A Case Study of Swine Wastewater Treatment via Electrochemical Oxidation by Ti4O7 Anode
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Analysis of Swine Wastewater
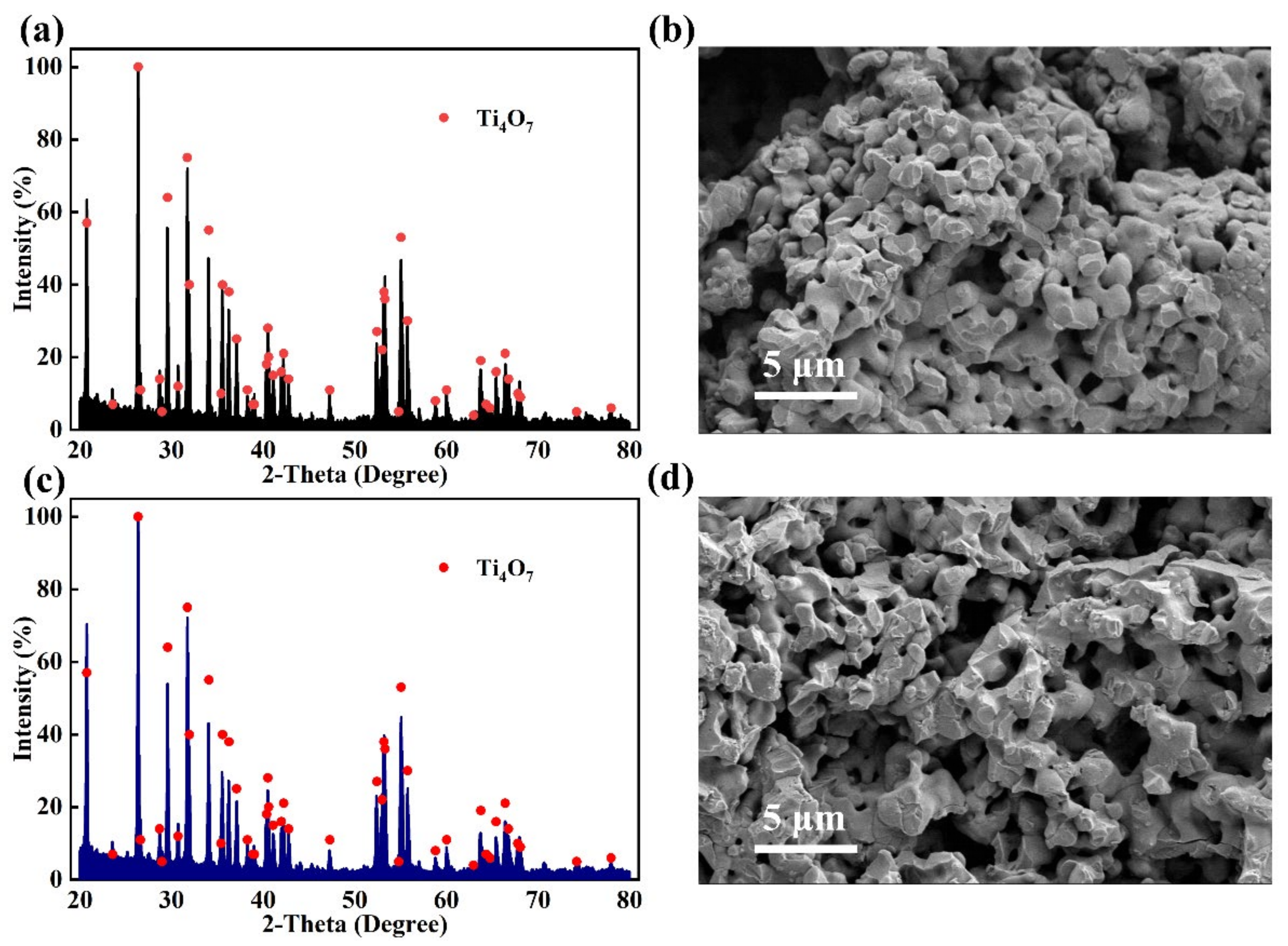
2.2. Ti4O7 Electrode Fabrication
2.3. Electrooxidation Experiments
3. Results and Discussion
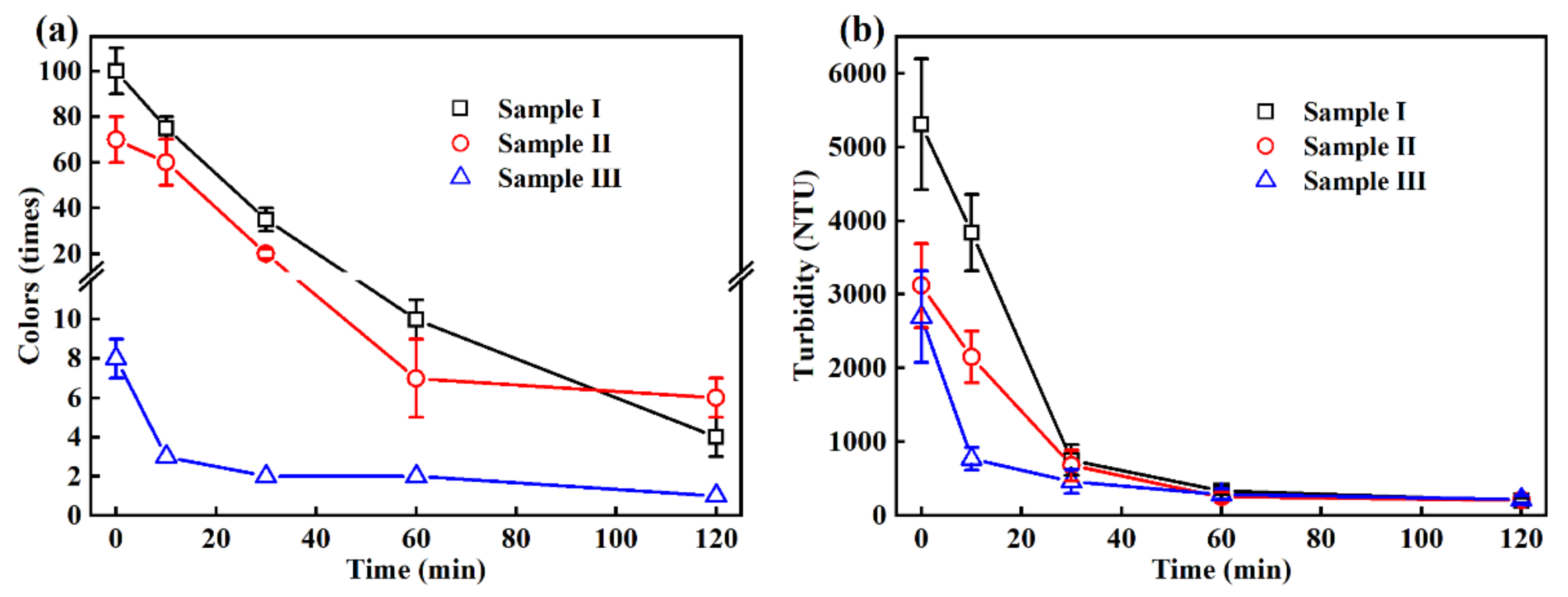
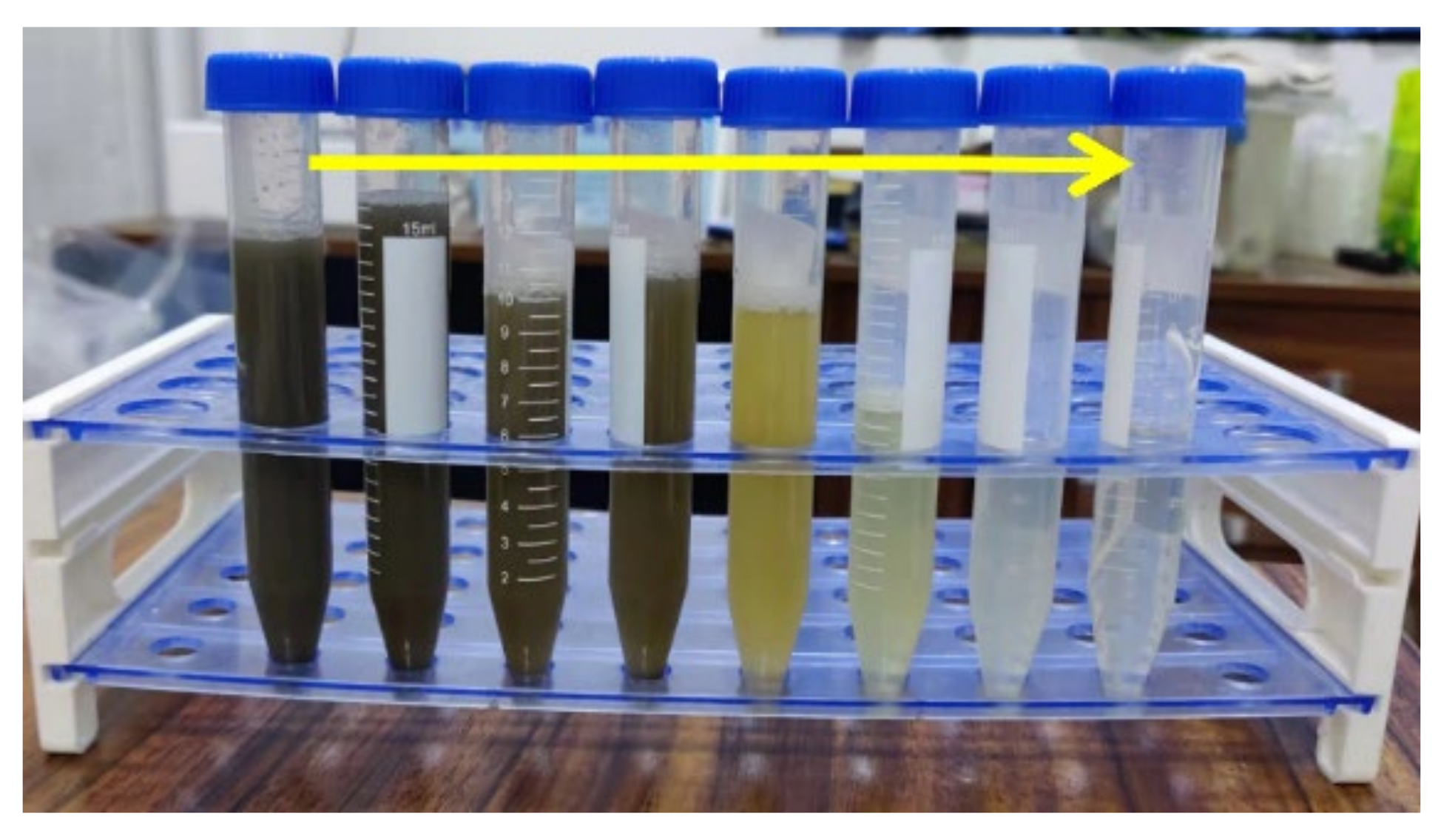
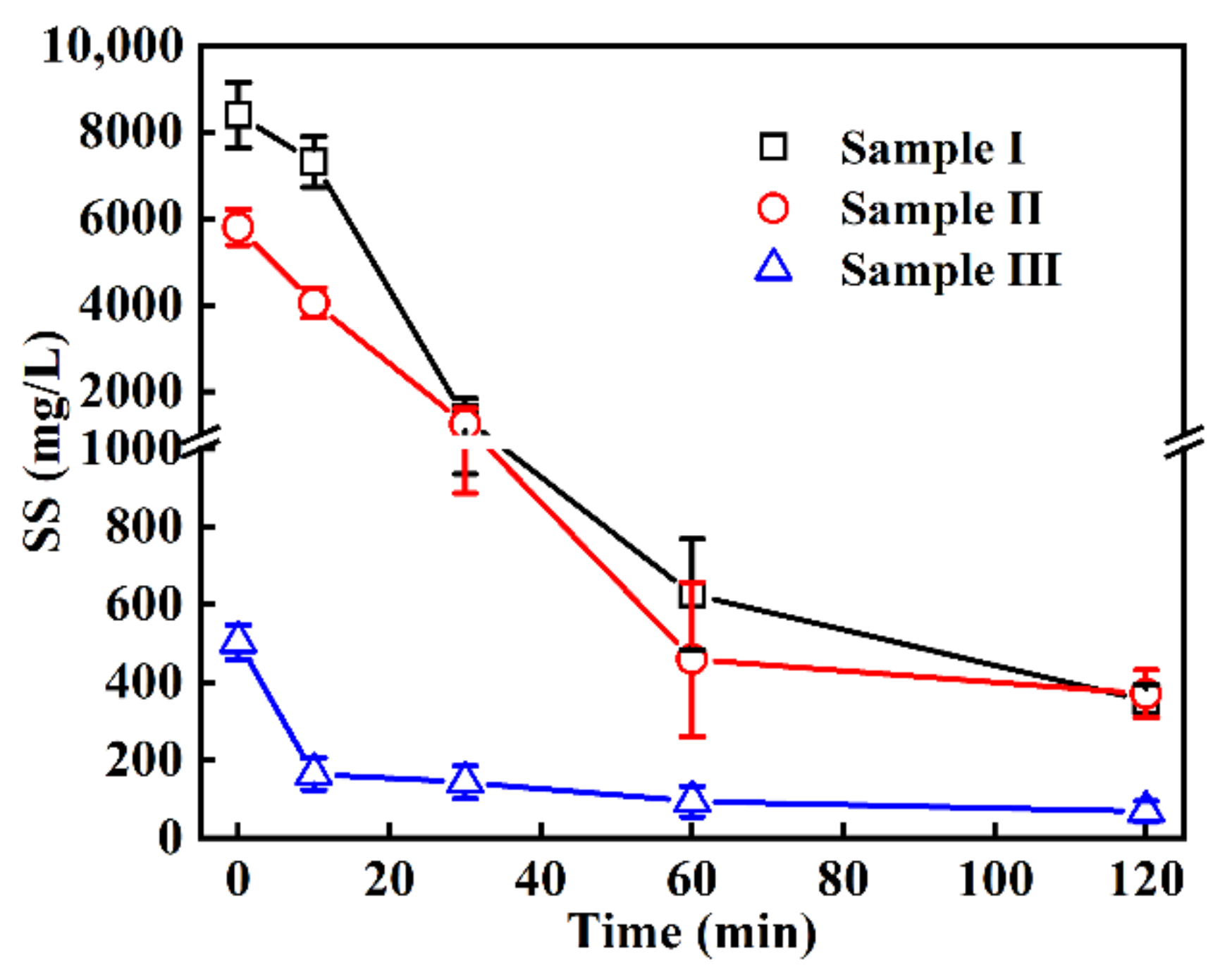
3.1. Changes in the Colors, Turbidity and SS in the EO Treatment
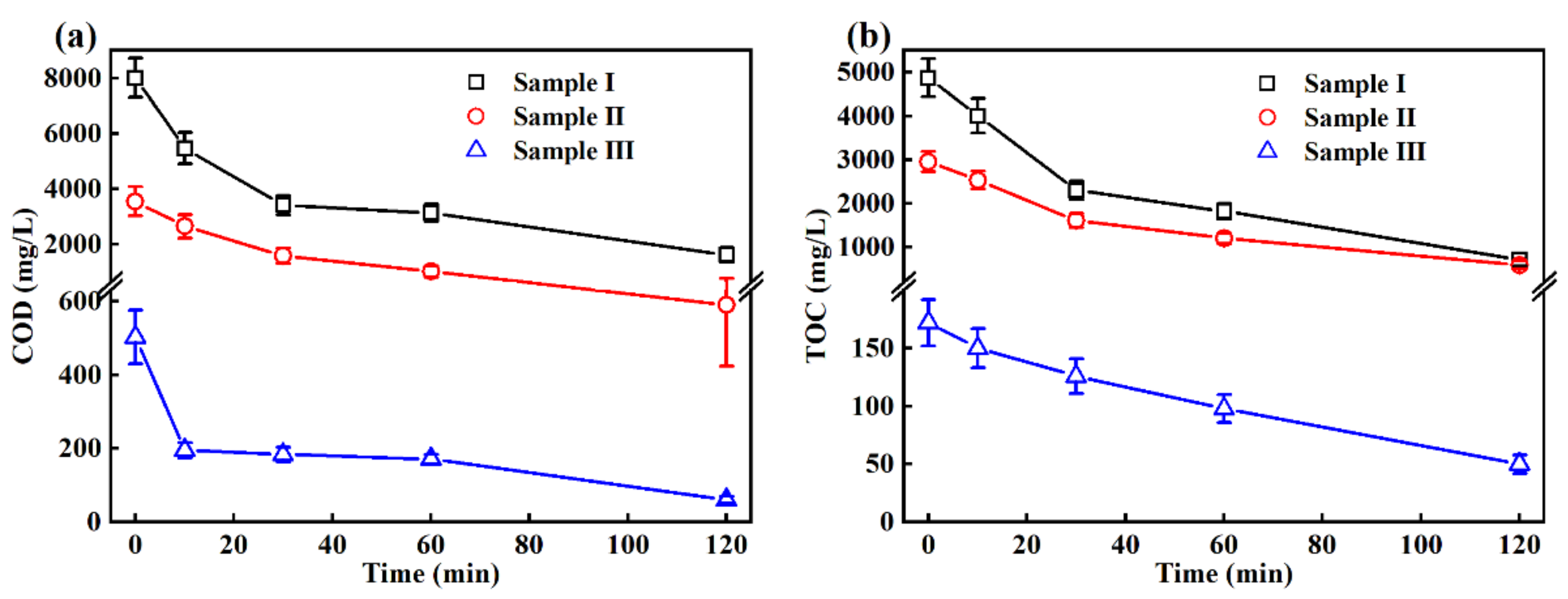
3.2. Removal of the COD and TOC in the EO Treatment
3.3. Removal of the NH3-N and TP in the EO Treatment
3.4. Changes in Excitation-Emission Matrix and UV-Vis Absorbance in EO Treatment
3.5. Stability Characterization of Anode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Feng, Y.; Li, Y.; Zhang, L.; Liu, J.; Li, N.; He, W. Effects of ammonia on electrochemical active biofilm in microbial electrolysis cells for synthetic swine wastewater treatment. Water Res. 2022, 219, 118570. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Ye, Z.L.; Chen, S.; Wei, Q.; Zhang, J.; Ye, X. Influences of dissolved organic matters on tetracyclines transport in the process of struvite recovery from swine wastewater. Water Res. 2018, 134, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, J.; Ye, Z.L.; Lin, L.; Hong, Z. Morphological crystal adsorbing tetracyclines and its interaction with magnesium ion in the process of struvite crystallization by using synthetic wastewater. Water Res. 2022, 215, 118253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Z.L.; Cai, J.; Lin, L. Quantification of DOM effects on tetracyclines transport during struvite recovery from swine wastewater. Water Res. 2021, 206, 117756. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.F.; Wang, Y.C. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Yang, Y.; Chen, C.; Xu, J. Effects of hydraulic loading rate on nutrients removal from anaerobically digested swine wastewater by multi soil layering treatment bioreactor. Int. J. Environ. Res. Public Health 2018, 15, 2688. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, H.; Liu, J.; Li, B.; Chang, Y.; Yao, D. A new green model for the bioremediation and resource utilization of livestock wastewater. Int. J. Environ. Res. Public Health 2021, 18, 8634. [Google Scholar] [CrossRef]
- Li, J.; Shao, B.; Shen, J.; Wang, S.; Wu, Y. Occurrence of chloramphenicol-resistance genes as environmental pollutants from swine feedlots. Environ. Sci. Technol. 2013, 47, 2892–2897. [Google Scholar] [CrossRef]
- Ye, Z.L.; Ghyselbrecht, K.; Monballiu, A.; Pinoy, L.; Meesschaert, B. Fractionating various nutrient ions for resource recovery from swine wastewater using simultaneous anionic and cationic selective-electrodialysis. Water Res. 2019, 160, 424–434. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Obotey Ezugbe, E.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO42− from oil refinery wastewater using a photo-catalytic system—Comparing TiO2 and zeolite efficiencies. Water 2020, 12, 214. [Google Scholar] [CrossRef]
- Gao, R.; Mosquera-Romero, S.; Ntagia, E.; Wang, X.; Rabaey, K.; Bonin, L. Review—Electrochemical separation of organic and inorganic contaminants in wastewater. J. Electrochem. Soc. 2022, 169, 033505. [Google Scholar] [CrossRef]
- Silva, D.B.; Arrais Junior, L.C.C.; Souza, A.A.G.; Silva, F.D.C.; Abrantes-Coutinho, V.E.; Santos, A.O.; Oliveira, T.M.B.F. Upcycling ferrous blast-furnace slag to design an effective ceramic anode for tartrazine yellow electrodegradation. Sustain. Mater. Technol. 2022, 31, e00373. [Google Scholar] [CrossRef]
- Balu, S.; Chuaicham, C.; Balakumar, V.; Rajendran, S.; Sasaki, K.; Sekar, K.; Maruthapillai, A. Recent development on core-shell photo(electro)catalysts for elimination of organic compounds from pharmaceutical wastewater. Chemosphere 2022, 298, 134311. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Ohashi, A.; Ozaki, N.; Shoiful, A.; Kindaichi, T. Pollutant removal from synthetic aqueous solutions with a combined electrochemical oxidation and adsorption method. Int. J. Environ. Res. Public Health 2018, 15, 1443. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.; Wang, J.; Jia, J. Improvement of electrochemical wastewater treatment through mass transfer in a seepage carbon nanotube electrode reactor. Environ. Sci. Technol. 2009, 43, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Vargas, R.; Borrás, C.; Méndez, D.; Mostany, J.; Scharifker, B.R. Electrochemical oxygen transfer reactions: Electrode materials, surface processes, kinetic models, linear free energy correlations, and perspectives. J. Solid State Electrochem. 2015, 20, 875–893. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Q.; Lei, L.; Ma, C.A.; Wang, D. Long life modified lead dioxide anode for organic wastewater treatment: Electrochemical characteristics and degradation mechanism. Environ. Sci. Technol. 2005, 39, 363–370. [Google Scholar] [CrossRef]
- Liang, S.; Lin, H.; Yan, X.; Huang, Q. Electro-oxidation of tetracycline by a Magnéli phase Ti4O7 porous anode: Kinetics, products, and toxicity. Chem. Eng. J. 2018, 332, 628–636. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Ye, J.; Wang, G. Electrochemical degradation of azo dye methyl orange by anodic oxidation on Ti4O7 electrodes. J. Mater. Sci. Mater. Electron. 2018, 29, 14065–14072. [Google Scholar] [CrossRef]
- Yu, M.; Saunders, T.; Grasso, S.; Mahajan, A.; Zhang, H.; Reece, M.J. Magnéli phase titanium suboxides by Flash Spark Plasma Sintering. Scr. Mater. 2018, 146, 241–245. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Lin, Z.; Yang, X. Electrochemical oxidation of methyl orange by a Magnéli phase Ti4O7 anode. Chemosphere 2020, 241, 125084. [Google Scholar] [CrossRef]
- Feng, H.; Chen, Z.; Wang, X.; Chen, S.; Crittenden, J. Electrochemical advanced oxidation for treating ultrafiltration effluent of a landfill leachate system: Impacts of organics and inorganics and economic evaluation. Chem. Eng. J. 2021, 413, 127492. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, P.; Ma, H.; Proietto, F.; Prestigiacomo, C.; Galia, A.; Scialdone, O. Electrochemical treatment of synthetic wastewaters contaminated by organic pollutants at Ti4O7 anode. Study of the role of operative parameters by experimental results and theoretical modelling. ChemElectroChem 2022, 9, e202101720. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, J.; Wang, J.; Luo, L.; Zhou, Y.; Zhou, Y. Electrochemical treatments of coking wastewater and coal gasification wastewater with Ti/Ti4O7 and Ti/RuO2-IrO2 anodes. J. Environ. Manag. 2020, 265, 110571. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Zhang, F.; Wang, Y.; Zhang, X.; Wang, J.; He, X. Comparison of Ti/Ti4O7, Ti/Ti4O7-PbO2-Ce, and Ti/Ti4O7 nanotube array anodes for electro-oxidation of p-nitrophenol and real wastewater. Sep. Purif. Technol. 2021, 266, 118600. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Kumar, A.; Syam Babu, D.; Scaria, J.; Suresh Kumar, M. Treatment of mixed industrial wastewater by electrocoagulation and indirect electrochemical oxidation. Chemosphere 2020, 251, 126437. [Google Scholar] [CrossRef]
- Yan-yang, C.; Yi, Q.; Mao-juan, B. Three advanced oxidation processes for the treatment of the wastewater from acrylonitrile production. Water Sci. Technol. 2009, 60, 2991–2999. [Google Scholar] [CrossRef]
- Leshem, E.N.; Pines, D.S.; Ergas, S.J.; Reckhow, D.A. Electrochemical oxidation and ozonation for textile wastewater reuse. J. Environ. Eng. 2006, 132, 324–330. [Google Scholar] [CrossRef]
- Fisher, R.; Le-Minh, N.; Alvarez-Gaitan, J.; Moore, S.; Stuetz, R. Emissions of volatile sulfur compounds (VSCs) throughout wastewater biosolids processing. Sci. Total Environ. 2018, 616, 622–631. [Google Scholar] [CrossRef]
- Cozic, A.; Viollier, E.; Chiffoleau, J.-F.; Knoery, J.; Rozuel, E. Interactions between volatile reduced sulfur compounds and metals in the Seine Estuary (France). Estuaries Coasts 2008, 31, 1063–1071. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, S.; Zhuang, P.; Xie, D.; Li, S.; Liu, D.; Li, Z.; Wang, F.; Xing, F. Assessment of the nutrient removal potential of floating native and exotic aquatic macrophytes cultured in swine manure wastewater. Int. J. Environ. Res. Public Health 2020, 17, 1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Zeng, W. Pretreatment for dissolved organic nitrogen testing by gas stripping. Toxicol. Environ. Chem. 2016, 98, 679–688. [Google Scholar] [CrossRef]
- Jafvert, C.T.; Valentine, R.L. Reaction scheme for the chlorination of ammoniacal water. Environ. Sci. Technol. 1992, 26, 577–586. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Ensano, B.M.B.; Yee, J.J. Fuzzy optimization for the remediation of ammonia: A case study based on electrochemical oxidation. Int. J. Environ. Res. Public Health 2021, 18, 2986. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Jiang, J.; Xia, G.; Chen, J. Process optimization of electrochemical treatment of COD and total nitrogen containing wastewater. Int. J. Environ. Res. Public Health 2022, 19, 850. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Xia, G.; Lu, Y.; Xu, D.; Sun, N.; Wang, J.; Chen, J. Process optimization of electrochemical oxidation of ammonia to nitrogen for actual dyeing wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 2931. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, H.; Wu, K.; Zhao, X.; Wang, F.; Liao, Q. Fines isolated from waste concrete as a new material for the treatment of phosphorus wastewater. Environ. Sci. Pollut. Res. Int. 2020, 27, 12539–12549. [Google Scholar] [CrossRef]
- Liu, D.; Quan, X.; Zhu, H.; Huang, Q.; Zhou, L. Evaluation of modified waste concrete powder used as a novel phosphorus remover. J. Clean. Prod. 2020, 257, 120646. [Google Scholar] [CrossRef]
- Hakimi, M.H.; Jegatheesan, V.; Navaratna, D. The potential of adopting struvite precipitation as a strategy for the removal of nutrients from pre-AnMBR treated abattoir wastewater. J. Environ. Manag. 2020, 259, 109783. [Google Scholar] [CrossRef]
- Dai, H.; Tan, X.; Zhu, H.; Sun, T.; Wang, X. Effects of commonly occurring metal ions on hydroxyapatite crystallization for phosphorus recovery from wastewater. Water 2018, 10, 1619. [Google Scholar] [CrossRef]
- Xing, C.; Shi, J.; Cui, F.; Shen, J.; Li, H. Fe2+/H2O2-Strengite method with the enhanced settlement for phosphorus removal and recovery from pharmaceutical effluents. Chemosphere 2021, 277, 130343. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, P.; Liu, Z.; Zhang, J.; Huang, X. Simultaneous recovery of phosphorus with nickel purification in nickel-plating wastewater via Fe/C activated H2O2 oxidation. Chem. Eng. J. 2020, 381, 122702. [Google Scholar] [CrossRef]
- Pan, Z.; Sheng, J.; Qiu, C.; Wei, H.; Yang, Q.; Pan, J.; Li, J. A magic filter filled with waste plastic shavings, loofah, and iron shavings for wastewater treatment. Polymers 2022, 14, 1410. [Google Scholar] [CrossRef]
- Chan, M.T.; Selvam, A.; Wong, J.W. Reducing nitrogen loss and salinity during ‘struvite’ food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef]
- Li, W.-T.; Chen, S.-Y.; Xu, Z.-X.; Li, Y.; Shuang, C.-D.; Li, A.-M. Characterization of dissolved organic matter in municipal wastewater using fluorescence PARAFAC analysis and chromatography multi-excitation/emission scan: A comparative study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef]
- Lin, H.; Peng, H.; Feng, X.; Li, X.; Zhao, J.; Yang, K.; Liao, J.; Cheng, D.; Liu, X.; Lv, S.; et al. Energy-efficient for advanced oxidation of bio-treated landfill leachate effluent by reactive electrochemical membranes (REMs): Laboratory and pilot scale studies. Water Res. 2021, 190, 116790. [Google Scholar] [CrossRef]
- Yan, S.; Liu, Y.; Lian, L.; Li, R.; Ma, J.; Zhou, H.; Song, W. Photochemical formation of carbonate radical and its reaction with dissolved organic matters. Water Res. 2019, 161, 288–296. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 8850. [Google Scholar] [CrossRef]
- Liu, H.J.; Luo, M.Q.; Yang, L.X.; Zeng, C.L.; Fu, C. A high strength and conductivity bulk Magnéli phase Ti4O7 with superior electrochemical performance. Ceram. Int. 2022, 48, 25538–25546. [Google Scholar] [CrossRef]
- Li, W.; Xiao, R.; Xu, J.; Lin, H.; Yang, K.; Li, W.; He, K.; Tang, L.; Chen, J.; Wu, Y.; et al. Interface engineering strategy of a Ti4O7 ceramic membrane via graphene oxide nanoparticles toward efficient electrooxidation of 1,4-dioxane. Water Res. 2022, 216, 118287. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect engineering on a Ti4O7 electrode by Ce3+ doping for the efficient electrooxidation of perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef] [PubMed]


| Water Parameters | Sample I | Sample II | Sample III | Discharge Standard (GB 18596–2001) |
|---|---|---|---|---|
| Colors (times) | 100 ± 10 | 70 ± 10 | 8 ± 1 | - |
| Turbidity (NTU) | 5310 ± 889 | 3119 ± 568 | 2697 ± 622 | - |
| SS (mg/L) | 8393 ± 759 | 5794 ± 407 | 503 ± 45 | 200 |
| COD (mg/L) | 8014 ± 708 | 3550 ± 532 | 503 ± 73 | 400 |
| TOC (mg/L) | 4875 ± 435 | 2958 ± 235 | 172 ± 20 | - |
| NH3-N (in N, mg/L) | 853 ± 61 | 684 ± 48 | 37 ± 3.8 | 80 |
| TP (in P, mg/L) | 316 ± 25 | 106 ± 10 | 106 ± 8.2 | 8 |
| ECCOD (kWh/kgCOD) | Sample I | Sample II | Sample III |
|---|---|---|---|
| period I | 8.15 | 20.42 | 125.39 |
| period II | 70.79 | 130.37 | 1024.39 |
| Wastewater | UV254 | UV410 | UV250/365 | |
|---|---|---|---|---|
| Sample I | Pristine | 0.627 | 0.165 | 2.947 |
| After 30 min treatment | 0.409 | 0.082 | 3.523 | |
| After 120 min treatment | 0.147 | 0.013 | 7.533 | |
| Sample II | Pristine | 0.547 | 0.148 | 2.824 |
| After 30 min treatment | 0.268 | 0.047 | 3.827 | |
| After 120 min treatment | 0.145 | 0.011 | 7.800 | |
| Sample III | Pristine | 0.778 | 0.184 | 2.979 |
| After 30 min treatment | 0.466 | 0.067 | 4.772 | |
| After 120 min treatment | 0.290 | 0.052 | 4.451 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Wang, R.; Wang, B.; Zhang, K.; Shi, H.; Wang, H. A Case Study of Swine Wastewater Treatment via Electrochemical Oxidation by Ti4O7 Anode. Int. J. Environ. Res. Public Health 2022, 19, 13840. https://doi.org/10.3390/ijerph192113840
Wan H, Wang R, Wang B, Zhang K, Shi H, Wang H. A Case Study of Swine Wastewater Treatment via Electrochemical Oxidation by Ti4O7 Anode. International Journal of Environmental Research and Public Health. 2022; 19(21):13840. https://doi.org/10.3390/ijerph192113840
Chicago/Turabian StyleWan, Hongyou, Ruifeng Wang, Beibei Wang, Kehao Zhang, Huanhuan Shi, and Hailong Wang. 2022. "A Case Study of Swine Wastewater Treatment via Electrochemical Oxidation by Ti4O7 Anode" International Journal of Environmental Research and Public Health 19, no. 21: 13840. https://doi.org/10.3390/ijerph192113840
APA StyleWan, H., Wang, R., Wang, B., Zhang, K., Shi, H., & Wang, H. (2022). A Case Study of Swine Wastewater Treatment via Electrochemical Oxidation by Ti4O7 Anode. International Journal of Environmental Research and Public Health, 19(21), 13840. https://doi.org/10.3390/ijerph192113840







