Pulsed-Xenon Ultraviolet Light Highly Inactivates Human Coronaviruses on Solid Surfaces, Particularly SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains
2.2. Cell Lines
2.3. Inactivation of Coronavirus by UV-Irradiation
2.4. Quantification of the Irradiance Supplied to the Experimental Samples
2.5. Statistical Analysis
3. Results
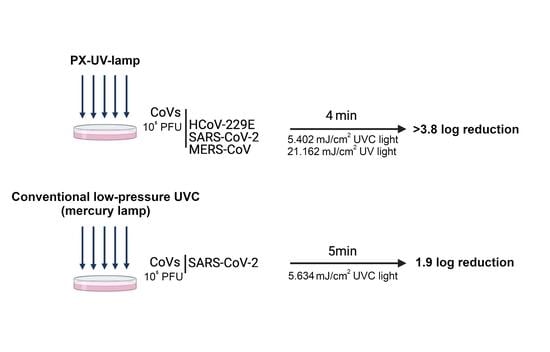
3.1. Inactivation of CoVs on Contaminated Surfaces Using PX-UV Irradiation
3.2. Comparison between PX-UV or Conventional UVC Radiation in the Inactivation of SARS-CoV-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rabenau, H.F.; Cinatl, J.; Morgenstern, B.; Bauer, G.; Preiser, W.; Doerr, H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005, 194, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ball, J.K.; Alexander, C.; Alexander, M.R. All Surfaces Are Not Equal in Contact Transmission of SARS-CoV-2. Matter 2020, 3, 1433–1441. [Google Scholar] [CrossRef]
- Hadi, J.; Dunowska, M.; Wu, S.; Brightwell, G. Control measures for SARS-CoV-2: A review on light-based inactivation of single-stranded RNA viruses. Pathogens 2020, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Ball, A.R.; Baptista, M.S.; Dai, T.; Hamblin, M.R.; Ribeiro, M.S.; Santos, A.L.; Sellera, F.P.; Tegos, G.P.; Wainwright, M. Light-based technologies for management of COVID-19 pandemic crisis. J. Photochem. Photobiol. B Biol. 2020, 212, 111999. [Google Scholar] [CrossRef]
- Browne, K. Brought to Light: How Ultraviolet Disinfection Can Prevent the Nosocomial Transmission of COVID-19 and Other Infectious Diseases. Appl. Microbiol. 2021, 1, 537–556. [Google Scholar] [CrossRef]
- Jean, J.; Rodríguez-López, M.I.; Jubinville, E.; Núñez-Delicado, E.; Gómez-López, V.M. Potential of pulsed light technology for control of SARS-CoV-2 in hospital environments. J. Photochem. Photobiol. B Biol. 2021, 215, 112106. [Google Scholar] [CrossRef]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Kowalski, W.J. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Springer: New York, NY, USA, 2009; ISBN 978-3-642-01998-2. [Google Scholar]
- Chiappa, F.; Frascella, B.; Vigezzi, G.P.; Moro, M.; Diamanti, L.; Gentile, L.; Lago, P.; Clementi, N.; Signorelli, C.; Mancini, N.; et al. The efficacy of ultraviolet light-emitting technology against coronaviruses: A systematic review. J. Hosp. Infect. 2021, 114, 63–78. [Google Scholar] [CrossRef]
- Stibich, M.; Stachowiak, J. The microbiological impact of pulsed xenon ultraviolet disinfection on resistant bacteria, bacterial spore and fungi and viruses. South. Afr. J. Infect. Dis. 2016, 31, 12–15. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.E.; Carrion, R.; Alfson, K.J.; Staples, H.M.; Jinadatha, C.; Jarvis, W.R.; Sampathkumar, P.; Chemaly, R.F.; Khawaja, F.; Povroznik, M.; et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: Implications for environmental COVID-19 control. Infect. Control Hosp. Epidemiol. 2021, 42, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Jureka, A.S.; Williams, C.G.; Basler, C.F. Pulsed Broad-Spectrum UV Light Effectively Inactivates SARS-CoV-2 on Multiple Surfaces and N95 Material. Viruses 2021, 13, 460. [Google Scholar] [CrossRef]
- Inagaki, H.; Saito, A.; Kaneko, C.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid inactivation of SARS-CoV-2 variants by continuous and intermittent irradiation with a deep-ultraviolet light-emitting diode (DUV-LED) device. Pathogens 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.M.; Romero, C.; Vergara-Alert, J.; Bello-Perez, M.; Rodon, J.; Honrubia, J.M.; Segalé, J.; Sola, I.; Enjuanes, L.; Gajardo, R. Cross-neutralization activity against SARS-CoV-2 is present in currently available intravenous immunoglobulins. Immunotherapy 2020, 12, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Snijder, E.J.; Decroly, E.; Ziebuhr, J. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv. Virus Res. 2016, 96, 59–126. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Wilbrink, B.; Pyrc, K.; Zaaijer, H.L.; Minor, P.D.; Franklin, S.; Berkhout, B.; Thiel, V.; van der Hoek, L. Human coronavirus 229E encodes a single ORF4 protein between the spike and the envelope genes. Virol. J. 2006, 3, 106. [Google Scholar] [CrossRef]
- Woo, P.C.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Coronavirus genomics and bioinformatics analysis. Viruses 2010, 2, 1804–1820. [Google Scholar] [CrossRef]
- Kandeel, M.; Ibrahim, A.; Fayez, M.; Al-Nazawi, M. From SARS and MERS CoVs to SARS-CoV-2: Moving toward more biased codon usage in viral structural and nonstructural genes. J. Med. Virol. 2020, 92, 660–666. [Google Scholar] [CrossRef]
- Lorenz, C.M.; Wolk, B.M.; Quan, C.P.; Alcala, E.W.; Eng, M.; McDonald, D.J.; Matthews, T.C. The effect of low intensity ultraviolet-C light on monoclonal antibodies. Biotechnol. Prog. 2009, 25, 476–482. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Chu, V.C.; Whittaker, G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA 2009, 106, 5871–5876. [Google Scholar] [CrossRef] [PubMed]
- Khazova, M.; Johnstone, L.; Naldzhiev, D.; O’Hagan, J.B. Survey of Home-Use UV Disinfection Products†. Photochem. Photobiol. 2021, 97, 560–565. [Google Scholar] [CrossRef]
- Darnell, M.E.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef] [PubMed]


| PX-UV-C Irradiation Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.5 | 1 | 2 | 3 | 4 | 5 | ||
| HCoV-229E | Mean titer (PFU/mL) | 2.1 × 105 ± 2.1 × 104 | np | 6.5 × 102 ± 71 | 9.5 × 10 ± 49 | 4.5 × 10 ± 71 | nd | nd |
| Log reduction | np | 2.5 | 3.4 | 3.7 | >4.0 | >4.0 | ||
| SARS-CoV-2 | Mean titer (PFU/mL) | 1.2 × 105 ± 4.9 × 103 | 4.0 × 104 ± 0.0 | 1.4 × 103 ± 13 | 1.0 × 102 ± 0.0 | nd | nd | nd |
| Log reduction | 0.5 | 0.9 | 3.1 | >3.8 | >3.8 | >3.8 | ||
| MERS-CoV | Mean titer (PFU/mL) | 2.2 × 105 ± 1.1 × 104 | 8.0 × 102 ± 28 | 6.0 × 10 ± 57 | nd | nd | nd | nd |
| Log reduction | 2.4 | 3.6 | >4.0 | >4.0 | >4.0 | >4.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello-Perez, M.; Esparza, I.; De la Encina, A.; Bartolome, T.; Molina, T.; Sanjuan, E.; Falco, A.; Enjuanes, L.; Sola, I.; Usera, F. Pulsed-Xenon Ultraviolet Light Highly Inactivates Human Coronaviruses on Solid Surfaces, Particularly SARS-CoV-2. Int. J. Environ. Res. Public Health 2022, 19, 13780. https://doi.org/10.3390/ijerph192113780
Bello-Perez M, Esparza I, De la Encina A, Bartolome T, Molina T, Sanjuan E, Falco A, Enjuanes L, Sola I, Usera F. Pulsed-Xenon Ultraviolet Light Highly Inactivates Human Coronaviruses on Solid Surfaces, Particularly SARS-CoV-2. International Journal of Environmental Research and Public Health. 2022; 19(21):13780. https://doi.org/10.3390/ijerph192113780
Chicago/Turabian StyleBello-Perez, Melissa, Iris Esparza, Arancha De la Encina, Teresa Bartolome, Teresa Molina, Elena Sanjuan, Alberto Falco, Luis Enjuanes, Isabel Sola, and Fernando Usera. 2022. "Pulsed-Xenon Ultraviolet Light Highly Inactivates Human Coronaviruses on Solid Surfaces, Particularly SARS-CoV-2" International Journal of Environmental Research and Public Health 19, no. 21: 13780. https://doi.org/10.3390/ijerph192113780
APA StyleBello-Perez, M., Esparza, I., De la Encina, A., Bartolome, T., Molina, T., Sanjuan, E., Falco, A., Enjuanes, L., Sola, I., & Usera, F. (2022). Pulsed-Xenon Ultraviolet Light Highly Inactivates Human Coronaviruses on Solid Surfaces, Particularly SARS-CoV-2. International Journal of Environmental Research and Public Health, 19(21), 13780. https://doi.org/10.3390/ijerph192113780