Diet Quality Influences the Occurrence of Food Aversions in Women Undergoing Adjuvant Chemotherapy for Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Dietary Assessment
2.3. Dietary Antioxidant Capacity Assessment
2.4. Diet Quality Assessment
2.5. Food Aversions Assessment
2.6. Oxidative Stress Biomarkers Analysis
2.7. Other Parameters
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2020: Incidência de Câncer no Brasil. 2020. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/estimativa-2020-incidencia-de-cancer-no-brasil.pdf (accessed on 10 July 2022).
- Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2020: Tipos de Câncer: Câncer de Mama. 2020. Available online: https://www.inca.gov.br/tipos-de-cancer/cancer-de-mama/profissional-de-saude (accessed on 10 July 2022).
- Gebremedhin, T.K.; Cherie, A.; Tolera, B.D.; Atinafu, B.T.; Demelew, T.M. Prevalence and risk factors of malnutrition among adult cancer patients receiving chemotherapy treatment in cancer center, Ethiopia: A cross-sectional study. Heliyon 2021, 7, e07362. [Google Scholar] [CrossRef]
- Drareni, K.; Bensafi, M.; Giboreau, A.; Dougkas, A. Chemotherapy-induced taste and smell changes influence food perception in cancer patients. Support. Care Cancer 2021, 29, 2125–2132. [Google Scholar] [CrossRef]
- Xu, L.; Peterson, L.L. The Impact of Diet on Breast Cancer Outcomes. Curr. Nutr. Rep. 2019, 8, 212–221. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, G.M.; Son, S.; Song, M.; Park, S.; Chung, H.C.; Lee, S.-M. Changes in taste and food preferences in breast cancer patients receiving chemotherapy: A pilot study. Support. Care Cancer 2020, 28, 1265–1275. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, L.; Zhang, Z.; Zhao, J.; Wan, C. Severe Zinc Deficiency Causes the Loss and Apoptosis of Olfactory Unsheathing Cells (OECs) and Olfactory Deficit. J. Mol. Neurosci. 2021, 71, 869–878. [Google Scholar] [CrossRef]
- Goraya, J.S. Vitamin B12 deficiency in Indian infants and children. Paediatr. Int. Child Health 2020, 40, 75–77. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity-Exploring the armory of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Reitz, L.K.; Baptista, S.d.L.; Santos, E.d.S.; Hinnig, P.F.; Rockenbach, G.; Vieira, F.G.K.; de Assis, M.A.A.; da Silva, E.L.; Boaventura, B.C.B.; Pietro, P.F.D. Diet Quality Is Associated with Serum Antioxidant Capacity in Women with Breast Cancer: A Cross Sectional Study. Nutrients 2020, 13, 115. [Google Scholar] [CrossRef]
- Reitz, L.K.; Schroeder, J.; Longo, G.Z.; Boaventura, B.C.B.; Di Pietro, P.F. Dietary Antioxidant Capacity Promotes a Protective Effect against Exacerbated Oxidative Stress in Women Undergoing Adjuvant Treatment for Breast Cancer in a Prospective Study. Nutrients 2021, 13, 4324. [Google Scholar] [CrossRef]
- Sichieri, R.; Everhart, J.E. Validity of a Brazilian food frequency questionnaire against dietary recalls and estimated energy intake. Nutr. Res. 1998, 18, 1649–1659. [Google Scholar] [CrossRef]
- Medeiros, N.I. Consumo Alimentar e Níveis de Antioxidantes Plasmáticos em Mulheres com Câncer de Mama. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2004. [Google Scholar]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs, and supplements used worldwide. Nutr. J. 2010, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- NEPA—Center for Studies and Research in Food. Brazilian Food Composition Database, 4th ed.; NEPA: Campinas, Brazil, 2011. [Google Scholar]
- U.S. Department of Agriculture: Agricultural Research Service (USDA). National Nutrient Database for Standard Reference, Release 18. Nutrient Data Laboratory, 2005. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 21 March 2022).
- U.S. Department of Agriculture: Agricultural Research Service (USDA). Database for the Flavonoid Content of Selected Foods, Release 3.2. 2015. Available online: https://data.nal.usda.gov/dataset/usda-database-flavonoid-content-selected-foods-release-32-november-2015 (accessed on 21 March 2022).
- U.S. Department of Agriculture: Agricultural Research Service (USDA). Database for the Isoflavone Content of Selected Foods, Release 2.1. 2015. Available online: https://data.nal.usda.gov/dataset/usda-database-isoflavone-content-selected-foods-release-21-november-2015 (accessed on 21 March 2022).
- U.S. Department of Agriculture: Agricultural Research Service (USDA). Database for the Proanthocyanidin Content of Selected Foods, Release 2. 2015. Available online: https://data.nal.usda.gov/dataset/usda-database-proanthocyanidin-content-selected-foods-release-2-2015 (accessed on 21 March 2022).
- Brasil, Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica. Guia Alimentar para a População Brasileira, 2nd ed; Ministério da Saúde: Brasília, Brazil, 2014. [Google Scholar]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Moritz, B.; Tramonte, V.L.C. Bioavailability of lycopene. Rev. Nutr. 2006, 19, 265–273. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Boado, L.S.; Fretes, R.M.; Brumovsky, L.A. Bioavailability and antioxidant effect of the Ilex Paraguariensis polyphenols. Nutr. Food Sci. 2015, 45, 326–335. [Google Scholar] [CrossRef]
- Previdelli, A.N.; De Andrade, S.C.; Pires, M.M.; Ferreira, G.R.; Fisberg, R.M.; Marchioni, D.M. Índice de Qualidade da Dieta Revisado para população brasileira. Rev. De Saúde Pública 2011, 45, 794–798. [Google Scholar] [CrossRef]
- Andrade, S.C.; Previdelli, A.N.; Marchioni, D.M.L.; Fisberg, R.M. Evaluation of the reliability and validity of the Brazilian Healthy Eating Index Revised. Rev. De Saúde Pública 2013, 47, 675–683. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde, Secretaria de Atenção à Saúde. Guia Alimentar para a População Brasileira: Promovendo a Alimentação Saudável; Secretaria de Atenção à Saúde: Brasília, Brazil, 2006. [Google Scholar]
- Pinheiro, A.B. Tabela Para Avaliação de Consumo Alimentar em Medidas Caseiras, 5th ed; Atheneu: São Paulo, Brazil, 2008. [Google Scholar]
- Rockenbach, G.; Di Pietro, P.F.; Ambrosi, C.; Boaventura, B.C.; Vieira, F.G.; Crippa, C.G.; Da Silva, E.L.; Fausto, M.A. Dietary intake and oxidative stress in breast cancer: Before and after treatments. Nutr. Hosp. 2011, 26, 737–744. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Esterbauer, H.; Zollner, H. Methods for determination of aldehydic lipid peroxidation products. Free. Radic. Biol. Med. 1989, 7, 197–203. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Wolff, S.P. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal. Biochem. 1994, 220, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Di Pietro, P.F.; Medeiros, N.I.; Vieira, F.G.; Fausto, M.A.; Belló-Klein, A. Breast cancer in southern Brazil: Association with past dietary intake. Nutr. Hosp. 2007, 22, 565–572. [Google Scholar] [PubMed]
- Petroski, E.L. Antropometria: Técnicas e Padronizações; Palotti: Porto Alegre, Brazil, 1999. [Google Scholar]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; WHO Technical Report Series 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio. Report of WHO Expert Consultation; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Dahlgren, D.; Sjöblom, M.; Hellström, P.M.; Lennernäs, H. Chemotherapeutics-Induced Intestinal Mucositis: Pathophysiology and Potential Treatment Strategies. Front. Pharmacol. 2021, 12, 681417. [Google Scholar] [CrossRef]
- Norde, M.M.; Fisberg, R.M.; Marchioni, D.; Rogero, M.M. Systemic low-grade inflammation-associated lifestyle, diet, and genetic factors: A population-based cross-sectional study. Nutrition 2020, 70, 110596. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Han, M.; Wang, C.; Liu, P.; Li, D.; Li, Y.; Ma, X. Dietary Fiber Gap and Host Gut Microbiota. Protein Pept. Lett. 2017, 24, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary Fat and Cancer-Which Is Good, Which Is Bad, and the Body of Evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef]
- Orchard, T.S.; Andridge, R.R.; Yee, L.D.; Lustberg, M.B. Diet Quality, Inflammation, and Quality of Life in Breast Cancer Survivors: A Cross-Sectional Analysis of Pilot Study Data. J. Acad. Nutr. Diet. 2018, 118, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Sahin, T.K.; Bilir, B.; Kucuk, O. Modulation of inflammation by phytochemicals to enhance efficacy and reduce toxicity of cancer chemotherapy. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Le Bitoux, M.A.; Stamenkovic, I. Tumor-host interactions: The role of inflammation. Histochem. Cell Biol. 2008, 130, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; de Haan-Du, J.; Sidorenkov, G.; Landman, G.W.D.; Jalving, M.; Zhang, Q.; de Bock, G.H. Type 2 Diabetes Mellitus and Clinicopathological Tumor Characteristics in Women Diagnosed with Breast Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4992. [Google Scholar] [CrossRef]
- American Cancer Society. Breast Cancer Early Detection and Diagnosis. Available online: https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/breast-biopsy.html (accessed on 6 August 2022).
- Bours, M.J.; Beijer, S.; Winkels, R.M.; van Duijnhoven, F.J.; Mols, F.; Breedveld-Peters, J.J.; Kampman, E.; Weijenberg, M.P.; van de Poll-Franse, L.V. Dietary changes and dietary supplement use, and underlying motives for these habits reported by colorectal cancer survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) registry. Br. J. Nutr. 2015, 114, 286–296. [Google Scholar] [CrossRef]
- Fassier, P.; Zelek, L.; Lécuyer, L.; Bachmann, P.; Touillaud, M.; Druesne-Pecollo, N.; Galan, P.; Cohen, P.; Hoarau, H.; Latino-Martel, P.; et al. Modifications in dietary and alcohol intakes between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Santé cohort. Int. J. Cancer 2017, 141, 457–470. [Google Scholar] [CrossRef]
- Verde, S.M.M.L.; São Pedro, B.M.O.; Netto, M.M.; Damasceno, N.R.T. Aversão alimentar adquirida e qualidade de vida em mulheres com neoplasia mamária. Rev. Nutr. 2009, 22, 795–807. [Google Scholar] [CrossRef][Green Version]
- Marinho, E.D.C.; Custódio, I.D.D.; Ferreira, I.B.; Crispim, C.A.; Paiva, C.E.; Maia, Y.C.D.P. Relationship between food perceptions and health-related quality of life in a prospective study with breast cancer patients undergoing chemotherapy. Clinics 2018, 73, e411. [Google Scholar] [CrossRef]
- Ford, K.L.; Arends, J.; Atherton, P.J.; Engelen, M.P.K.J.; Gonçalves, T.J.M.; Laviano, A.; Lobo, D.N.; Phillips, S.M.; Ravasco, P.; Deutz, N.E.; et al. The importance of protein sources to support muscle anabolism in cancer: An expert group opinion. Clin. Nutr. 2022, 41, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Caan, B.; Feliciano, E.M.C.; Meyerhardt, J.A.; Kroenke, C.H.; Baracos, V.E.; Weltzien, E.; Kwan, M.L.; Alexeeff, S.E.; Castillo, A.L.; et al. The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am. J. Clin. Nutr. 2019, 109, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The Underappreciated Role of Low Muscle Mass in the Management of Malnutrition. J. Am. Med. Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef] [PubMed]
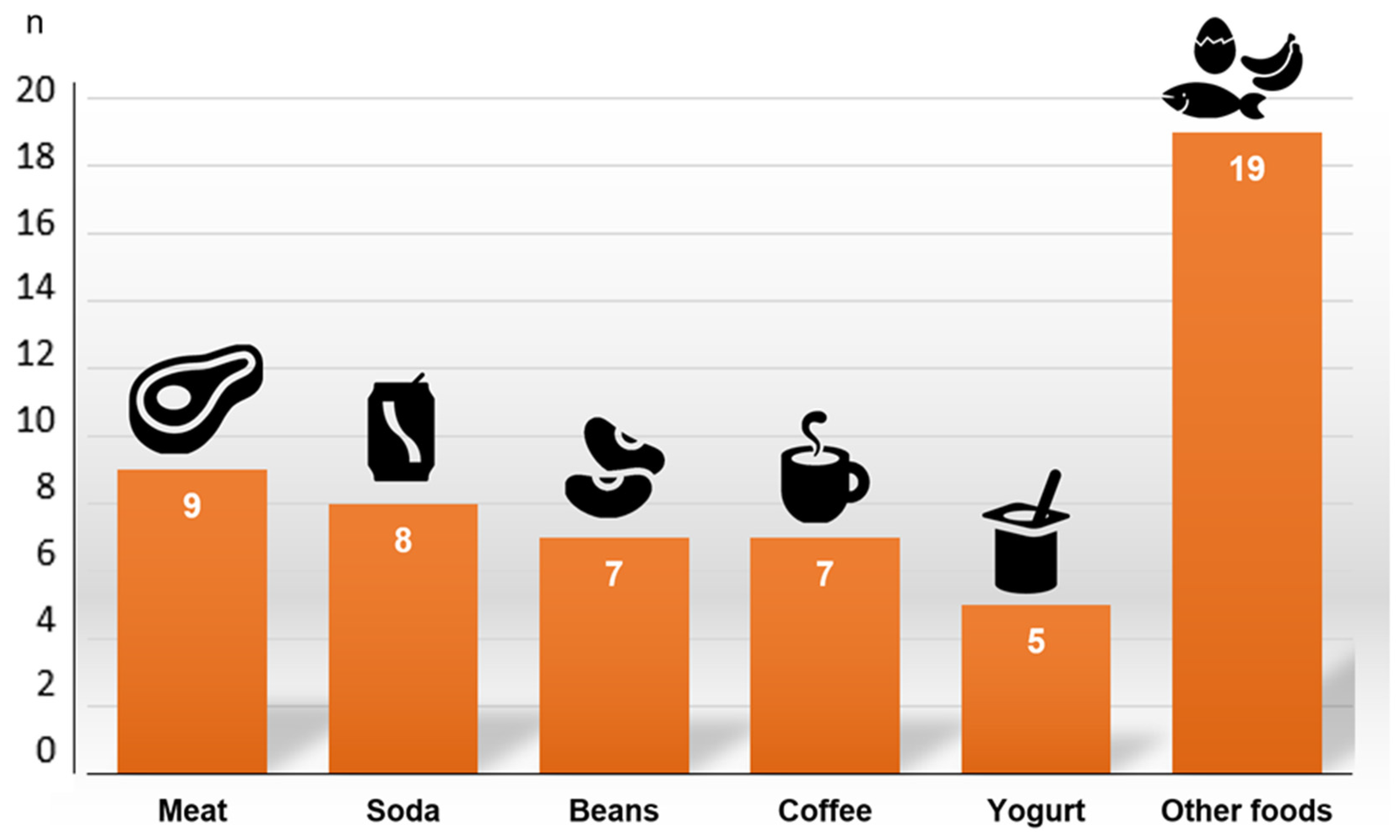
| Occurrence of Food Aversion (n = 24) | Non-Occurrence of Food Aversion (n = 49) | p | |
|---|---|---|---|
| Age (years) b | 50.6 (10.2) | 52.5 (12.3) | 0.518 $ |
| Ethnic group, n (%) | 0.453 # | ||
| White | 22 (91.7) | 47 (95.9) | |
| Non-white | 2 (8.3) | 2 (4.1) | |
| Schooling | 4 (2, 8) | 7 (4, 11) | 0.104 & |
| (years of study) a | |||
| Smoking, n (%) | 0.875 # | ||
| Yes | 5 (20.8) | 11 (22.4) | |
| No | 19 (79.2) | 38 (77.6) | |
| Alcohol intake, n (%) | 0.725 # | ||
| Yes | 2 (8.3) | 3 (6.10) | |
| No | 22 (91.7) | 46 (93.9) | |
| BMI (kg/m2) a | 26.3 (24.2, 31.8) | 27.3 (23.7, 30.4) | 0.809 & |
| Waist circumference (cm) a | 87.5 (80.5, 101) | 84 (78.5, 98) | 0.537 & |
| Tumor stage, n (%) | 0.072 # | ||
| 0–I | 6 (25) | 23 (46.9) | |
| II–III | 18 (75) | 26 (56.1) | |
| Number of chemotherapy sessions | 6 (6, 8) | 6 (6, 8) | 0.430 & |
| Number of radiotherapy sessions | 28.5 (28, 32) | 32 (28, 33) | 0.187 & |
| Hormone therapy, n (%) | 0.261 # | ||
| Yes | 18 (75) | 42 (85.7) | |
| No | 6 (25) | 7 (14.3) | |
| Surgery type, n (%) | 0.360 # | ||
| Partial mastectomy | 10 (41.7) | 26 (53.1) | |
| Total mastectomy | 14 (58.3) | 23 (46.9) | |
| Tumor size (cm) a | 2.4 (1.8, 3.5) | 1.5 (1, 2.5) | 0.004 & |
| Occurrence of Food Aversion (n = 24) | Non-Occurrence of Food Aversion (n = 49) | p | |
|---|---|---|---|
| DaC (mmol/d) a | 9.8 (7.3, 12.1) | 8.4 (6.12, 12.2) | 0.565 & |
| aC from whole cereals, legumes, tubers, and roots (mmol/d) a | 0.79 (0.44, 155) | 0.81 (0.54, 113.6) | 0.721 & |
| aC from total fruits (mmol/d) a | 1.21 (0.68, 1.99) | 1.02 (0.51, 1.78) | 0.711 & |
| aC from cruciferous vegetables (mmol/d) a | 0.13 (0.04, 0.29) | 0.12 (0.04, 0.24) | 0.995 & |
| aC from orange and dark green vegetables and fruits (mmol/d) a | 0.50 (0.28, 1.13) | 0.39 (0.20, 0.75) | 0.142 & |
| aC from citric fruits (mmol/d) a | 0.47 (0.19, 0.98) | 0.35 (0.08, 1.19) | 0.762 & |
| aC from red vegetables and fruits (mmol/d) a | 0.13 (0.03, 0.41) | 0.17 (0.05, 0.48) | 0.442 & |
| aC from polyphenol-rich foods and beverages (mmol/d) a | 8.24 (5.2, 11.2) | 7.25 (4.5, 11.5) | 0.607 & |
| aC from coffee (mmol/d) a | 7.42 (3.72, 11.2) | 5.58 (3.72, 7.44) | 0.967 & |
| Total BHEI-R score | 71.8 (11.2) | 75.4 (8.5) | 0.285 & |
| Total fruits (0–5) a | 5 (3.5, 5) | 5 (3.9, 5) | 0.802 & |
| Whole fruits (0–5) a | 5 (4.1, 5) | 5 (4.9, 5) | 0.691 & |
| Total vegetables (0–5) a | 4.7 (2.7, 5) | 5 (3, 5) | 0.482 & |
| Dark green and orange vegetables and legumes (0–5) a | 5 (2.8, 5) | 5 (4, 5) | 0.951 & |
| Total grains (0–5) a | 5 (4.4, 5) | 5 (5, 5) | 0.175 & |
| Whole grains (0–5) a | 0 (0, 0.185) | 0 (0, 0.35) | 0.739 & |
| Milk and dairy products (0–10) b | 4.6 (0.530) | 5.1 (0.434) | 0.554 $ |
| Meat, eggs, and legumes (0–10) a | 8.7 (6.5, 10) | 9.1 (7.1, 10) | 0.350 & |
| Oils (0–10) a | 10 (10, 10) | 10 (10, 10) | 0.061 & |
| Saturated fat (0–10) a | 7.2 (3.5, 8.7) | 6.5 (3.8, 9.4) | 1.000 & |
| Sodium (0–10) a | 9.0 (8.0, 9.8) | 8.5 (6.9, 9.3) | 0.244 & |
| TBARS (μmol/L) a | 4.9 (4.3, 5.6) | 4.8 (4.1, 5.9) | 0.850 & |
| HL (μmol/L) a | 4.3 (3.3, 5.3) | 3.9 (2.9, 6.2) | 0.752 & |
| GSH (μmol/L) b | 76.7 (19.6) | 78.8 (22.1) | 0.698 $ |
| FRAP (μmol/L) b | 589.3 (118.1) | 648.6 (164.9) | 0.122 $ |
| CP (μmol/L) a | 0.7 (0.6, 1.1) | 0.7 (0.6, 1.2) | 0.504 & |
| Non-Occurrence of Food Aversion | ||||||
|---|---|---|---|---|---|---|
| OR (Crude) | CI95% | p | OR (Adjusted) | CI95% | p | |
| BHEI-R score | 1.04 | 0.987–1.096 | 0.137 | 1.080 * | 1.003–1.154 | 0.041 |
| DaC (mmol/day) | 0.992 | 0.908–1.083 | 0.801 | 0.986 * | 0.883–1.102 | 0.811 |
| TBARS (μmol/L) | 0.98 | 0.878–1.095 | 0.729 | 0.963 # | 0.855–1.085 | 0.583 |
| LH (μmol/L) | 0.992 | 0.831–1.184 | 0.932 | 0.924 $ | 0.761–1.123 | 0.932 |
| GSH (μmol/L) | 1.005 | 0.981–1.029 | 0.693 | 1.002 + | 0.977–1.028 | 0.862 |
| FRAP (μmol/L) | 1.002 | 0.999–1.006 | 0.124 | 1.003 + | 0.999–1.007 | 0.131 |
| CP (μmol/L) | 2.192 | 0.560–8.580 | 0.259 | 2.540 $ | 0.543–11.867 | 0.236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitz, L.K.; Schroeder, J.; Raick, M.; de Fragas Hinnig, P.; Vieira, F.G.K.; De Assis, M.A.A.; Da Silva, E.L.; Di Pietro, G.; Di Pietro, P.F. Diet Quality Influences the Occurrence of Food Aversions in Women Undergoing Adjuvant Chemotherapy for Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 13915. https://doi.org/10.3390/ijerph192113915
Reitz LK, Schroeder J, Raick M, de Fragas Hinnig P, Vieira FGK, De Assis MAA, Da Silva EL, Di Pietro G, Di Pietro PF. Diet Quality Influences the Occurrence of Food Aversions in Women Undergoing Adjuvant Chemotherapy for Breast Cancer. International Journal of Environmental Research and Public Health. 2022; 19(21):13915. https://doi.org/10.3390/ijerph192113915
Chicago/Turabian StyleReitz, Luiza Kuhnen, Jaqueline Schroeder, Marina Raick, Patricia de Fragas Hinnig, Francilene Gracieli Kunradi Vieira, Maria Alice Altenburg De Assis, Edson Luiz Da Silva, Giuliano Di Pietro, and Patricia Faria Di Pietro. 2022. "Diet Quality Influences the Occurrence of Food Aversions in Women Undergoing Adjuvant Chemotherapy for Breast Cancer" International Journal of Environmental Research and Public Health 19, no. 21: 13915. https://doi.org/10.3390/ijerph192113915
APA StyleReitz, L. K., Schroeder, J., Raick, M., de Fragas Hinnig, P., Vieira, F. G. K., De Assis, M. A. A., Da Silva, E. L., Di Pietro, G., & Di Pietro, P. F. (2022). Diet Quality Influences the Occurrence of Food Aversions in Women Undergoing Adjuvant Chemotherapy for Breast Cancer. International Journal of Environmental Research and Public Health, 19(21), 13915. https://doi.org/10.3390/ijerph192113915







