2. PPD, BOP, SUP, periodontal phenotype, implant mobility, plaque, amount of radiographic bone loss | 1. PPD ≥ 4 mm and radiographic bone loss with more than 20%, but no more than half (50%) of the implant length when compared to baseline radiograph taken at least one year prior to baseline. 2. In health, 11 maxillary and 13 mandibular. In PIP, 7 maxillary and 17 mandibular | - Healthy implants vs. access flap surgery in PIP with anti- microbial therapy. - Prophylactic AB prior to surgery; Amoxicillin/azithromycin - Full thickness flap on buccal and palatal, debridement, 30 sec Tetracycline paste application - Post-operative AB for 7 days. | 1. Biomarker selection using Luminex IS-100 instrument |
| De Mendonga et al., 2009 [15] (Brazil) | 1. Case series 2. No arm 3. NR | 1. 10; 5/5 2. 50/50 3. 62.3 ± 8.4 4. Guarulhos University 5. NR | 1. Total amounts of TNF-a in the PICF 2. PI, mucosal marginal bleeding (MB), BOP, SUP, PPD, PD reduction, relative clinical attachment level (rCAL) | PPD ≥ 5 mm with BOP and/or SUP and concomitant radiographic bone loss involving at least three threads compared to the radiograph taken at the time of prostheses placement | Access flap surgery, debridement with abrasive sodium carbonate air-powder, and resin curettes CHX mw for 7 days. | Biomarker assessment with ELISA |
| Duarte et al., 2009 [16] (Brazil) | 1. Case control 2. PIH, PIM and PIP 3. NR | 1. 40; 15/20 2. 10 classified as PIH, 10 classified as PIM, and 20 classified as PIP 3. 53.4 ± 16.2 4. Guarulhos University | 1.Visible plaque accumulation, marginal bleeding, BOP, SUP, and PPD 2. The total amounts of gene expression interleukin IL-4, IL-10, and IL-12, TNF-a, RANKL, and OPG in PICF | Presence of PD ≥ 5 mm with BOP and/or SUP and concomitant radiographic bone loss involving at least three threads of the implant but no more than half of the implant length. | PIM: mechanical debridement using abrasive sodium carbonate air-powder and resin curettes. PIP: open surgical debridement using abrasive sodium carbonate air-powder and resin curettes. | ELISA |
| Esberg et al., 2019 [17] (Sweden) | 1. Pilot RCT 2. Surgical treatment of PIP without EMD 3. Pilot study-no sample size calculation. | 1. 29 (4 drop out; at the end 25), gender not reported (not present nor original study). 2. 15 EMD group and 14 to the non-EMD group 3. No mean reported. The median age at implant installation was 70.0 years (min–max, 61–81) in the EMD group and 73.5 years (67–83) in the non-EMD group. 4. Not specified, all patients referred to periodontology department (Gävle County Hospital). 5. Most of the patients in this study had a history of periodontitis that had been successfully treated before peri-implant surgery, and 50% of the patients stated that tooth loss was due to periodontitis. | 1. PICF proteome profile before and at 3, 6 and 12 months after the treatment of active peri-implantitis 2. PICF proteome profile relation with implant loss, BOP, PPD and adjunctive EMD treatment. smoking and implant loading time | 1. PIP was defined as PD ≥ 5 mm with BOP and/or SUP and progressive angular peri-implant bone loss ≥ 3 mm as measured on radiographs 2. NR 3. Screw retained | Initial hygiene phase when needed. Access flap for mechanical cleaning using an ultrasonic cleaner with a special implant tip and titanium instruments combined with rinsing with sodium chloride solution (9 mg/mL, 2 × 20 mL). The randomization disclosed the allocation to either adjunctive EMD or no EMD at the implant site before closure of the flap.PICF Collection: Paper were placed for 30 sec at the implant mucosal sulcus site with the deepest pocket | Proteome: protein function and protein–protein interaction networks |
| Gershenfeld et al., 2018 [18] (Australia) | 1. RCT 2. Study has one arm: AZM + NSPT vs. Placebo + NSPT 3. No SS calculation performed | 1. 17; 8/9 2. Randomization 3. 61.9 (59.7 for test group and 64.4 for control group) 4. NR, Consecutive patients referred to the Westmead Centre for Oral Health (Westmead, Sydney, NSW, Australia) 5. In total 8 of total patients In test group: 5 In control group: 3 | 1. BOP and SUP, PPD and gingival margin retraction (recession in mm), radiographic bone loss 2. GI, PI and microbiological and IL-1 Beta results, AZM in PICF | 1. Defined as having a PPD of 5 mm or more with BOP with or without SUP, and radiographic bone loss of more than 2 mm after abutment connection. 2. NR 3. NR | OHI and mechanical debridement for all pts.; test: AZM 500 mg once daily and control placebo for 3 days. Review at days 3, 7, 21, 90 and 180 after mechanical debridement for follow-up. PICF collected. | The total viable aerobic and anaerobic microbiological counts by conventional culturing methods. The AZM level in PICF was assayed using the Driscoll method (Carbonnelle et al., 2011). ELISA for IL-1b |
| Granfeldt et al., 2010 [19] (Norway) | 1. Case control 2. No Arm 3. NR | 1. 36; NR 2. NR 3. NR 4. NR | 1. MMP-8 concentration before and 12 months after surgical treatment of PIP | 1. NR 2. NR 3. NR | OFD alone vs. OFD + porous titanium particles | PICF sample analysis by ELISA |
| Hallstrom et al., 2016 [20] (Sweden) | 1. Double-blind randomized placebo-controlled trial 2. One arm, effect of NSPT in PIM 3. Sample size calculation indicated that 23 patients in each group. | 1. 46; 18/31 2. The study groups were slightly, but non-significantly, imbalanced with respect to general health and tobacco use at baseline; the proportion of smokers was higher in the test group, while the proportion of healthy patients was higher in the placebo group. The groups were, however, balanced regarding age and sex. 3. Placebo 63.3 years and test: 53.7 years 4. NR 5. NR | 1. PPD 2. PI, BOP, SUP collected at baseline and after 1, 2, 4, 12 and 26 weeks, PICF (IL-1b, IL-1RA, IL-4, IL-6, IL-8, IL-17A, CCL5, TNF-a, IFN-g and GM- CSF) Plaque samples (Porphyromonas gingivalis, Prevotella intermedia Prevotella nigrescens, Tannerella forsythia, A. actinomycetemcomitans, Fusobacterium nucleatum, Treponema. Denticola, Parvimonas micra, Campylobacter rectus, Porphymonas endodontis, Filifactor alocis, Prevotella tannerae) Collected at baseline and after 1, 2, 4, 12 and 26 weeks. | 1. PPD ≥ 4 mm combined with bleeding and/or pus on probing using a probing force of 0.2 N. 2. 31 maxillary implant (17 placebo and 14 test group) and 18 mandibular implants (8 placebo and 10 test group). 3. NR | After initial mechanical debridement and OHI, the patients received a topical oil application (active or placebo) followed by twice-daily intake of lozenges (active or placebo) for 3 months. The active products contained a mix of two strains of Lactobacillus reuteri (probiotics) | PICF Volume was recorded using a Perio- trone 8000.Biomarker concentration assessment by Bio-Plex Cytokine Assay. |
| Hentenaar et al., 2021 [21] (Netherlands) | 1.Case control 2. 3 arms; healthy implant and PIP before and after treatment. 3. 40 implant (20/20) from 36 patients, average effect size of 0.9 and power of 80%. | 1. 36; 22/14 2. Equal number of implant distribution between groups (20/20) 3. 60.2 4. University setting 5. NR | 1. level of biomarkers IL-1β, IL-6, TNF-α, MCP-1/CCL2, MIP-1α/CCL3, IFN-γ, MMP-8, sRANKL, OPG and G-CSF in healthy implants and PIP before and 3 months after nonsurgical therapy. 2. PPD, BOP%, SOP%, Pi%, full mouth PPD (mm), full mouth SOP%, full mouth Bop%, full mouth PI%, MBL, Mean PICF Volume, mean periotron value. | 1. Progressive loss of marginal bone ≥ 2 mm, as compared to baseline radiograph in combination with bleeding and or suppuration on probing. 2. NR 3. NR | PICF samples and clinical data recorded at baseline and 3 months after non-surgical treatment using Airflow Master Piezon (EMS) | Luminex assay to analyse biomarkers in the PICF |
| Kalos et al., 2015 [22](Australia) | 1. Pilot prospective double-blind placebo controlled randomized clinical trial. 2. Study has 2 arms, NSPT and AZM vs. NSPT alone and compare to PIH 3. NR | 1. 22 cases (17 PIP and 5 PIH) 9 in the test (4 M and 5 F) and 8 participants in the control (3 M and 5 F) 2. NSSD 3. For the test and control groups were 59.7 (SD = 13.1) and 64.4 (SD = 8.5) 4. Private practice 5. 8 patients in total, NSSD between groups | 1. Mean counts and mean changes from baseline levels in the anaerobic and aerobic microbiological counts (CFU/mL) and the pro inflammatory cytokine Il-1β levels (pg/mL) over time. 2. AZM in PICF over time (expressed as a positive or negative result) To determine the frequency of “positive responders” to treatment. This was determined by the % frequency of patients who displayed a decrease in microbiological and immunological parameters from baseline. | 1. Pocket probing depth of ≥5 mm with bleeding on probing with or without suppuration and radiographic bone loss of >2 mm after abutment connection 2. NR 3. NR | NSPT with and without AZM | aerobic vs. anaerobic, bacterial complexes by culture technique ELISA for IL-1 analysis Batch testing for presence or absence of AZM |
| Komatsu et al., 2018 [23](Japan) | 1. RCT 2. Has 2 arms: Er:YAG and MC 3. 40 in total, 20 patients per group, effect size = 0.80, alpha = 0.05, and power at 80%) | 1. 40 in total; 6/12 (laser) 9/10 (MC) 2.NR 3. 64.1 + −8.5 (laser) 64.8 + −7.2 (MC) 4. University setting 5. NR | 1. Clinical parameter: PPD, CAL, BOP, Mo, and BL. IL-1α, IL-1β, IL-6, IL-8, TNF-α, and MMP-1, 3, 9, and 13, CRP 2. The count of G + and G-in both groups (P. intermedia, P. gingivalis, T. forsythia, T. denticola, F. nucletum) | 1. PID: PPD greater than 5 mm and concomitant BOP from at least two sites. 2. NR 3. NR | Treatment (Er: YAG) irradiation Vs. locally delivered minocycline hydrochloride (MC) | PICF: biomarker detection by multiplex suspension array system, whereas C-reactive protein (CRP) Sub-gingival plaque: PCR |
| Malik et al., 2015 [24] (India) | 1. Case control 2. Study has 2 arms 3. NR | 1. 30 participants 20/10 2. NR 3. 41.83 ± 13.67 4. University setting 5. NR | 1. PICF concentration of MPO and ALP 2. PI, GI, mPI, mBI, mGI, and PPD | 1. Presence of supra- gingival plaque; mBI score >1, mPI score >1, mGI score >1; redness and swelling of peri-implant mucosa; radiographic evidence of bone loss higher than two-thirds length of the first step of implant or exposure of ≥2 threads of the implant; probing depth ≥4 mm in at least 1 site around the fixture. 2. NR 3. NR | Healthy implant Vs, NS anti-infective therapy (supra and sub gingival scaling and local irrigation with 0.2% CHX. Post-operative CHX gel for 4 weeks.) | ALP Liquid Stable Reagent Kit using modified DGKC method; MPO using spectrophotometric MPO assay. |
| Peres Pimentel et al., 2019 [25] (Brazil) | 1. Double-blind, randomized, crossover study 2. Random assignment to Triclosan/fluoride (n: 13) or fluoride toothpaste (n: 13) 3. NR | 1. 26; 15/11 2. Triclosan/Fluoride Toothpaste (n = 13) or Fluoride Toothpaste (n = 13) 3. 49.62 ± 16.01 years 4. University setting 5. Confounding factors: NR | 1. Biomarker Levels of IFN-γ, IL-17, IL-1β, IL-10, IL-6, IL-8, TNF-α, OPG, osteocalcin (OC), osteopontin (OPN), MMP-2, MMP-96, TGF-β7, RANKL. 2. PI, BOP, PPM, PD and RCAL at experimental sites at baseline, 3-, 7-, 14- and 21-day follow-ups. | 1. PIH: PDD < 4 mm with no Bop and no evidence of radiographic bone loss beyond bone remodelling (AAP, 2013) 2. NR 3. NR | All smoker patients 3 weeks not performing mechanical plaque removal and after 3 weeks randomly assigned to 2 groups: group 1: Triclosan fluoride toothpaste, group 2: only fluoride toothpaste 3DS. Clinical and biomarker assessment at days 0, 3, 7, 14, 21. | Biomarker assessment by MAGpixTM instrument (Luminex) |
| Renvert et al., 2017 [26] (Sweden) | 1. Case series 2. No arm 3. NR | 1. 41; NR 2. NR 3. NR 4. NR 5. NR | 1. Pro- and anti-inflammatory cytokines IL-1β, IL-1ra, IL-6, IL-8, IL-17A, IP-10, MIP-1α, PDGFBB, TNF-α, and VEGF 2. Microbiome. Aeruginosa, S. aureus and T. forsythia. Clinical characteristics: BOP; sup, Bone level, PPD; at baseline and 6 months after treatment | 1. Bone loss > 3 mm and probing pocket ≥ 5 mm, and with bleeding/pus on probing at the implant. 2. NR 3. NR | Nonsurgical therapy by either with the PerioFlow® device or by an Er: YAG laser (KAVO, Biberach, Germany. Outcomes in stable (no further bone loss, probing pocket depth decrease ≥0.5 mm, no bleeding/suppuration) and unstable patients after 6 months. | Luminex magnet bead technology and checkerboard DNA-DNA hybridization |
| Ribeiro et al., 2018 [27] (Brazil) | 1. Double-blind, randomized, crossover study No arm 2. NR | 1. 22 Gender/Sex: 8/13 2. Triclosan (n = 11) or placebo (n = 11) 3. 48.45 ± 13.64 years 4. University setting 5. NR | 1.Biomarker: IL-4, IL-17, IL-6, IL-23, INF-γ, TNF-α, MMP-2, MMP-9 2. Clinical evaluation of PI/%, BI, position of the peri-implant margin (PPM/mm): distance from the stent to the peri-implant margin, RCAL, PPD; at baseline and at 3, 7, 14, and 21 days | 1. NR 2. NR 3. NR | 3 weeks not performing mechanical plaque removal and after 3 weeks randomly assigned to 2 groups: group 1: Triclosan Fluoride toothpaste, group 2: Fluoride toothpaste | PICF analysis by MAGpix™ instrument10 (Luminex) |
| Thierbach et al., 2016 [28] (Germany) | 1. case-control 2. PIP, PIH 3. NR | 1. 29 patients; male n: 11 (healthy), male n: 14 20:8 (peri-implantitis) 2. Distribution between groups: age: 55.5 (PIH), 56.4 (PIP) 3. Age range: 4. German military hospital setting 5. 18 patients had periodontitis and 11 patients had healthy/gingivitis periodontium | 1. PISF MMP-8 Levels in Peri-Implantitis 2. PPD, BOP, age of implant, smoking status | 1. PPD > 5 mm in at least one site, exhibiting BOP and/or SUP, and having RBL in at least one site were considered implants with peri-implantitis. 2. NR 3. NR | Treatment: All patients underwent (aPDT) using a Low-Intensity Laser Treatment (LILT) laser of the implant pockets. The defects were then exposed to laser light with a wavelength of 660 nm for ten seconds using fibre optics. Light was delivered to six sites per implant. 4 months later the patients underwent access flap surgery of the PIP sites included in the study. The clinical treatment result was evaluated six months after the flap surgery. | ELISA for biomarker assessment |
| Wohlfahrt et al., 2014 [29](Norway) | 1. Prospective, randomized, test-control, clinical study 2. Arm:2 3. NR | 1. 32; NR 2. Distribution between groups: NR 3. NR 4. University and private 5. NR | 1. MMP-8, IL-6, OPG, osteocalcin, leptin, osteopontin, parathyroid hormone, TNF-α, adiponectin and insulin, total protein content 2. PPD, BOP | 1. NR1.NR 2. NR 3. NR | Surgery, comparing OFD and surface decontamination with titanium curettes and 24% EDTA gel (n = 16), or additional insertion of porous titanium granules. | MMP-8: ELISA, Luminex for the rest of biomarkers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moaven, H.; Giacaman, A.; Beltrán, V.; Sam, Y.H.; Betancur, D.; Mainas, G.; Tarjomani, S.A.; Donos, N.; Sousa, V. Biomarker Expression of Peri-Implantitis Lesions before and after Treatment: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14085. https://doi.org/10.3390/ijerph192114085
Moaven H, Giacaman A, Beltrán V, Sam YH, Betancur D, Mainas G, Tarjomani SA, Donos N, Sousa V. Biomarker Expression of Peri-Implantitis Lesions before and after Treatment: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(21):14085. https://doi.org/10.3390/ijerph192114085
Chicago/Turabian StyleMoaven, Haniyeh, Annesi Giacaman, Víctor Beltrán, Ye Han Sam, Daniel Betancur, Giuseppe Mainas, Seyed Ali Tarjomani, Nikolaos Donos, and Vanessa Sousa. 2022. "Biomarker Expression of Peri-Implantitis Lesions before and after Treatment: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 21: 14085. https://doi.org/10.3390/ijerph192114085
APA StyleMoaven, H., Giacaman, A., Beltrán, V., Sam, Y. H., Betancur, D., Mainas, G., Tarjomani, S. A., Donos, N., & Sousa, V. (2022). Biomarker Expression of Peri-Implantitis Lesions before and after Treatment: A Systematic Review. International Journal of Environmental Research and Public Health, 19(21), 14085. https://doi.org/10.3390/ijerph192114085






