Long-Term Examination of Water Chemistry Changes Following Treatment of Cyanobacterial Bloom with Coagulants and Minerals
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Water Sampling
2.2. Coagulants and Minerals
2.3. Jar Test
2.4. Experimental Methods
2.5. Analytical Methods
3. Results and Discussion
3.1. Cyanobacterial Removal Efficiencies of the Coagulants and Minerals
3.2. Changes in Water Chemistry Following Treatment with Coagulants and Minerals
3.2.1. Turbidity
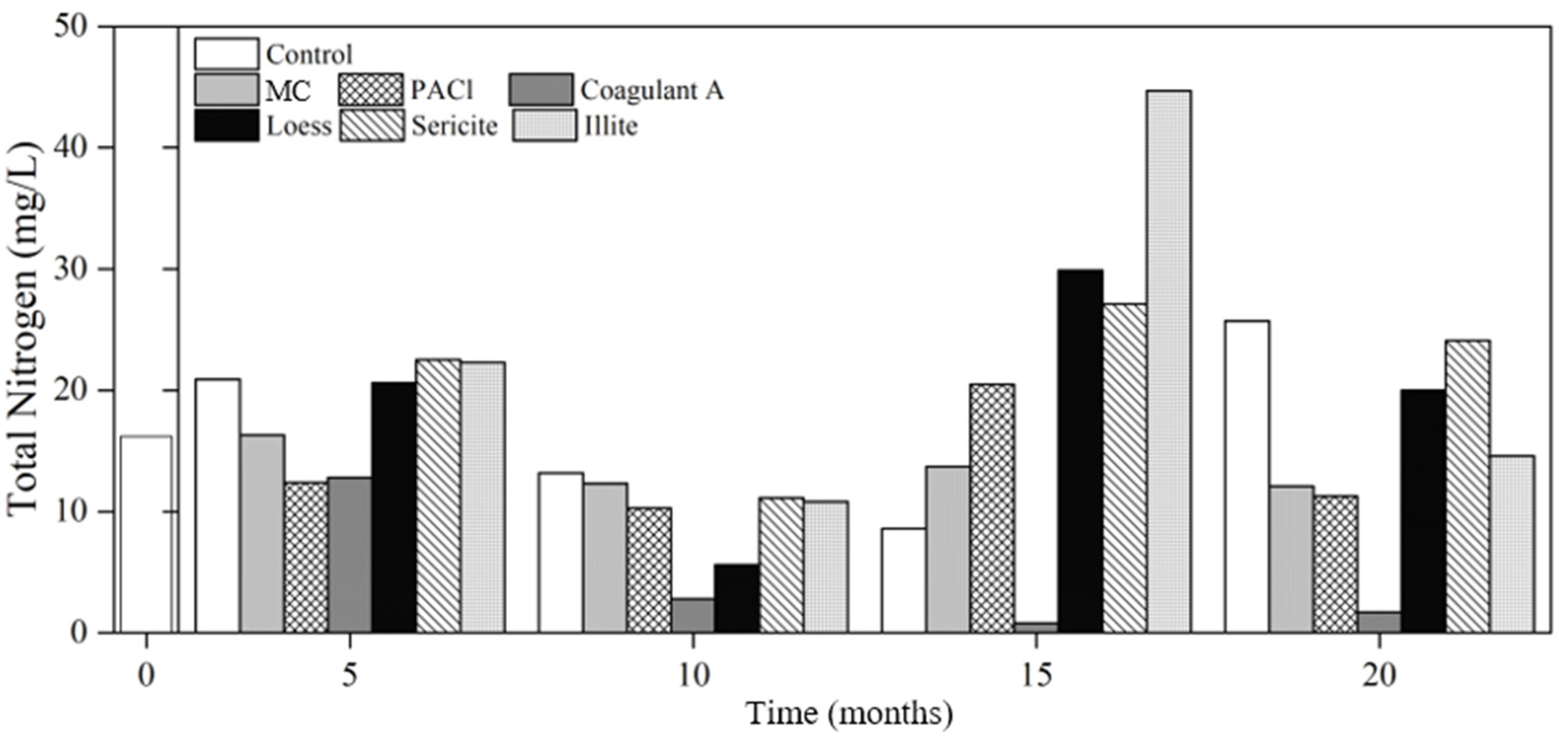
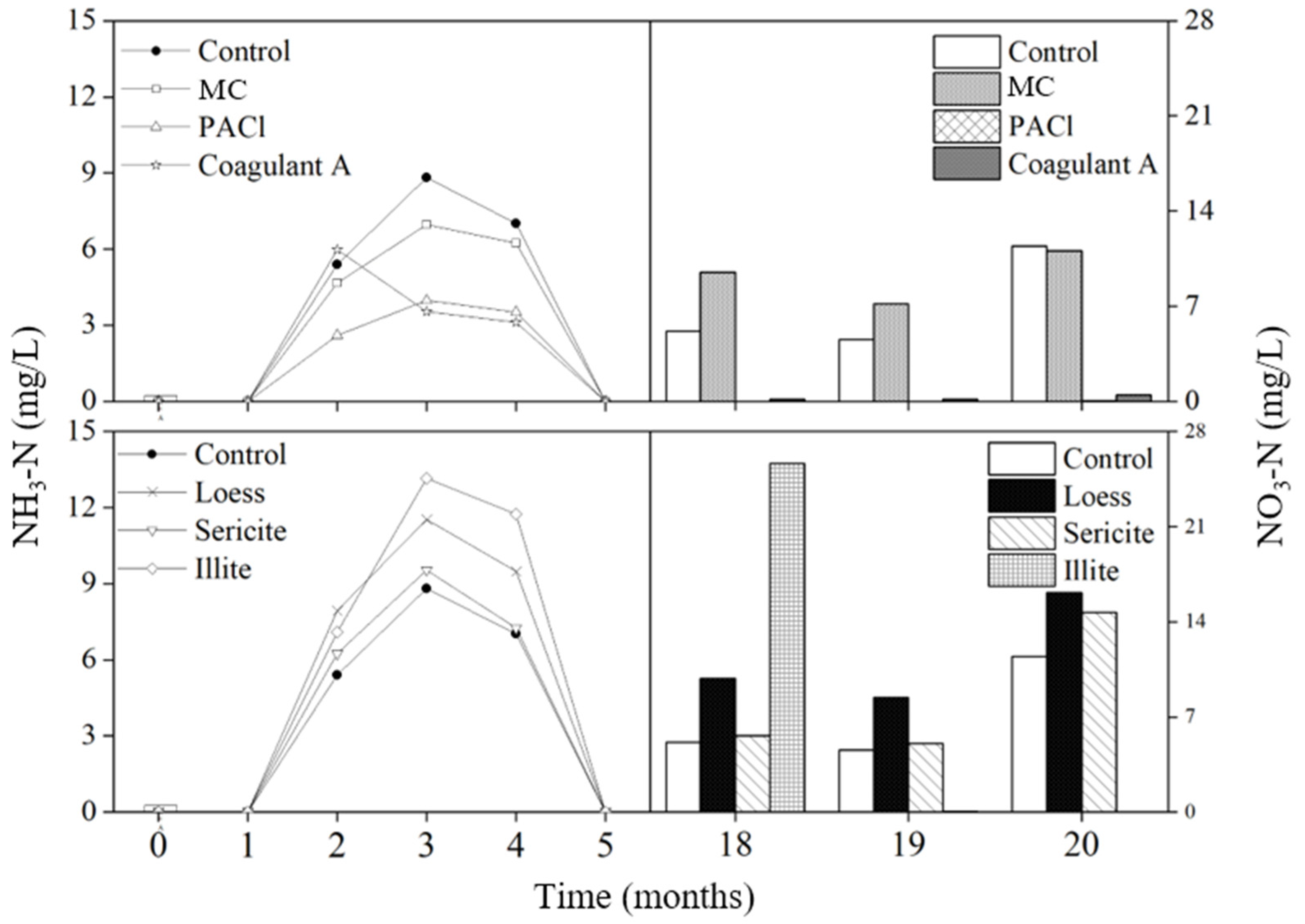
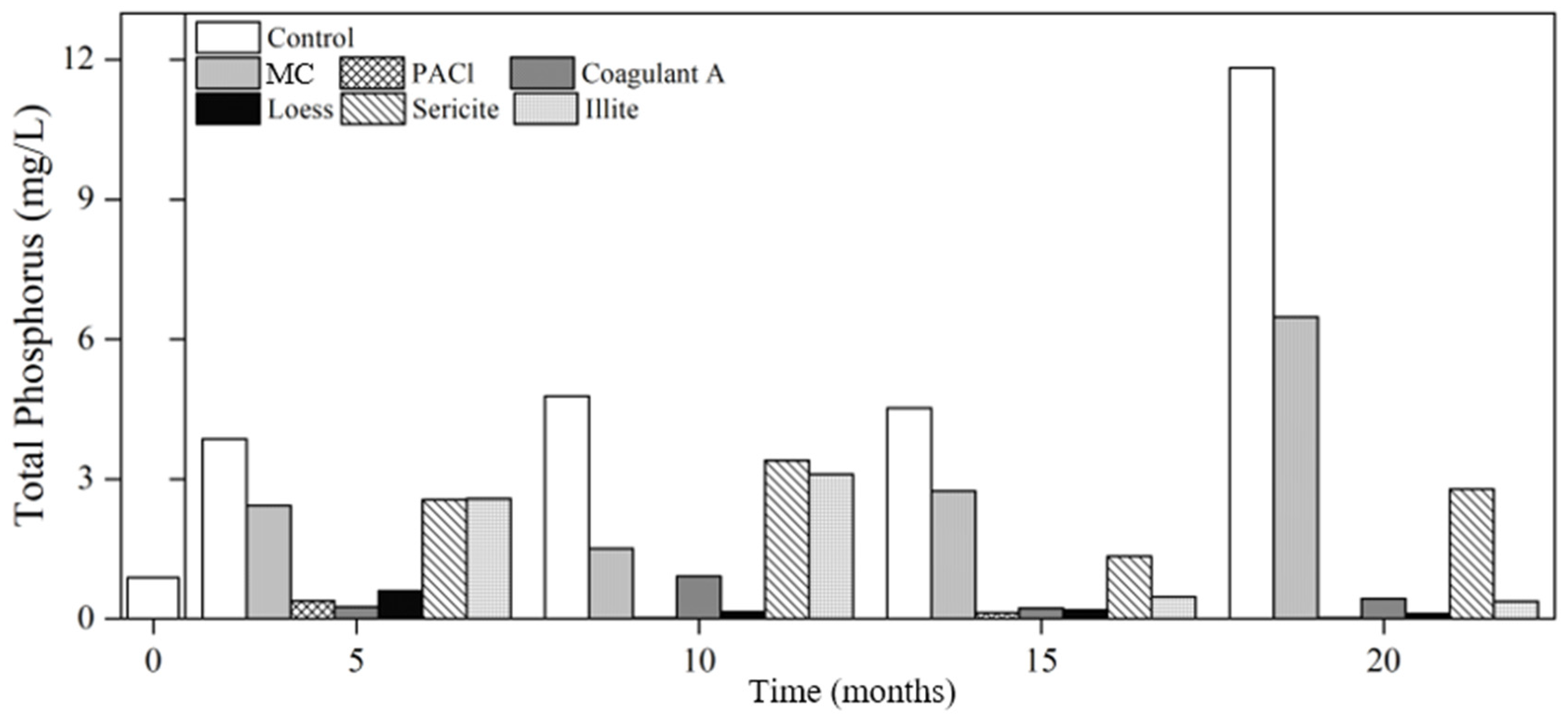
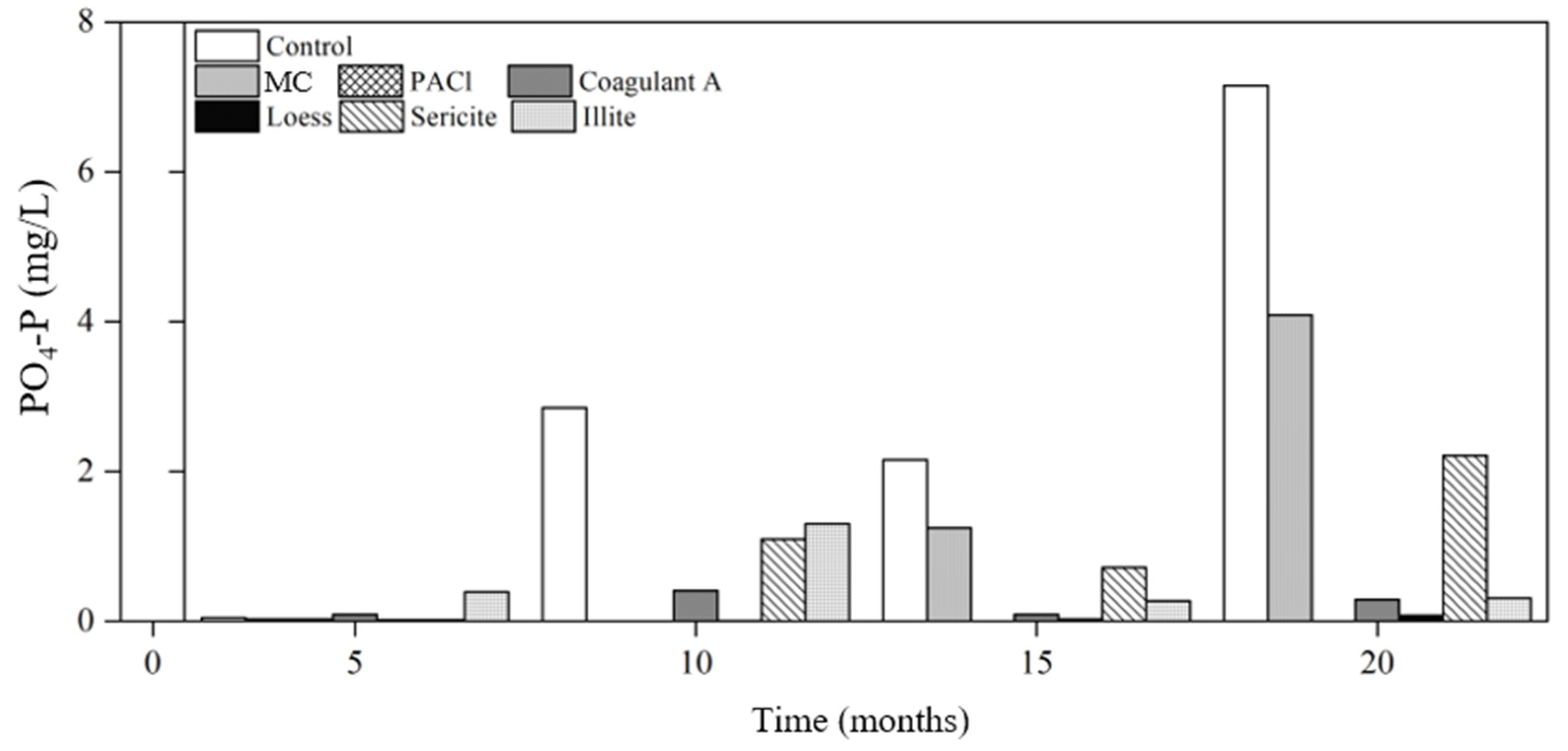
3.2.2. Nutrients
Nitrogen
Phosphorus
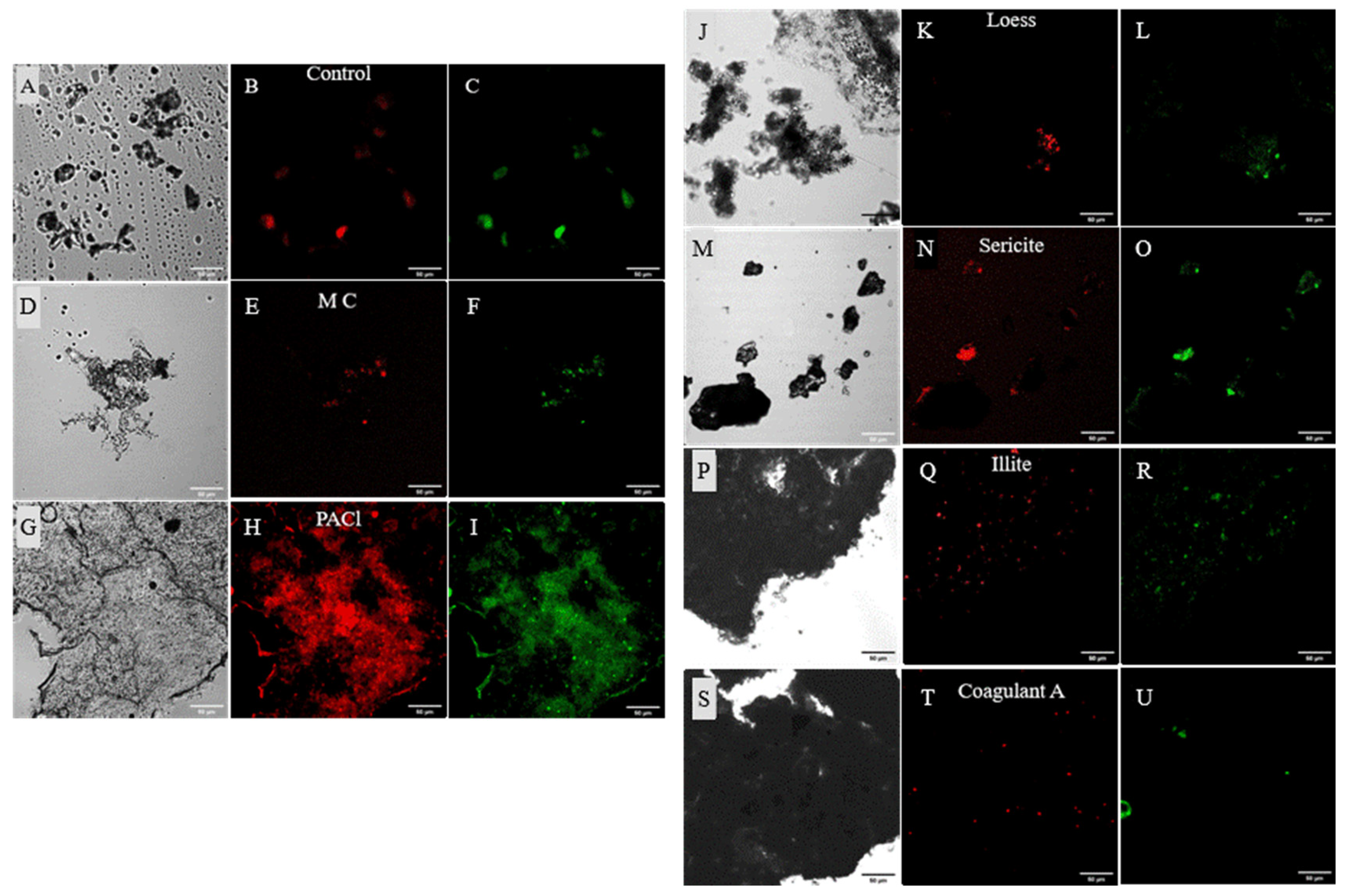
3.3. Effects of Coagulants and Minerals on Cell Integrity
4. Conclusions
- The phosphorus concentration of the raw water without removing cyanobacteria (control) increased by 4.5 times after 5 months (3.93 mg/L) and 13 times after 20 months (11.82 mg/L) compared to the initial concentration (0.88 mg/L). This increases the risk of eutrophication when cyanobacterial cells remained in the water. During the 20-month study period, the longer the cyanobacterial cells remained in the water, the more the phosphorus concentration increased, thus intensifying the risk of eutrophication.
- The addition of coagulants and minerals enabled nutrient removal via complex formation and adsorption; however, the effect on other intracellular matter remains to be investigated.
- Cell integrity analysis revealed cellular damage in all samples after 20 months. This highlights the importance of removing cyanobacterial cells from water to prevent eutrophication and cyanotoxin pollution.
- Since the nutrient concentrations of the treated columns were lower than the control in the early stages, we propose the removal of coagulated and flocculated cyanobacterial cells immediately after treatment in order to reduce eutrophication and manage water quality.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Michaloudi, E.; Kormas, K.A.; Tryfon, E.; Mihalatou, H.; Gkelis, S.; Lanaras, T. Plankton Food Web Structure in a Eutrophic Polymictic Lake with a History of Toxic Cyanobacterial Blooms. Limnol. Oceanogr. 2006, 51, 715–727. [Google Scholar] [CrossRef]
- Briland, R.D.; Stone, J.P.; Manubolu, M.; Lee, J.; Ludsin, S.A. Cyanobacterial Blooms Modify Food Web Structure and Interactions in Western Lake Erie. Harmful Algae 2020, 92, 101586. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.A.; Hunt, R.W. Capitalizing on harmful algal blooms: From problems to products. Algal Res. 2021, 55, 102265. [Google Scholar] [CrossRef]
- Guo, T.; Nisbet, E.C.; Martin, J.F. Identifying mechanisms of environmental decision-making: How ideology and geographic proximity influence public support for managing agricultural runoff to curb harmful algal blooms. J. Environ. Manag. 2019, 241, 264–272. [Google Scholar] [CrossRef]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef]
- Drábková, M.; Admiraal, W.; Maršálek, B. Combined exposure to hydrogen peroxide and Light-selective effects on cyanobacteria, green algae, and diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Van Hullebusch, E.V.; Deluchat, V.; Chazal, P.M.; Baudu, M. Environmental impact of two successive chemical treatments in a small shallow eutrophied lake: Part II. Case of copper sulfate. Environ. Pollut. 2002, 120, 627–634. [Google Scholar] [CrossRef]
- Bauzá, L.; Aguilera, A.; Echenique, R.; Andrinolo, D.; Giannuzzi, L. Application of hydrogen peroxide to the control of eutrophic lake systems in laboratory assays. Toxins 2014, 6, 2657–2675. [Google Scholar] [CrossRef]
- Fan, J.; Ho, L.; Hobson, P.; Brookes, J. Evaluating the effectiveness of copper sulphate, chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res. 2013, 47, 5153–5164. [Google Scholar] [CrossRef]
- Zamyadi, A.; Greenstein, K.E.; Glover, C.M.; Adams, C.; Rosenfeldt, E.; Wert, E.C. Impact of hydrogen peroxide and copper sulfate on the delayed release of microcystin. Water 2020, 12, 1105. [Google Scholar] [CrossRef]
- Choi, H.-J. Application of hybrid material, modified sericite and pine needle extract, for blue-green algae removal in the lake. Environ. Eng. Res. 2018, 23, 364–373. [Google Scholar] [CrossRef]
- Arend, K.K.; Beletsky, D.; Depinto, J.V.; Ludsin, S.A.; Roberts, J.J.; Rucinski, D.K.; Scavia, D.; Schwab, D.J.; Höök, T.O. Seasonal and Interannual Effects of Hypoxia on Fish Habitat Quality in Central Lake Erie. Freshw. Biol. 2011, 56, 366–383. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wei, Z.; Zhang, S. Potent Removal of Cyanobacteria with Controlled Release of Toxic Secondary Metabolites by a Titanium Xerogel Coagulant. Water Res. 2018, 128, 341–349. [Google Scholar] [CrossRef]
- Sengco, M.R.; Anderson, D.M. Controlling Harmful Algal Blooms through Clay Flocculation1. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef]
- Li, H.; Pei, H.; Xu, H.; Jin, Y.; Sun, J. Behavior of Cylindrospermopsis raciborskii during coagulation and sludge storage—Higher potential risk of toxin release than Microcystis aeruginosa? J. Hazard. Mater. 2018, 347, 307–316. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Li, X.-Q.; Ma, C.-X.; Pei, R.-T. The cell damage of Microcystis aeruginosa in PACl coagulation and floc storage processes. Sep. Purif. Technol. 2013, 115, 123–128. [Google Scholar] [CrossRef]
- Han, J.; Jeon, B.S.; Futatsugi, N.; Park, H.D. The effect of alum coagulation for in-lake treatment of toxic Microcystis and other cyanobacteria related organisms in microcosm experiments. Ecotoxicol. Environ. Saf. 2013, 96, 17–23. [Google Scholar] [CrossRef]
- Zhou, S.; Shao, Y.; Gao, N.; Deng, Y.; Qiao, J.; Ou, H.; Deng, J. Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Sci. Total Environ. 2013, 463–464, 111–119. [Google Scholar] [CrossRef]
- Boyer, J.N.; Kelble, C.R.; Ortner, P.B.; Rudnick, D.T. Phytoplankton bloom status: Chlorophyll-a biomass as an indicator of water quality condition in the southern estuaries of Florida, USA. Ecol. Indic. 2009, 9, S56–S67. [Google Scholar] [CrossRef]
- Klauth, P.; Wilhelm, R.; Klumpp, E.; Poschen, L.; Groeneweg, J. Enumeration of soil bacteria with the green fluorescent nucleic acid dye Sytox green in the presence of soil particles. J. Microbiol. Methods 2004, 59, 189–198. [Google Scholar] [CrossRef]
- Mucci, M.; Guedes, I.A.; Faassen, E.J.; Lürling, M. Chitosan as a coagulant to remove Cyanobacteria Can Cause microcystin release. Toxins 2020, 12, 711. [Google Scholar] [CrossRef]
- Son, H.; Bae, S.; Lee, G.; You, C. Comparison of microalgae removal efficiency using the coagulants. J. Korean Soc. Environ. Technol. 2020, 21, 101–106. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Dexter, Z.D.; Joseph, C.G.; Hilal, N. Effective coagulation-flocculation treatment of highly polluted palm oil mill biogas plant wastewater using dual coagulants: Decolourisation, kinetics and phytotoxicity studies. J. Water Process Eng. 2017, 16, 258–269. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe vera as an organic coagulant for improving drinking water quality. Water 2021, 13, 2024. [Google Scholar] [CrossRef]
- Jung, B.; O’Carroll, D.; Sleep, B. The influence of humic acid and clay content on the transport of polymer-coated iron nanoparticles through sand. Sci. Total Environ. 2014, 496, 155–164. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa: Part I: The key operating conditions. Sep. Purif. Technol. 2006, 52, 84–94. [Google Scholar] [CrossRef]
- Malam Issa, O.; Le Bissonnais, Y.; Défarge, C.; Trichet, J. Role of a Cyanobacterial Cover on Structural Stability of Sandy Soils in the Sahelian Part of Western Niger. Geoderma 2001, 101, 15–30. [Google Scholar] [CrossRef]
- Sepehr, A.; Hassanzadeh, M.; Rodriguez-Caballero, E. The protective role of cyanobacteria on soil stability in two aridisols in northeastern Iran. Geoderma Reg. 2019, 16, e00201. [Google Scholar] [CrossRef]
- Reddy, D.H.; Lee, S.-M.; Kim, J.-O. A Review Onemerging Applications of Naturalsericite and Its Composites. World Appl. Sci. J. 2013, 27, 1514–1523. [Google Scholar] [CrossRef]
- Lim, S.; Jeon, W.; Lee, J.; Lee, K.; Kim, N. Engineering properties of water/wastewater-treatment sludge modified by hydrated lime, fly ash and loess. Water Res. 2002, 36, 4177–4184. [Google Scholar] [CrossRef]
- Park, J.; Chun, Y.W.; Trung, H.T.; Kim, Y.U. Effect of ultrasonic energy on self-weight consolidation of clay minerals. KSCE J. Civ. Eng. 2014, 18, 971–974. [Google Scholar] [CrossRef]
- Won, J.; Kim, T.; Kang, M.; Choe, Y.; Choi, H. Kaolinite and illite colloid transport in saturated porous media. Colloids Surf. A Physicochem. Eng. Aspects 2021, 626, 127052. [Google Scholar] [CrossRef]
- Lee, D. A study on the geological occurrence, the mineralogical and physico-chemical properties of the Yucheon sericite ore in Chungha area, Kyungsangbuk-do. J. Mineral. Soc. Korea 1997, 10, 114–125. [Google Scholar]
- Lürling, M.; Kang, L.; Mucci, M.; van Oosterhout, F.; Noyma, N.P.; Miranda, M.; Huszar, V.L.M.; Waajen, G.; Marinho, M.M. Coagulation and precipitation of cyanobacterial blooms. Ecol. Eng. 2020, 158, 106032. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506–509, 135–145. [Google Scholar] [CrossRef]
- Yuan, L.L.; Pollard, A.I.; Pather, S.; Oliver, J.L.; D’Anglada, L. Managing microcystin: Identifying national-scale thresholds for total nitrogen and chlorophylla. Freshw. Biol. 2014, 59, 1970–1981. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Ryoo, K.S.; Choi, J.-H. A Comparative Study on Adsorption Characteristics of Total Nitrogen and Phosphorous in Water Using Various Adsorbents. J. Korean Chem. Soc. 2012, 56, 700–705. [Google Scholar] [CrossRef][Green Version]
- Xu, P.; Xiao, E.R.; He, F.; Xu, D.; Zhang, Y.; Wu, Z. Microbial fuel cell improves restoration of Hydrilla verticillata in an algae-rich sediment microcosm system. Chemosphere 2021, 266, 128987. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Lee, L.-L.; Chang, C.-N.; Chao, A.C. Control of carbon and ammonium ratio for simultaneous nitrification and denitrification in a sequencing batch bioreactor. Int. Biodeterior. Biodegrad. 2007, 59, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Wang, D.; Li, H.; Wang, Q.; Liu, Y.; Peng, L.; Yang, Q.; Li, X.; Zeng, G.; et al. Understanding the mechanisms of how poly aluminium chloride inhibits short-chain fatty acids production from anaerobic fermentation of waste activated sludge. Chem. Eng. J. 2018, 334, 1351–1360. [Google Scholar] [CrossRef]
- Wang, Z.; Akbar, S.; Sun, Y.; Gu, L.; Zhang, L.; Lyu, K.; Huang, Y.; Yang, Z. Cyanobacterial dominance and succession: Factors, mechanisms, predictions, and managements. J. Environ. Manag. 2021, 297, 113281. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.P.; Salmaso, N.; Paerl, H.W. Mitigating harmful cyanobacterial blooms: Strategies for control of nitrogen and phosphorus loads. Aquat. Ecol. 2016, 50, 351–366. [Google Scholar] [CrossRef]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae 2021, 109, 102099. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, D.I. Removal of total phosphorus (TP) from municipal wastewater using loess. Desalination 2011, 269, 104–110. [Google Scholar] [CrossRef]
- Guan, B.; Yao, X.; Jiang, J.; Tian, Z.; An, S.; Gu, B.; Cai, Y. Phosphorus removal ability of three inexpensive substrates: Physicochemical properties and application. Ecol. Eng. 2009, 35, 576–581. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Review: Phosphorus removal and recovery technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Bae, S.; Seo, D. Changes in algal bloom dynamics in a regulated large river in response to eutrophic status. Ecol. Model. 2021, 454, 109590. [Google Scholar] [CrossRef]
- Tang, X.; Wu, M.; Li, R. Phosphorus distribution and bioavailability dynamics in the mainstream water and surface sediment of the Three Gorges Reservoir between 2003 and 2010. Water Res. 2018, 145, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Chuai, X.; Ding, W.; Chen, X.; Wang, X.; Miao, A.; Xi, B.; He, L.; Yang, L. Phosphorus release from cyanobacterial blooms in Meiliang Bay of Lake Taihu, China. Ecol. Eng. 2011, 37, 842–849. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Zhang, K.; Xu, P.; Lu, Y. Phosphorus release from cyanobacterial blooms during their decline period in eutrophic Dianchi Lake, China. Environ. Sci. Pollut. Res. Int. 2018, 25, 13579–13588. [Google Scholar] [CrossRef] [PubMed]
| Parameter (Unit) | Value |
|---|---|
| pH | 8.9 |
| Turbidity (NTU) | 3924 |
| Chlorophyll-a (mg/m3) | 48 |
| Total nitrogen (mg/L) | 16.2 |
| Ammoniacal nitrogen (mg/L) | N.D. |
| Nitrate nitrogen (mg/L) | 0.09 |
| Total phosphorus (mg/L) | 0.8 |
| Phosphate (mg/L) | N.D. |
| Coagulant and Minerals | Phase | Major Components |
|---|---|---|
| MC | Liquid | Na, Ca, Mg, K, Al, Cl, and pine needle extract |
| PACl | Liquid | Al2(OH)3Cl3 and Al2O3 |
| Coagulant A | Powder | CaO, Al2O3, MgO, K2O, Fe2O3, and SiO2 |
| Loess | Powder | Al2O3 and Fe2O3 |
| Sericite | Powder | SiO2, Al2O3, CaO, and MgO |
| Illite | Powder | SiO2, Al2O3, Fe2O3, and K2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Kang, H.; Oh, H.-c.; Ahn, J.; Park, S.; Yun, S.-L.; Kim, S. Long-Term Examination of Water Chemistry Changes Following Treatment of Cyanobacterial Bloom with Coagulants and Minerals. Int. J. Environ. Res. Public Health 2022, 19, 13577. https://doi.org/10.3390/ijerph192013577
Lee B, Kang H, Oh H-c, Ahn J, Park S, Yun S-L, Kim S. Long-Term Examination of Water Chemistry Changes Following Treatment of Cyanobacterial Bloom with Coagulants and Minerals. International Journal of Environmental Research and Public Health. 2022; 19(20):13577. https://doi.org/10.3390/ijerph192013577
Chicago/Turabian StyleLee, Bokjin, Heejun Kang, Hye-cheol Oh, Jaehwan Ahn, Saerom Park, Sang-Leen Yun, and Seogku Kim. 2022. "Long-Term Examination of Water Chemistry Changes Following Treatment of Cyanobacterial Bloom with Coagulants and Minerals" International Journal of Environmental Research and Public Health 19, no. 20: 13577. https://doi.org/10.3390/ijerph192013577
APA StyleLee, B., Kang, H., Oh, H.-c., Ahn, J., Park, S., Yun, S.-L., & Kim, S. (2022). Long-Term Examination of Water Chemistry Changes Following Treatment of Cyanobacterial Bloom with Coagulants and Minerals. International Journal of Environmental Research and Public Health, 19(20), 13577. https://doi.org/10.3390/ijerph192013577






