Integrating Genomic Information with Tumor-Immune Microenvironment in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
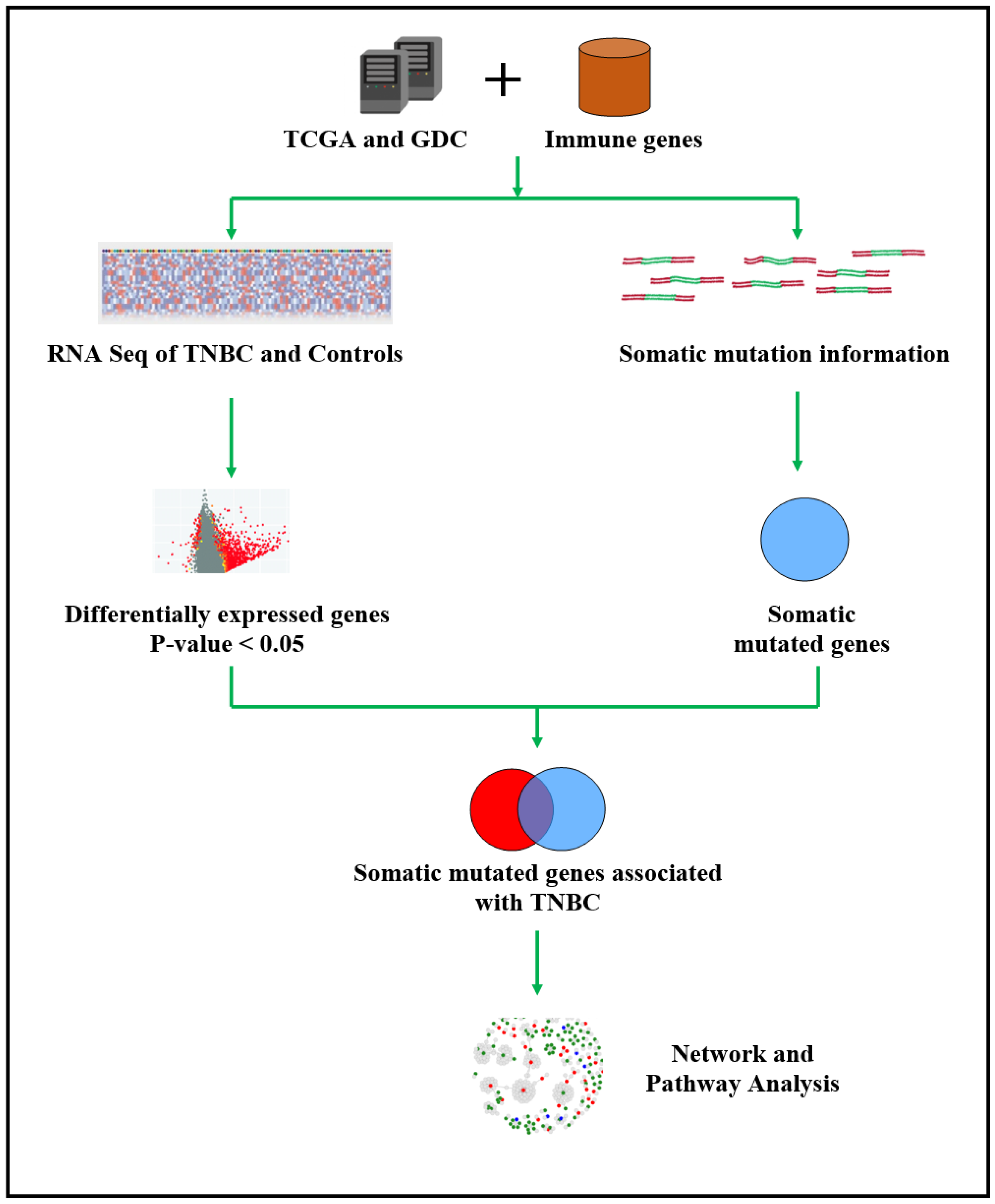
2.1. Project Design and Execution Strategy
2.2. Sources of Genomics Data and Immune-Modulated Genes
2.3. Bioinformatics and Statistical Data Analysis and Integration
2.4. Functional Analysis and Validation
3. Results
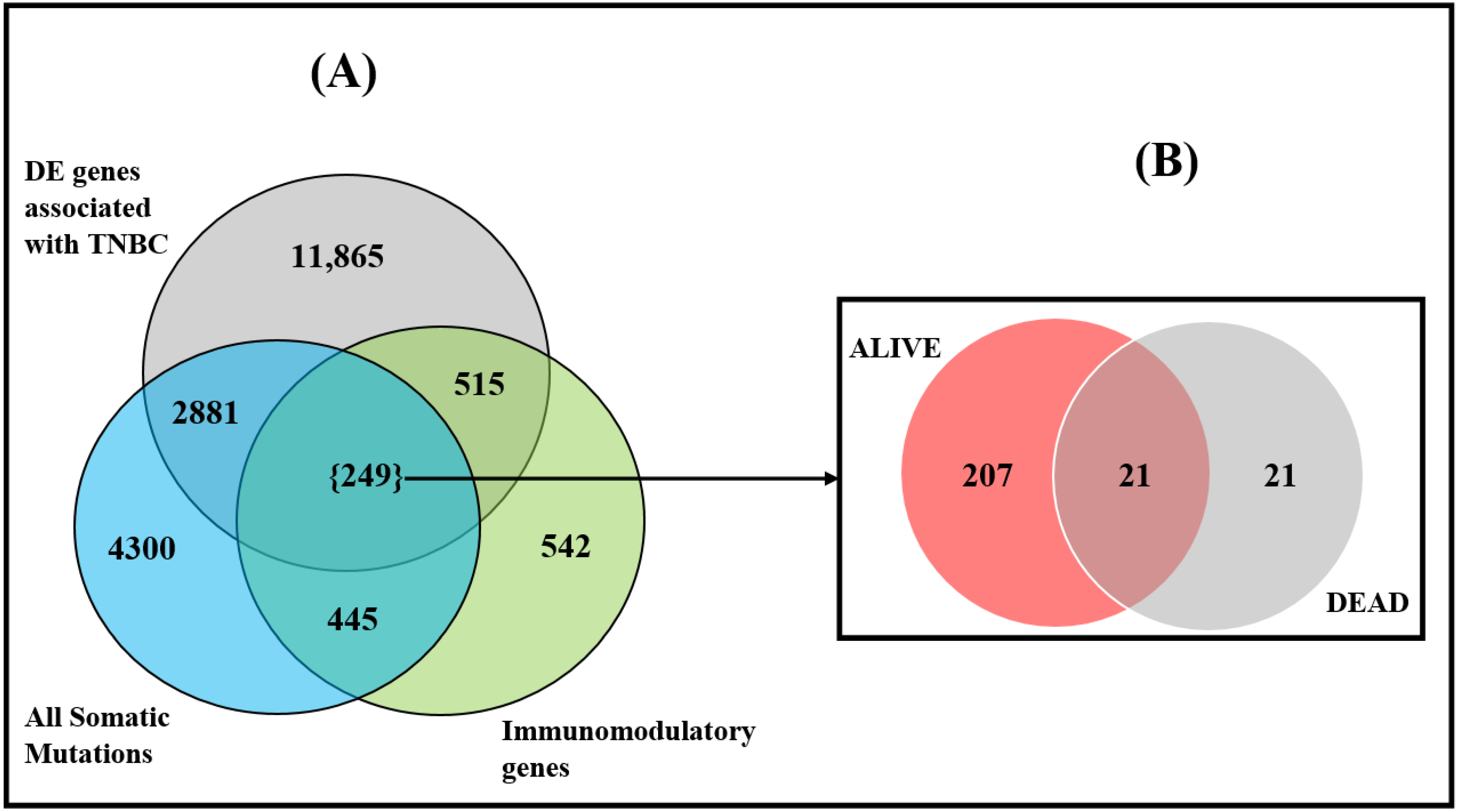
3.1. Discovery of Gene Expression and Somatic Mutated Signatures
3.2. Differences in Somatic Mutation Burden between Deceased and Alive
3.3. Discovery of Somatic Mutated Immune Modulated Gene Signatures and Pathways
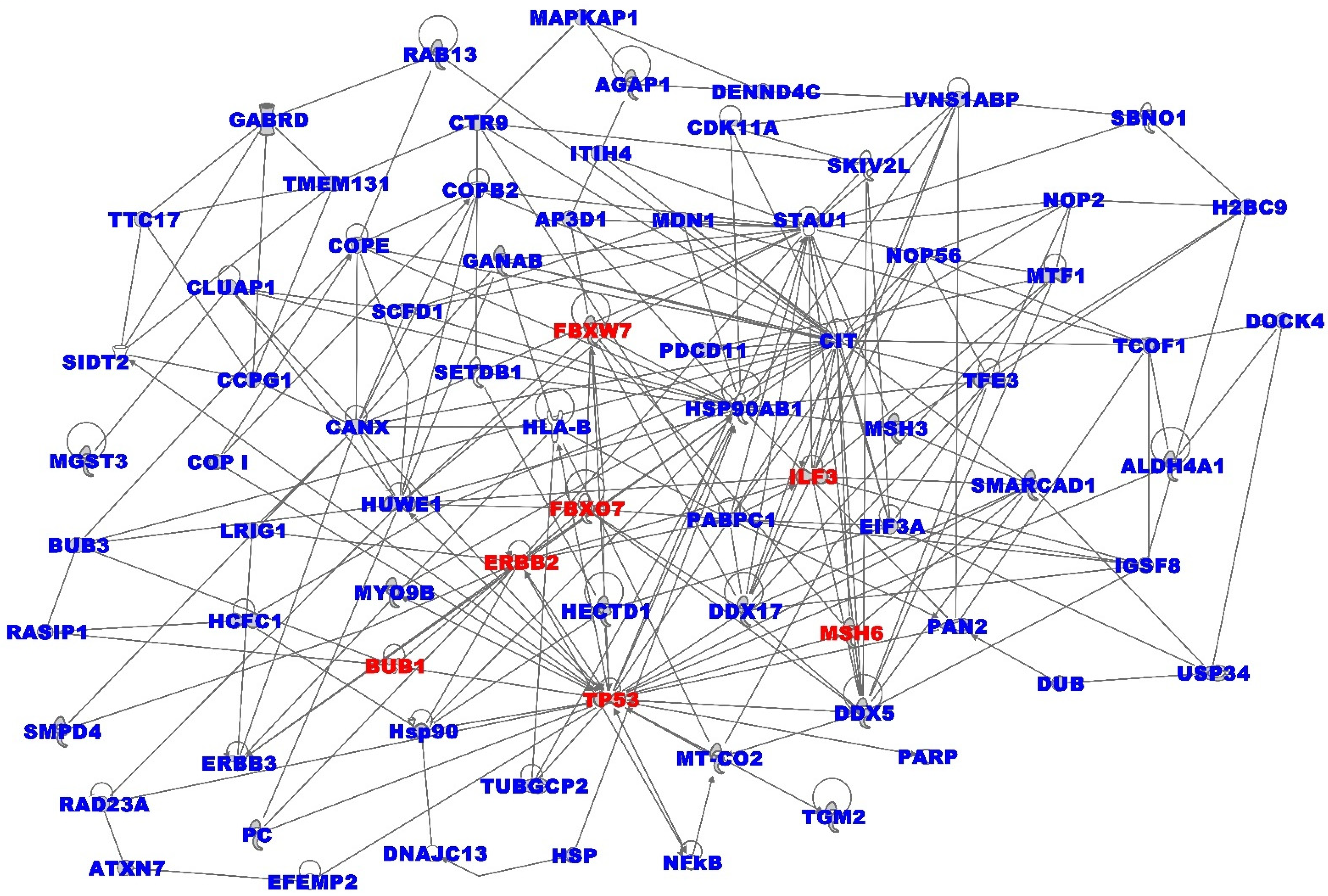
3.4. Oncogenic Interactions between Somatic and Immune Microenvironment
3.5. Validation and Potential Clinical and Translational Impact
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plasilova, M.L.; Hayse, B.; Killelea, B.K.; Horowitz, N.R.; Chagpar, A.B.; Lannin, D.R. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine 2016, 95, e4614. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Dietze, E.C.; Sistrunk, C.; Miranda-Carboni, G.; O’Regan, R.; Seewaldt, V.L. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat. Rev. Cancer 2015, 15, 248–254. [Google Scholar] [CrossRef]
- Dietze, E.C.; Chavez, T.A.; Seewaldt, V.L. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. Am. J. Pathol. 2018, 188, 280–290. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef]
- Katayama, H.; Boldt, C.; Ladd, J.J.; Johnson, M.M.; Chao, T.; Capello, M.; Suo, J.; Mao, J.; Manson, J.E.; Prentice, R.; et al. An Autoimmune Response Signature Associated with the Development of Triple-Negative Breast Cancer Reflects Disease Pathogenesis. Cancer Res. 2015, 75, 3246–3254. [Google Scholar] [CrossRef]
- Crosby, E.J.; Wei, J.; Yang, X.Y.; Lei, G.; Wang, T.; Liu, C.X.; Agarwal, P.; Korman, A.J.; Morse, M.A.; Gouin, K.; et al. Complimentary mechanisms of dual checkpoint blockade expand unique T-cell repertoires and activate adaptive anti-tumor immunity in triple-negative breast tumors. Oncoimmunology 2018, 7, e1421891. [Google Scholar] [CrossRef]
- Nederlof, I.; Horlings, H.; Curtis, C.; Kok, M. A High-Dimensional Window into the Micro-Environment of Triple Negative Breast Cancer. Cancers 2021, 13, 316. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, S.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, Y.; Tang, X.; Wu, R. Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: Preclinical and clinical studies and mechanism of action. Expert Opin. Drug Deliv. 2021, 18, 187–203. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; Van De Vijver, K.K.; De Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Zhu, H.; Du, C.; Yuan, M.; Fu, P.; He, Q.; Yang, B.; Cao, J. PD-1/PD-L1 counterattack alliance: Multiple strategies for treating triple-negative breast cancer. Drug Discov. Today 2020, 25, 1762–1771. [Google Scholar] [CrossRef]
- Picornell, A.C.; Echavarria, I.; Alvarez, E.; López-Tarruella, S.; Jerez, Y.; Hoadley, K.; Parker, J.S.; del Monte-Millán, M.; Ramos-Medina, R.; Gayarre, J.; et al. Breast cancer PAM50 signature: Correlation and concordance between RNA-Seq and digital multiplexed gene expression technologies in a triple negative breast cancer series. BMC Genom. 2019, 20, 452. [Google Scholar] [CrossRef]
- Yam, C.; Yen, E.-Y.; Chang, J.T.; Bassett, R.L.; Alatrash, G.; Garber, H.; Huo, L.; Yang, F.; Philips, A.V.; Ding, Q.-Q.; et al. Immune Phenotype and Response to Neoadjuvant Therapy in Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 5365–5375. [Google Scholar] [CrossRef]
- Audeh, W.; Blumencranz, L.; Kling, H.; Trivedi, H.; Srkalovic, G. Prospective Validation of a Genomic Assay in Breast Cancer: The 70-gene MammaPrint Assay and the MINDACT Trial. Acta Med. Acad. 2019, 48, 18–34. [Google Scholar] [CrossRef]
- Ricks-Santi, L.J.; McDonald, J.T. Low utility of Oncotype DX in the clinic. Cancer Med. 2017, 6, 501–507. [Google Scholar] [CrossRef]
- Zhao, Y.; Schaafsma, E.; Cheng, C. Gene signature-based prediction of triple-negative breast cancer patient response to Neoadjuvant chemotherapy. Cancer Med. 2020, 9, 6281–6295. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. Cancer Genome Atlas Research Network. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Hudson, T.J.; Anderson, W.; Artez, A.; Barker, A.D.; Bell, C.; Bernabé, R.R.; Bhan, M.K.; Calvo, F.; Eerola, I.; Gerhard, D.S.; et al. The International Cancer Genome Consortium International network of cancer genome projects. Nature 2010, 464, 993–998. [Google Scholar]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef]
- Bareche, Y.; Venet, D.; Ignatiadis, M.; Aftimos, P.; Piccart, M.; Rothe, F.; Sotiriou, C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 2018, 29, 895–902. [Google Scholar] [CrossRef]
- Wu, J.; Mamidi, T.K.K.; Zhang, L.; Hicks, C. Integrating Germline and Somatic Mutation Information for the Discovery of Biomarkers in Triple-Negative Breast Cancer. Int. J. Environ. Res. Public Health 2019, 16, 1055. [Google Scholar] [CrossRef]
- Wu, J.; Mamidi, T.K.K.; Zhang, L.; Hicks, C. Unraveling the Genomic-Epigenomic Interaction Landscape in Triple Negative and Non-Triple Negative Breast Cancer. Cancers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Wu, J.; Mamidi, T.K.K.; Zhang, L.; Hicks, C. Deconvolution of the Genomic and Epigenomic Interaction Landscape of Triple-Negative Breast Cancer. Cancers 2019, 11, 1692. [Google Scholar] [CrossRef]
- O’Meara, T.; Safonov, A.; Casadevall, D.; Qing, T.; Silber, A.; Killelea, B.; Hatzis, C.; Pusztai, L. Immune microenvironment of triple-negative breast cancer in African-American and Caucasian women. Breast Cancer Res. Treat. 2019, 175, 247–259. [Google Scholar] [CrossRef]
- The Genomics Data Commons. Available online: https://gdc.cancer.gov/ (accessed on 28 July 2021).
- AmpliSeq for Illumina Immune Response Panel; Illumina Inc.: San Diego, CA, USA, 2019.
- QIAseq Immune Repertoire RNA Library Kits; Qiagen Inc.: Venlo, The Netherlands, 2019.
- Liu, Z.; Li, M.; Jiang, Z.; Wang, X. A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer. Transl. Oncol. 2018, 11, 311–329. [Google Scholar] [CrossRef]
- Hu, S.; Qu, X.; Jiao, Y.; Hu, J.; Wang, B. Immune Classification and Immune Landscape Analysis of Triple-Negative Breast Cancer. Front. Genet. 2021, 12, 710534. [Google Scholar] [CrossRef]
- Guberman, J.M.; Ai, J.; Arnaiz, O.; Baran, J.; Blake, A.; Baldock, R.; Chelala, C.; Croft, D.; Cros, A.; Cutts, R.J.; et al. BioMart Central Portal: An open database network for the biological community. Database 2011, 2011, bar041. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Morrissey, E.R.; Diaz-Uriarte, R. Pomelo II: Finding differentially expressed genes. Nucleic Acids Res. 2009, 37 (Suppl. 2), W581–W586. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ingenuity Pathways Analysis (IPA). Ingenuity Pathways Analysis (IPA) System. Ingenuity Systems; Qiagen, Inc.: Redwood, CA, USA, 2007; Available online: http://www.ingenuity.com/ (accessed on 13 August 2021).
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Oshi, M.; Kawaguchi, T.; Yan, L.; Peng, X.; Qi, Q.; Tian, W.; Schulze, A.; McDonald, K.A.; Narayanan, S.; Young, J.; et al. Immune cytolytic activity is associated with reduced intra-tumoral genetic heterogeneity and with better clinical outcomes in triple negative breast cancer. Am. J. Cancer Res. 2021, 11, 3628–3644. [Google Scholar]
- Marczyk, M.; Qing, T.; O’Meara, T.; Yagahoobi, V.; Pelekanou, V.; Bai, Y.; Reisenbichler, E.; Cole, K.S.; Li, X.; Gunasekharan, V.; et al. Tumor immune microenvironment of self-identified African American and non-African American triple negative breast cancer. NPJ Breast Cancer 2022, 8, 88. [Google Scholar] [CrossRef]
- Ross, J.S. Multigene classifiers, prognostic factors, and predictors of breast cancer clinical outcome. Adv. Anat. Pathol. 2009, 16, 204–215. [Google Scholar] [CrossRef]
- Ross, J.S.; Hatzis, C.; Symmans, W.F.; Pusztai, L.; Hortobágyi, G.N. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist 2008, 13, 477–493. [Google Scholar] [CrossRef]
- Vural, S.; Wang, X.; Guda, C. Classification of breast cancer patients using somatic mutation profiles and machine learning approaches. BMC Syst. Biol. 2016, 10 (Suppl. 3), 62. [Google Scholar] [CrossRef]
- Jiang, Q.; Jin, M. Feature Selection for Breast Cancer Classification by Integrating Somatic Mutation and Gene Expression. Front. Genet. 2021, 12, 629946. [Google Scholar] [CrossRef]
- Salvadores, M.; Mas-Ponte, D.; Supek, F. Passenger mutations accurately classify human tumors. PLoS Comput. Biol. 2019, 15, e1006953. [Google Scholar] [CrossRef]
- Disis, M.L.; Stanton, S.E. Triple-negative breast cancer: Immune modulation as the new treatment paradigm. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e25–e30. [Google Scholar] [CrossRef]
- Isaacs, J.; Anders, C.; McArthur, H.; Force, J. Biomarkers of Immune Checkpoint Blockade Response in Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2021, 22, 38. [Google Scholar] [CrossRef]
- Stagg, J.; Allard, B. Immunotherapeutic approaches in triple-negative breast cancer: Latest research and clinical prospects. Ther. Adv. Med. Oncol. 2013, 5, 169–181. [Google Scholar] [CrossRef]
- Hendrickx, W.; Simeone, I.; Anjum, S.; Mokrab, Y.; Bertucci, F.; Finetti, P.; Curigliano, G.; Seliger, B.; Cerulo, L.; Tomei, S.; et al. Identification of genetic determinants of breast cancer immune phenotypes by integrative genome-scale analysis. Oncoimmunology 2017, 6, e1253654. [Google Scholar] [CrossRef]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef]
- Clark, C.A.; Yang, E.S. Harnessing DNA Repair Defects to Augment Immune-Based Therapies in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 703802. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Medici, N.; Bilancio, A.; Migliaccio, A.; Castoria, G. Breast cancer stem cells: The role of sex steroid receptors. World J. Stem Cells 2019, 11, 594–603. [Google Scholar] [CrossRef]
- Ma, F.; Li, H.; Li, Y.; Ding, X.; Wang, H.; Fan, Y.; Lin, C.; Qian, H.; Xu, B. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Medicine 2017, 96, e6561. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Song, Y.; Wang, S.; Huang, X.; Xuan, Q.; Kang, X.; Zhang, Q. CD44+/CD24- phenotype predicts a poor prognosis in triple-negative breast cancer. Oncol. Lett. 2017, 14, 5890–5898. [Google Scholar] [CrossRef]
- Jin, J.; Krishnamachary, B.; Mironchik, Y.; Kobayashi, H.; Bhujwalla, Z.M. Phototheranostics of CD44-positive cell populations in triple negative breast cancer. Sci. Rep. 2016, 6, 27871. [Google Scholar] [CrossRef] [PubMed]
- Masili-Oku, S.M.; Almeida, B.G.L.; Bacchi, C.E.; Filassi, J.R.; Baracat, E.C.; Carvalho, F.M. Lymphocyte-predominant triple-negative breast carcinomas in premenopausal patients: Lower expression of basal immunohistochemical markers. Breast 2017, 31, 34–39. [Google Scholar] [CrossRef]
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018, 68, 102–110. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.-D.; Liu, Y.-R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell. 2019, 35, 428–440.e5. [Google Scholar] [CrossRef]
- Memorial Sloan-Kettering Cancer Center and National Cancer Institute (NCI). Bicalutamide in Treating Patients with Metastatic Breast Cancer. NIH Web Site. Available online: http://clinicaltrials.gov/ct2/show/NCT00468715 (accessed on 18 October 2022).
- Zhu, A.; Li, Y.; Song, W.; Xu, Y.; Yang, F.; Zhang, W.; Yin, Y.; Guan, X. Antiproliferative Effect of Androgen Receptor Inhibition in Mesenchymal Stem-Like Triple-Negative Breast Cancer. Cell Physiol. Biochem. 2016, 38, 1003–1014. [Google Scholar] [CrossRef]
- Pidsley, R.; Lawrence, M.G.; Zotenko, E.; Niranjan, B.; Statham, A.; Song, J.; Chabanon, R.M.; Qu, W.; Wang, H.; Richards, M.; et al. Enduring epigenetic landmarks define the cancer microenvironment. Genome Res. 2018, 28, 625–638. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, Y.; Zhang, X.S. Identification of mutated core cancer modules by integrating somatic mutation, copy number variation, and gene expression data. BMC Syst. Biol. 2013, 7 (Suppl. 2), S4. [Google Scholar] [CrossRef]
- Omilian, A.R.; Wei, L.; Hong, C.C.; Bandera, E.V.; Liu, S.; Khoury, T.; Ambrosone, C.B.; Yao, S. Somatic mutations of triple-negative breast cancer: A comparison between Black and White women. Breast Cancer Res. Treat. 2020, 182, 503–509. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Chrom Position | Ex-p-Value | SNVs | Deletions | Inserts | Total |
|---|---|---|---|---|---|---|
| CCDC74B | 2q21.1 | 1.00 × 10−6 | 1 | 0 | 0 | 1 |
| DCUN1D2 | 13q34 | 1.00 × 10−6 | 1 | 1 | 0 | 2 |
| MAGEA4 | Xq28 | 1.00 × 10−6 | 1 | 0 | 0 | 1 |
| ANKRD34C | 15q25.1 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| CAT | 11p13 | 1.10 × 10−6 | 0 | 1 | 0 | 1 |
| CCDC74A | 2q21.1 | 1.10 × 10−6 | 3 | 0 | 0 | 3 |
| EFR3B | 2p23.3 | 1.10 × 10−6 | 4 | 0 | 0 | 4 |
| FXYD3 | 19q13.12 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| GYG2 | Xp22.33 | 1.10 × 10−6 | 4 | 0 | 0 | 4 |
| IGLL5 | 22q11.22 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| LYST | 1q42.3 | 1.10 × 10−6 | 6 | 0 | 0 | 6 |
| MAPK8IP3 | 16p13.3 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| MT-CO2 | Mitochondria | 1.10 × 10−6 | 2 | 0 | 0 | 2 |
| SMARCE1 | 17q21.2 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| SPECC1L | 22q11.23 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| VWA2 | 10q25.3 | 1.10 × 10−6 | 1 | 0 | 0 | 1 |
| C15orf39 | 15q24.2 | 1.20 × 10−6 | 5 | 0 | 1 | 6 |
| DYNC1LI1 | 3p22.3 | 1.20 × 10−6 | 0 | 0 | 1 | 1 |
| FLNB | 3p14.3 | 1.20 × 10−6 | 1 | 0 | 0 | 1 |
| GLYATL2 | 11q12.1 | 1.20 × 10−6 | 1 | 0 | 0 | 1 |
| LINC00634 | 22q13.2 | 1.20 × 10−6 | 0 | 1 | 0 | 1 |
| MT-ND4 | Mitochondria | 1.20 × 10−6 | 1 | 0 | 0 | 1 |
| CNNM2 | 10q24.32 | 1.30 × 10−6 | 3 | 0 | 0 | 3 |
| DIEXF | 1q32.2 | 1.30 × 10−6 | 1 | 0 | 0 | 1 |
| DOCK1 | 10q26.2 | 1.30 × 10−6 | 1 | 0 | 0 | 1 |
| ERH | 14q24.1 | 1.30 × 10−6 | 1 | 0 | 0 | 1 |
| FREM2 | 13q13.3 | 1.30 × 10−6 | 4 | 0 | 0 | 4 |
| LAMP3 | 3q27.1 | 1.30 × 10−6 | 1 | 1 | 0 | 2 |
| NSD1 | 5q35.3 | 1.30 × 10−6 | 4 | 1 | 0 | 5 |
| SLCO4C1 | 5q21.1 | 1.30 × 10−6 | 1 | 0 | 0 | 1 |
| Gene Symbols | Chromosome Position | Mutation Frequency in the Alive Group | Mutation Frequency in the Deceased Group |
|---|---|---|---|
| DST | 6p12.1 | 8 | |
| RYR1 | 19q13.2 | 8 | |
| CSMD2 | 1p35.1 | 7 | |
| MUC5B | 11p15.5 | 7 | |
| MYO18B | 22q12.1 | 8 | |
| DYNC1H1 | 14q32.31 | 6 | |
| ARID1B | 6q25.3 | 6 | |
| SACS | 13q12.12 | 6 | |
| CACNA1B | 9q34.3 | 6 | |
| RYR2 | 1q43 | 6 | |
| ASPM | 1q31.3 | 7 | |
| MXRA5 | Xp22.33 | 6 | |
| CSMD3 | 8q23.3 | 7 | |
| LRP1 | 12q13.3 | 6 | |
| PREX1 | 2p14 | 5 | |
| DSCAM | 20q13.13 | 5 | |
| SCN11A | 21q22.2 | 5 | |
| FHOD3 | 3p22.2 | 5 | |
| DNAH5 | 18q12.2 | 5 | |
| DIDO1 | 5p15.2 | 5 | |
| HSPG2 | 20q13.33 | 5 | |
| R3HCC1L | 10q24.2 | 2 | |
| ZNF689 | 16p11.2 | 2 | |
| SYN2 | 3p25.2 | 2 | |
| C1orf167 | 1p36.22 | 2 | |
| PAQR8 | 6p12.2 | 2 | |
| NT5DC2 | 3p21.1 | 1 | |
| EYA1 | 8q13.3 | 1 | |
| LTK | 15q15.1 | 1 | |
| SUGP2 | 19p13 | 1 | |
| CCDC39 | 3q26.33 | 1 | |
| CEACAM5 | 19q13.2 | 1 | |
| LZTS2 | 10q24.31 | 1 | |
| ARMC5 | 16p11.2 | 1 | |
| FAM161A | 2p15 | 1 | |
| CPEB2 | 4p15.32 | 1 | |
| ITGB1 | 10p11.22 | 1 | |
| ZNF860 | 3p24.1 | 1 | |
| CANX | 5q35.3 | 1 | |
| KRT8P12 | 3q25.33 | 1 | |
| HAUS3 | 4p16.3 | 1 | |
| SEL1L3 | 4p15.2 | 1 |
| Parameters | Alive Group (n = 97) | Deceased Group (n = 18) |
|---|---|---|
| Average number of mutations per patient across the 274 genes common to the outcomes (mean ± standard deviation) | 6.63 ± 2.5 | 16.78 ± 4.6 * |
| Metastasis occurrence | 0 | 2 |
| Predominant tumor stage | Stage 1 & 2 | Stage 3 & 4 |
| Gene Symbols | Chromosome Position | Mutation Frequency in the Alive Group | Mutation Frequency in the Deceased Group |
|---|---|---|---|
| BMP5 | 12p13.33 | 0 | 1 |
| BCL6 | 20q13.33 | 0 | 1 |
| IFI35 | 12p13.31 | 0 | 1 |
| HLA-DMA | 3p21.31 | 0 | 1 |
| NGF | 14q22.1 | 0 | 1 |
| EYA1 | 17q25.3 | 0 | 1 |
| MAVS | 15q26.1 | 0 | 1 |
| MFGE8 | 8q21.11 | 0 | 1 |
| FBXO7 | 19p13.2 | 0 | 1 |
| ADA | 4q31.3 | 0 | 1 |
| FCER1A | 8p12 | 0 | 1 |
| IL21R | 4q13.3 | 0 | 1 |
| LTK | 6p21.31 | 0 | 1 |
| SFRP2 | 12p13.33 | 0 | 1 |
| MGMT | 12q13.3 | 0 | 1 |
| PAK3 | 14q12 | 0 | 1 |
| ALCAM | 5p15.33 | 0 | 1 |
| KNG1 | 5q31.1 | 0 | 1 |
| CEACAM5 | 15q25.2 | 0 | 1 |
| TSHR | 7p13 | 0 | 1 |
| KLRF1 | 19q13.11 | 0 | 1 |
| TP53 * | 17p13.1 | 64 | 13 |
| ACE * | 17q23.3 | 4 | 1 |
| LAMB4 * | 7q31.1 | 3 | 1 |
| ITGAX * | 16p11.2 | 3 | 1 |
| LRP2 * | 2q31.1 | 4 | 1 |
| FBXW7 * | 4q31.3 | 4 | 1 |
| BRCA1 * | 17q21.31 | 4 | 1 |
| NSD1 * | 5q35.3 | 2 | 2 |
| NCOR1 * | 17p12-p11.2 | 2 | 1 |
| NLRC5 * | 16q13 | 1 | 1 |
| CENPF * | 1q41 | 1 | 1 |
| MSH6 * | 2p16.3 | 2 | 1 |
| ILF3* | 19p13.2 | 1 | 1 |
| CDH1 * | 16q22.1 | 1 | 2 |
| ERBB2 * | 19p13.3 | 1 | 1 |
| APC * | 17q12 | 2 | 1 |
| CAPN2 * | 5q22.2 | 1 | 1 |
| NFATC4 * | 1q41 | 1 | 1 |
| BUB1 * | 14q12 | 1 | 1 |
| HRAS * | 2q13 | 1 | 1 |
| ARHGEF1 * | 19q13.2 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otohinoyi, D.; Kuchi, A.; Wu, J.; Hicks, C. Integrating Genomic Information with Tumor-Immune Microenvironment in Triple-Negative Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 13901. https://doi.org/10.3390/ijerph192113901
Otohinoyi D, Kuchi A, Wu J, Hicks C. Integrating Genomic Information with Tumor-Immune Microenvironment in Triple-Negative Breast Cancer. International Journal of Environmental Research and Public Health. 2022; 19(21):13901. https://doi.org/10.3390/ijerph192113901
Chicago/Turabian StyleOtohinoyi, David, Aditi Kuchi, Jiande Wu, and Chindo Hicks. 2022. "Integrating Genomic Information with Tumor-Immune Microenvironment in Triple-Negative Breast Cancer" International Journal of Environmental Research and Public Health 19, no. 21: 13901. https://doi.org/10.3390/ijerph192113901
APA StyleOtohinoyi, D., Kuchi, A., Wu, J., & Hicks, C. (2022). Integrating Genomic Information with Tumor-Immune Microenvironment in Triple-Negative Breast Cancer. International Journal of Environmental Research and Public Health, 19(21), 13901. https://doi.org/10.3390/ijerph192113901






