Glycemic and Insulinemic Responses of Healthy Humans to a Nutrition Bar with or without Added Fibersym® RW, a Cross-Linked Phosphorylated RS4-Type Resistant Wheat Starch
Abstract
1. Introduction
2. Materials and Methods
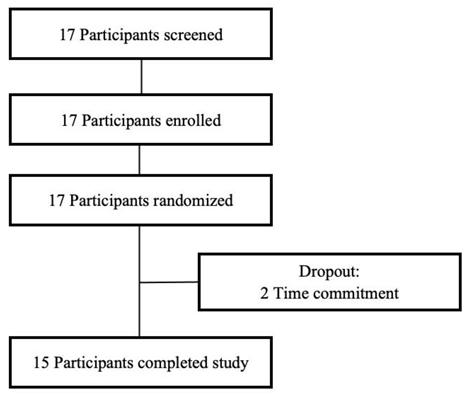
2.1. Recruitment of Participants
2.2. Blood Analysis
2.3. Statistical Analysis
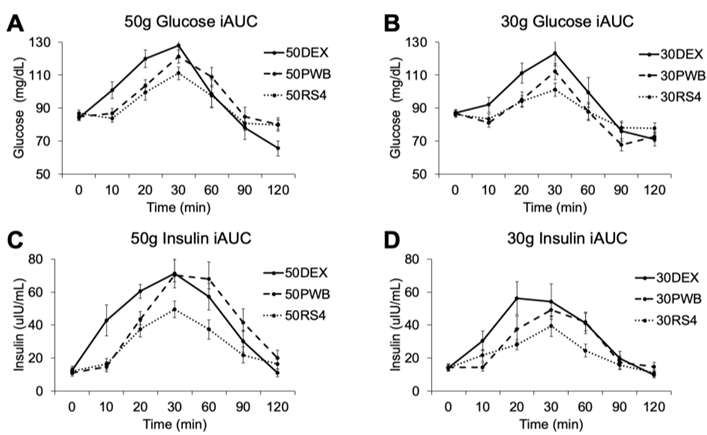
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weickert, M.; Pfeiffer, A.F.H. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Dayib, M.; Larson, J.; Slavin, J. Dietary fibers reduce obesity-related disorders: Mechanisms of action. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans, 2020–2025, 9th ed.; US Department of Health and Human Services and US Department of Agriculture: Washington, DC, USA, 2020. [Google Scholar]
- Merenkova, S.; Zinina, O.; Stuart, M.; Okuskhanova, E.; Androsova, N. Effects of Dietary Fiber on Human Health: A review. Human. Sport. Med. 2020, 20, 106–113. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch-Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- US Food and Drug Administration, 21 C.F.R Sect. 101.9(c)(6)(i). Docket No. FDA-2016-P-3620-0017: Third Addendum to Citizen Petition Requesting that FDA Amend the Definition of Dietary Fiber at 21 C.F.R. §101.9(c)(6)(i) to Include Modified Wheat Starch (Fibersym® RW and FiberRite® RW). 2019. Available online: https://www.regulations.gov/docket/FDA-2016-P-3620 (accessed on 16 September 2022).
- Al-Tamimi, E.K.; Seib, P.A.; Snyder, B.S.; Haub, M.D. Consumption of cross-linked resistant starch (RS4XL) on glucose and insulin responses in humans. J. Nutr. Metab. 2010, 2010, 651063. [Google Scholar] [CrossRef] [PubMed]
- Emilien, C.H.; Hsu, W.H.; Hollis, J.H. Effect of resistant wheat starch on subjective appetite and food intake in healthy adults. Nutrition 2017, 43–44, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gourineni, V.; Stewart, M.L.; Wilcox, M.L.; Maki, K.C. Nutritional Bar with Potato-Based Resistant Starch Attenuated Post-Prandial Glucose and Insulin Response in Healthy Adults. Foods 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Haub, M.D.; Hubach, K.L.; Al-Tamimi, E.K.; Ornelas, S.; Seib, P.A. Different Types of Resistant Starch Elicit Different Glucose Reponses in Humans. J. Nutr. Metab. 2010, 2010, 230501. [Google Scholar] [CrossRef] [PubMed]
- Haub, M.D.; Louk, J.A.; Lopez, T.C. Novel Resistant Potato Starches on Glycemia and Satiety in Humans. J. Nutr. Metab. 2012, 2012, 478043. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.J.; Maningat, C.C.; Seib, P.A.; Haub, M.D.; Rosenkranz, S.K. Metabolic Responses to Native Wheat Starch (MidsolTM 50) versus Resistant Wheat Starch Type 4 (Fibersym® RW): Standard versus Marketplace Testing Protocols. Curr. Dev. Nutr. 2021, 5, nzab011. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Zimmer, J.P. A High Fiber Cookie Made with Resistant Starch Type 4 Reduces Post-Prandial Glucose and Insulin Responses in Healthy Adults. Nutrients 2017, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Wilcox, M.L.; Bell, M.; Buggia, M.A.; Maki, K.C. Type-4 Resistant Starch in Substitution for Available Carbohydrate Reduces Postprandial Glycemic Response and Hunger in Acute, Randomized, Double-Blind, Controlled Study. Nutrients 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Zimmer, J.P. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: A double-blind, randomized, controlled trial. Nutrition 2018, 53, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Garcia-Campayo, V.; Liska, D. Substitution of Corn Starch with Resistant Starch Type 4 in a Breakfast Bar Decreases Postprandial Glucose and Insulin Responses: A Randomized, Controlled, Crossover Study. Curr. Dev. Nutr. 2018, 2, nzy066. [Google Scholar] [CrossRef] [PubMed]
- Tachibe, M.; Nishibata, T.; Oga, H.; Ebihara, K. Resistant starch type 4, cross-linked phosphate starch and hydroxypropyl distarch phosphate attenuate rapid glycemic response in men. Jpn. Pharmacol. Ther. 2010, 38, 731–736. [Google Scholar]
- Du, Y.; Wu, Y.; Xiao, D.; Guzman, G.; Stewart, M.L.; Gourineni, V.; Burton-Freeman, B.; Edirisinghe, I. Food prototype containing resistant starch type 4 on postprandial glycemic response in healthy adults. Food Funct. 2020, 11, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Planning of Experiments; John Wiley & Sons: New York, NY, USA, 1958. [Google Scholar]
- Cochrane, W.G.; Cox, G.M. Experimental Designs; John Wiley & Sons: New York, NY, USA, 1957. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lenith, R. Estimated Marginal Means, Aka Least-Squares Means. R package Version 1.8.1-1. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 19 October 2022).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed; Springer: New York, NY, USA, 2002. [Google Scholar]
- Purves, R.D. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-themoment-curve (AUMC). J. Pharm. Biopharm 1992, 20, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Office of Nutrition and Food Labeling; Center for Food Safety and Applied Nutrition; U.S Food and Drug Administration. Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments: Guidance for Industry Small Entity Compliance Guide; FDA-2004-N-0258; 2018. Available online: http://www.fda.gov/FoodGuidances (accessed on 16 September 2022).
- U.S. Food and Drug Administration. Health Claims: General Requirements; 21 CFR 101.14; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- Morgan, L.M.; Shi, J.-W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
| Component | 50PWB | 50RS4 | 30PWB | 30RS4 |
|---|---|---|---|---|
| Ash (g) | 1.7 | 1.7 | 1.1 | 0.9 |
| Moisture (g) | 14.9 | 16.9 | 8.9 | 10.0 |
| Carbohydrate (g) | 62.0 | 79.7 | 37.3 | 47.8 |
| Digestible carbohydrate (g) 1 | 50.0 | 50.0 | 30.0 | 30.0 |
| Dietary fiber (g) | 12.0 | 29.7 | 7.3 | 17.9 |
| Fat (g) | 3.3 | 2.1 | 2.0 | 1.3 |
| Protein (g) | 9.9 | 6.0 | 5.8 | 3.6 |
| Total calories (KJ) (kcal) | 1126.9 (269.2) | 1017.2 (243.0) | 676.0 (161.5) | 609.9 (145.7) |
| Weight provided to participants (g) | 91.7 | 106.4 | 55.0 | 63.8 |
| All (n = 15) | |
|---|---|
| Sex (Male;Female) | 8;7 |
| Age (years) | 26.1 ± 4.8 |
| Height (cm) | 174.0 ± 8.8 |
| Weight (kg) | 76.1 ± 16.8 |
| BMI (kg/m2) | 24.9 ± 4.0 |
| Body Fat (DXA, %) 1 | 23.6 ± 9.3 |
| Fasting Glucose (mg/dL) | 84.0 ± 5.77 |
| Fasting Insulin (μIU/mL) | 12.6 ± 9.29 |
| Treatment | Dose | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DEX | PWB | RS4 | 30 g | 50 g | p | |
| Glucose | iAUC (mg/dL × 2 h) | 1677 * (1134, 2325) | 1160 * (721, 1703) | 692 (365, 1123) | 824 (480, 1260) | 1508 (1020, 2093) | <0.001 |
| Peak (mg/dL) | 132 * (124, 142) | 119 * (111, 127) | 108 (101, 115) | 115 (108, 122) | 123 (116, 132) | 0.01 | |
| Baseline-to-peak (mg/dL) | 41.1 * (29.2, 58.0) | 27.8 * (19.8, 39.1) | 17.1 (12.2, 24.1) | 22.0 (16.1, 30.0) | 33.1 (24.0, 45.5) | 0.005 | |
| Time-to-peak (Minutes) | 27.5 (23.3, 32.6) | 34.3 (29.0, 40.5) | 32.0 (27.0, 37.8) | 32.9 (28.3, 38.3) | 29.5 (25.2, 34.4) | 0.12 | |
| Insulin | iAUC (μIU/mL × 2 h) | 2642 * (1993, 3382) | 2574 * (1934, 3305) | 1419 (953, 1978) | 1593 (1126, 2142) | 2840 (2198, 3564) | <0.001 |
| Peak (μIU/mL) | 67.3 * (54.8, 82.7) | 62.2 * (50.6, 76.4) | 45.6 (37.1, 56.0) | 51.0 (41.9, 62.1) | 65 (53.3, 79.2) | <0.001 | |
| Baseline-to-peak (μIU/mL) | 54.4 * (43.8, 67.4) | 50.6 * (40.9, 62.5) | 31.3 (25.3, 38.8) | 35.8 (29.5, 43.4) | 54.5 (44.6, 66.4) | <0.001 | |
| Time-to-peak (Minutes) | 27.6 (23.3, 32.7) | 38.6 (32.7, 45.6) | 33.0 (27.9, 39.0) | 31.3 (27.2, 35.9) | 34.3 (29.6, 39.8) | 0.30 | |
| Indexes | HOMA-IR 1 | 2.50 (1.88, 3.31) | 2.34 (1.77, 3.10) | 2.40 (1.81, 3.18) | 2.66 (2.03, 3.49) | 2.18 (1.66, 2.87) | 0.01 |
| Insulin:Glucose 2 | 1.95 (1.09, 3.49) | 3.01 (1.69, 5.36) | 2.75 (1.54, 4.89) | 2.55 (1.48, 4.39) | 2.50 (1.44, 4.34) | 0.92 | |
| 30 g | 50 g | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DEX | PWB | RS4 | DEX | PWB | RS4 | |
| Glucose | iAUC (mg/dL × 2 h) | 1432 * (883, 2114) | 693 * (331, 1188) | 481 (186, 914) | 1940 * (1249, 2783) | 1746 * (1109, 2528) | 941 (501, 1519) |
| Peak (mg/dL) | 130 * (120, 140) | 114 * (105, 123) | 103 (95.5, 112) | 135 * (124,147) | 125 * (114.7, 135) | 112 (103.2, 121) | |
| Baseline-to-peak (mg/dL) | 34.5 * (23.1, 51.5) | 22.0 * (14.7, 32.8) | 14.0 (9.3, 21.1) | 49.0 * (31.7, 75.8) | 35.1 * (23.0, 53.7) | 21.0 (13.9, 31.6) | |
| Time-to-peak (Minutes) | 31.8 * (26.1, 38.7) | 32.6 * (26.8, 39.8) | 34.3 (28.0, 42.1) | 23.9 * (19.2, 29.7) | 36 * (29.2, 44.4) | 29.7 (24.2, 36.4) | |
| Insulin | iAUC (μIU/mL × 2 h) | 2184 * (1519, 2970) | 1789 * (1193, 2506) | 943 (519, 1493) | 3143 * (2317, 4095) | 3501 * (2625, 4502) | 1993 (1347, 2764) |
| Peak (μIU/mL) | 59.1 * (47.0, 74.3) | 55.4 * (44.0, 69.6) | 40.6 (32.1, 51.2) | 76.8 * (60.8, 96.9) | 69.8 * (55.3, 88.2) | 51.2 (40.5, 64.6) | |
| Baseline-to-peak (μIU/mL) | 45.7 * (35.4, 58.8) | 40.7 * (31.6, 52.4) | 24.7 (19.1, 32.1) | 64.7 * (49.1, 85.4) | 62.9 * (48.1, 82.2) | 39.7 (30.4, 52.9) | |
| Time-to-peak (Minutes) | 29.5 * (23.8, 36.7) | 36.1 * (29.1, 44.8) | 28.7 (22.9, 35.9) | 25.8 * (20.3, 32.9) | 41.3 * (32.7, 52.0) | 38 (30.1, 47.9) | |
| Indexes | HOMA-IR 1 | 2.64 (1.94, 3.5) | 2.70 (1.98, 3.67) | 2.65 (1.95, 3.60) | 2.36 (1.73, 3.23) | 2.03 (1.50, 2.77) | 2.17 (1.59, 2.96) |
| Insulin:Glucose 2 | 1.98 (1.03, 3.80) | 3.48 (1.81, 6.68) | 2.41 (1.24, 4.69) | 1.92 (0.95, 3.86) | 2.60 (1.32, 5.15) | 3.13 (1.61, 6.10) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steele, T.J.; Steele, C.C.; Maningat, C.C.; Seib, P.A.; Haub, M.D.; Rosenkranz, S.K. Glycemic and Insulinemic Responses of Healthy Humans to a Nutrition Bar with or without Added Fibersym® RW, a Cross-Linked Phosphorylated RS4-Type Resistant Wheat Starch. Int. J. Environ. Res. Public Health 2022, 19, 13804. https://doi.org/10.3390/ijerph192113804
Steele TJ, Steele CC, Maningat CC, Seib PA, Haub MD, Rosenkranz SK. Glycemic and Insulinemic Responses of Healthy Humans to a Nutrition Bar with or without Added Fibersym® RW, a Cross-Linked Phosphorylated RS4-Type Resistant Wheat Starch. International Journal of Environmental Research and Public Health. 2022; 19(21):13804. https://doi.org/10.3390/ijerph192113804
Chicago/Turabian StyleSteele, Trevor J., Catherine C. Steele, Clodualdo C. Maningat, Paul A. Seib, Mark D. Haub, and Sara K. Rosenkranz. 2022. "Glycemic and Insulinemic Responses of Healthy Humans to a Nutrition Bar with or without Added Fibersym® RW, a Cross-Linked Phosphorylated RS4-Type Resistant Wheat Starch" International Journal of Environmental Research and Public Health 19, no. 21: 13804. https://doi.org/10.3390/ijerph192113804
APA StyleSteele, T. J., Steele, C. C., Maningat, C. C., Seib, P. A., Haub, M. D., & Rosenkranz, S. K. (2022). Glycemic and Insulinemic Responses of Healthy Humans to a Nutrition Bar with or without Added Fibersym® RW, a Cross-Linked Phosphorylated RS4-Type Resistant Wheat Starch. International Journal of Environmental Research and Public Health, 19(21), 13804. https://doi.org/10.3390/ijerph192113804







