A Cost-Utility Analysis of Mesh Prophylaxis in the Prevention of Incisional Hernias following Stoma Closure Surgery
Abstract
1. Introduction
1.1. Background
1.2. Motivation and Rationale
1.3. Study Objectives
1.4. Literature Review
2. Materials and Methods
2.1. Choice of Analysis
2.2. Choice of Perspective
2.3. Competing Alternatives
2.4. Time Period
2.5. Costs
2.5.1. Procedure and Intervention Costs
2.5.2. Complications Costs
2.5.3. Assumptions
- A cost for seroma drainage was not obtainable; instead the procedural cost for an abscess drainage was sourced from the NHS reference costs and utilised as a proxy. This is justified as the management of a seroma and abscess are the same, with both requiring single, percutaneous abdominal drainage.
- NHS reference costs for stoma closure, abscess drainage and hernia repair surgery are dependent upon patient comorbidity and complication (CC) scores. Based on the patient data in the ROCSS study, a CC score of 2 was assigned to all patients and the corresponding procedural cost was used.
- Diagnostic costs for each complication were omitted as these could not be reliably estimated based on the study data. This is justified as the majority of complications in this study can be visibly diagnosed.
2.5.4. Viewpoints and Net Present Values
2.6. Benefits
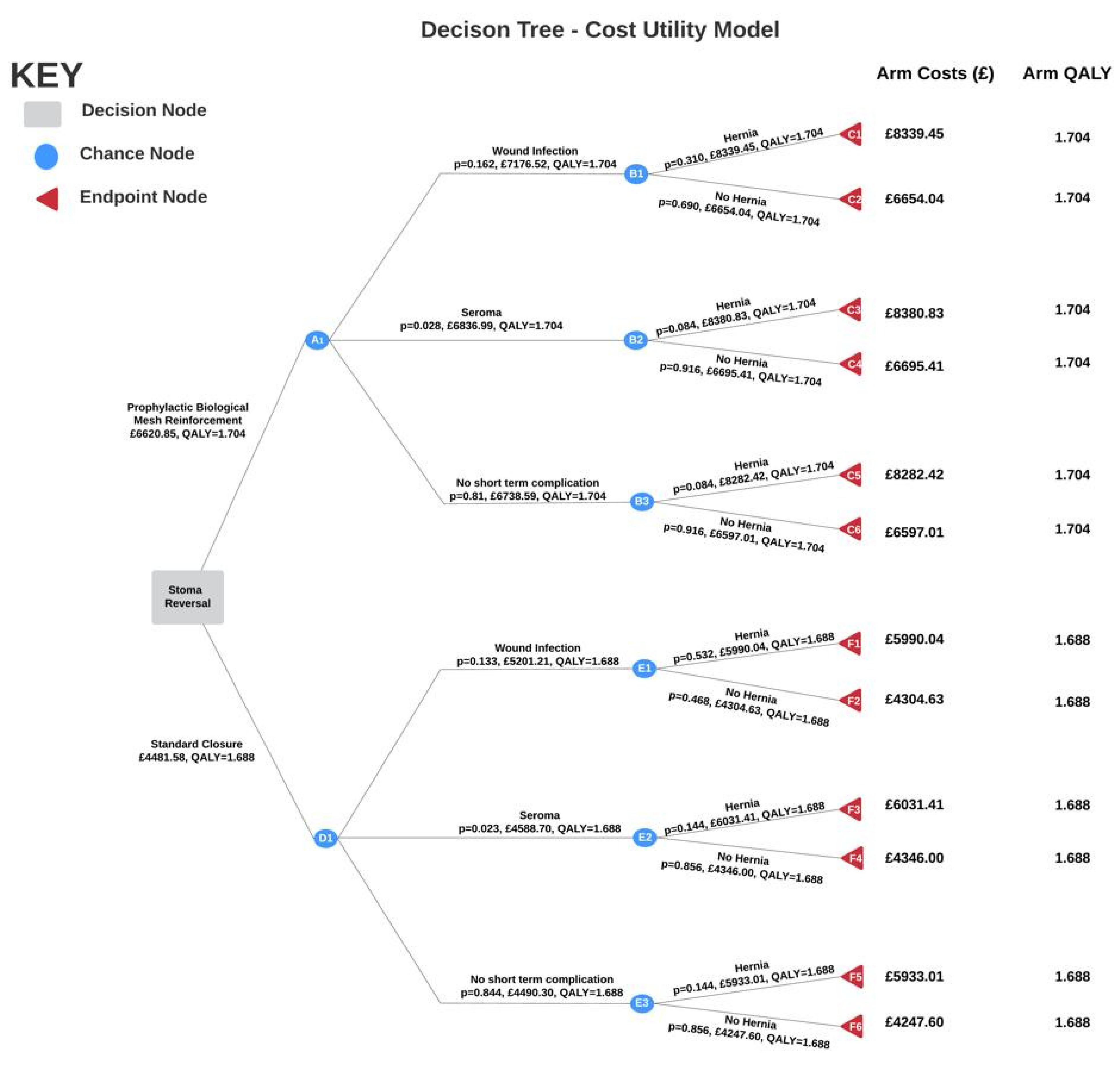
2.7. Modelling
- The ROCSS study data suggests that the probability of developing an incisional hernia is independent of any complications, which is contradictory to hernia pathophysiology. Wound infection directly contributes to development of hernia [6]. Using our medical knowledge and existing literature, a probability of hernia given wound infection was calculated (see Appendix G).
- The probabilities for both wound infection and seroma formation are mutually exclusive. This is justified as the ROCSS trial did not provide conditional probabilities for each complication.
- EQ-5D-3L scores for wound breakdown, seroma and no complication in this study are the same for each arm. Although the mean EQ-5D-3L scores provided in the study ignore the impact of complications, however these complications are short-term and are therefore unlikely to be detrimental to quality of life.
- Although death is a consideration when conducting surgical procedures, no patient deaths were reported in the study, so deaths were not investigated as an outcome in the tree. Moreover, the NHS reports stoma reversal surgery as a relatively straightforward procedure with a low probability of serious complications [24].
3. Results
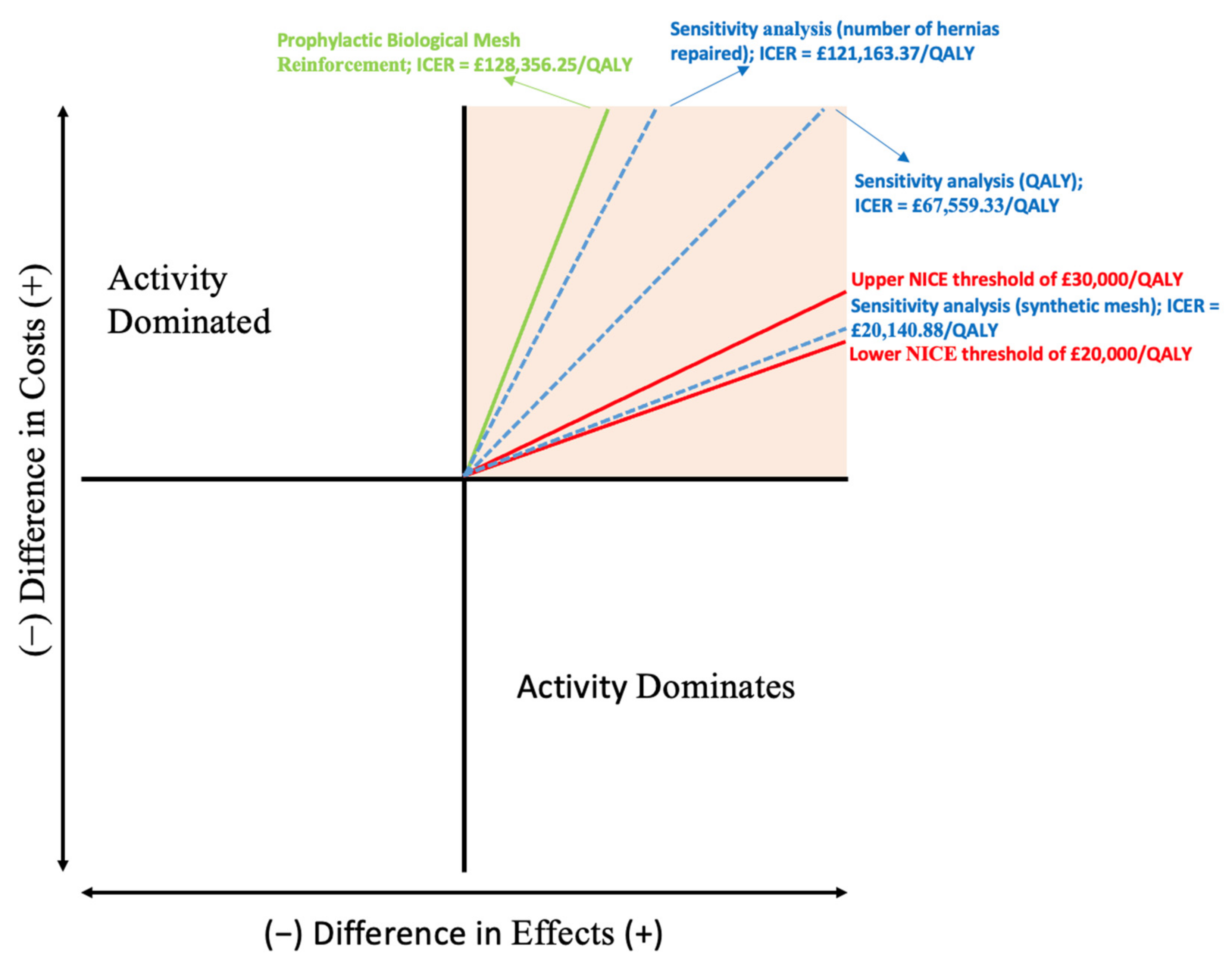
3.1. Incremental Cost-Effectiveness Ratio
3.2. Net Monetary and Health Benefit
3.3. Sensitivity Analysis
4. Discussion
4.1. Limitations
4.2. Contribution to the Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
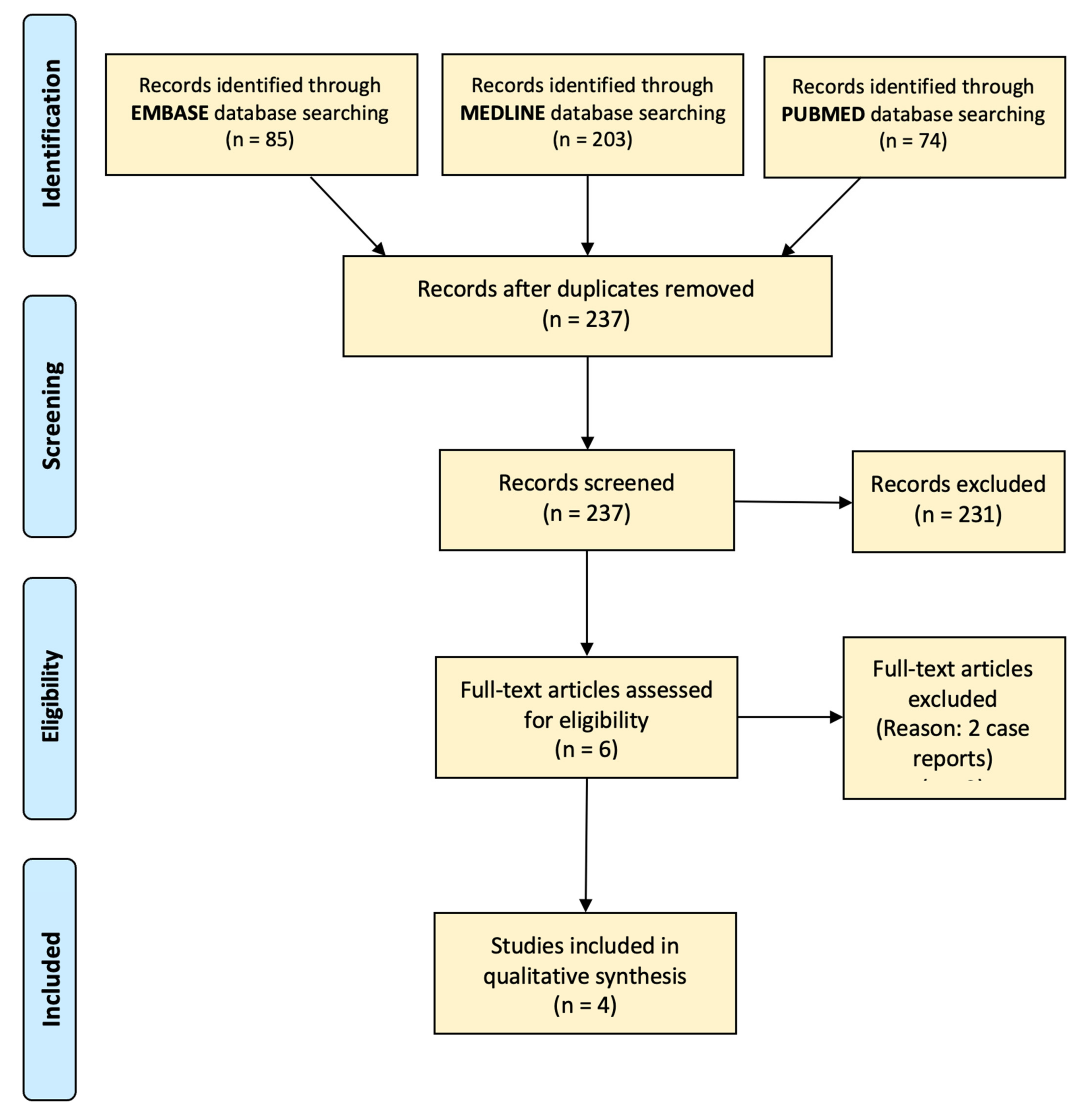
Appendix B
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomised controlled trials | Non-English studies |
| Retrospective cohort studies | Case reports |
| Cost analyses | Case series |
| Conference Abstracts | |
| Studies comparing mesh prophylaxis and closure with sutures alone for procedures other than stoma closure surgery |
Appendix C
| Treatment Component | Unit Cost [GBP] (Year) | Explanation of Calculation | Discount Rate | Present Value (GBP) | Reference |
|---|---|---|---|---|---|
| Stoma Closure | £4247.60 (2021) | Weighted average of FF34B Distal Colon Procedures and FF22C Major Small Intestine Procedures based on the proportion of ileostomy and colostomy closure | - | £4247.60 | [32] |
| Stoma Closure with Prophylactic Biological Mesh Reinforcement (Mesh) | £1650 (2017) | The average cost of biological mesh | 1.035 | £1893.41 | [21] |
| Stoma Closure with Prophylactic Synthetic Mesh Reinforcement (Mesh) | £33 (2017) | The average cost of a synthetic mesh | 1.035 | £37.87 | [21] |
| Stoma Closure with Prophylactic Biological Mesh Reinforcement (Extra time) | £20 (2019) £16 (2009) | The median extra duration of surgery (20 min) was multiplied by an average of the different estimated costs/min of operating theatres. Average cost/min = £22.80 Total cost = £456 | 1.035 1.035 | £21.42 £24.18 | [32] [33] |
| GP Consultation | £37.40 (2017) | Average cost of a 9 min GP consultation | 1.035 | £42.92 | [34] |
| Co-amoxiclav | £1.69 (2021) | The standard treatment for stoma site wound infection. Modal drug tariff price. (Pack of 21, 1 tablet 3 times daily for 7 days) | - | £1.69 | [23] |
| Wound dressings | £12 (2020) | Average cost of wound dressings | 1.035 | £12.42 | [35] |
| Seroma drainage | £1419 (2021) | Most seromas need no treatment. Abscess drainage is used as a proxy for the costs of seroma drainage. Percutaneous Single Drainage of Abdominal Abscess | - | £1419 | [35] |
| Incisional hernia repair | £3085 (2021) | Stoma site incisional hernias are by definition complex hernias. FF60C Complex Hernia Procedures with CC Score 1–2 | - | £3085 | [35] |
Appendix D
| Complication | Cost Calculation | Total (GBP) |
|---|---|---|
| Wound infection + hernia (C1) | (0.2 * × [Colostomy Closure + additional time costs] + 0.8 × [Ileostomy Closure + additional time costs]) + Cost of biological mesh + Cost of GP Consultation + Cost of co-amoxiclav + Cost of wound dressings + 0.51 × Cost of incisional hernia repair | £8339.45 |
| Wound infection + no hernia (C2) | (0.2 × [Colostomy Closure + additional time costs] + 0.8 × [Ileostomy Closure + additional time costs]) + Cost of biological mesh + Cost of GP Consultation + Cost of co-amoxiclav + Cost of wound dressings | £6654.04 |
| Seroma + hernia (C3) | (0.2 × [Colostomy Closure + additional time costs] + 0.8 × [Ileostomy Closure + additional time costs]) + Cost of biological mesh + 0.067 × Cost of seroma drainage + 0.51 × Cost of incisional hernia repair | £8380.83 |
| Seroma + no hernia (C4) | (0.2 × [Colostomy Closure + additional time costs] + 0.8 × [Ileostomy Closure + additional time costs]) + Cost of biological mesh + 0.067 × Cost of seroma drainage | £6695.41 |
| No short-term complication + Hernia (C5) | (0.2 × [Colostomy Closure + additional time costs] + 0.8 × [Ileostomy Closure + additional time costs]) + Cost of biological mesh + 0.51 × Cost of incisional hernia repair | £8282.42 |
| No complication (C6) | (0.2 × Colostomy Closure + additional time costs] + 0.8 × [Ileostomy Closure + additional time costs]) + Cost of biological mesh | £6957.01 |
| Complication | Cost Calculation | Total (GBP) |
|---|---|---|
| Wound infection + hernia (F1) | (0.2 × Colostomy Closure + 0.8 × Ileostomy Closure) + Cost of GP Consultation + Cost of co-amoxiclav + Cost of wound dressings + 0.51 × Cost of incisional hernia repair | £5990.04 |
| Wound infection + no hernia (F2) | (0.2 × Colostomy Closure + 0.8 × Ileostomy Closure) + Wound infection costs (GP Consultation + co-amoxiclav + wound dressings) | £4304.63 |
| Seroma + hernia (F3) | (0.2 × Colostomy Closure + 0.8 × Ileostomy Closure) + 0.067 × Cost of seroma drainage + 0.51 × Cost of incisional hernia repair | £6031.41 |
| Seroma + no hernia (F4) | (0.2 × Colostomy Closure + 0.8 × Ileostomy Closure) + 0.067 × Cost of seroma drainage | £4346.00 |
| No short-term complication + Hernia (F5) | (0.2 × Colostomy Closure + 0.8 × Ileostomy Closure) + 0.51 × Cost of incisional hernia repair | £5933.01 |
| No complication (F6) | (0.2 × Colostomy Closure + 0.8 × Ileostomy Closure) | £4247.60 |
Appendix E
| Treatment Option | QALY Calculation | Total QALY |
|---|---|---|
| Prophylactic Biological Mesh Reinforcement | 1.70416 | |
| Standard Closure | 1.6875 |
Appendix F
| Secondary Outcome | Reason for Exclusion as Specific Node | Group into Other Outcomes |
|---|---|---|
| Wound infection at 12 months | Most wound infections show up within the first 30 days after surgery. The outcome table in the study reflects this. | No |
| Radiological hernia at 12 months | Demographic details for patients included in the analysis for the radiological hernia outcome showed no clinically important differences | No |
| Symptomatic hernia at 12 months | Hernia already measured in primary outcome | No |
| Symptomatic hernia at 24 months | Hernia already measured in primary outcome | No |
| Surgical re-intervention at stoma site | Reason for re-intervention is not specified | No |
Appendix G
Appendix H
| Incidence of Hernia | Hernia Effect on QoL Score(Source) | Calculation |
|---|---|---|
| 25% decrease [37] |
Appendix I
| ∆ | ICER (GBP/QALY) | NMB (GBP) | Calculation | |
|---|---|---|---|---|
| Original model | ∆C = £2139.27 ∆E = 0.016 | £128,356.25 | −£1639.27 | = (30,000 × 0.016) − 2139.27 |
| Sensitivity analysis with adjusted QALYs | ∆C = £2139.27 ∆E = 0.0317 | £67,559.33 | −£1189.32 | = (30,000 × 0.0317) − 2139.27 |
| Sensitivity analysis with all incisional hernias treated | ∆C = £2019.39 ∆E = 0.016 | £121,163.37 | −£1519.39 | = (30,000 × 0.016) − 2019.39 |
| Sensitivity analysis using synthetic mesh prophylaxis | ∆C = £335.68 ∆E = 0.016 | £20,140.88 | +£144.32 | = (30,000 × 0.016) − 335.68 |
| ∆ | ICER (GBP/QALY) | NHB (GBP) | Calculation | |
|---|---|---|---|---|
| Original model | ∆C = £2139.27 ∆E = 0.016 | £128,356.25 | −0.055 | |
| Sensitivity analysis with adjusted QALYs | ∆C = £2139.27 ∆E = 0.0317 | £67,559.33 | −0.040 | |
| Sensitivity analysis with all incisional hernias treated | ∆C = £2019.39 ∆E = 0.016 | £121,163.37 | −0.051 | |
| Sensitivity analysis with synthetic mesh | ∆C = £335.68 ∆E = 0.016 | £20,140.88 | +0.0048 |
References
- Pine, J.; Stevenson, L. Ileostomy and Colostomy. Surgery 2014, 32, 212–217. [Google Scholar] [CrossRef]
- Shabbir, J.; Britton, D.C. Stoma Complications: A Literature Overview. Color. Dis. 2010, 12, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Deen, K.I.; Madoff, R.D.; Goldberg, S.M.; Rothenberger, D.A. Surgical Management of Left Colon Obstruction: The University of Minnesota Experience. J. Am. Coll. Surg. 1998, 187, 573–576. [Google Scholar] [CrossRef]
- Oxford University Hospitals. Common Concerns for People with a Stoma. Available online: https://www.ouh.nhs.uk/patient-guide/leaflets/files/13174Pconcerns.pdf (accessed on 20 August 2022).
- Sherman, K.L.; Wexner, S.D. Considerations in Stoma Reversal. Clin. Colon. Rectal. Surg. 2017, 30, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Nepogodiev, D.; Ives, N.; Magill, L.; Glasbey, J.; Forde, C.; Bisgaard, T.; Handley, K.; Mehta, S.; Morton, D.; et al. Prophylactic Biological Mesh Reinforcement versus Standard Closure of Stoma Site (ROCSS): A Multicentre, Randomised Controlled Trial. Lancet 2020, 395, 417–426. [Google Scholar] [CrossRef]
- Hospital Episode Statistics (HES)—NHS Digital. Available online: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed on 20 August 2022).
- Lorenz, A.; Kogler, P.; Kafka-Ritsch, R.; Öfner, D.; Perathoner, A. Incisional Hernia at the Site of Stoma Reversal-Incidence and Risk Factors in a Retrospective Observational Analysis. Int. J. Colorectal. Dis. 2019, 34, 1179–1187. [Google Scholar] [CrossRef]
- Sharma, P.; Bowyers, D.; Scott, N.; Hernandes, R.; Fraser, C.; Cruickshank, M. Background—The Clinical Effectiveness and Cost-Effectiveness of Open Mesh Rpairs in Adults Presenting with a Clinically Diagnosed Primary Unilateral Inguinal Hernia Who Are Operated in an Elective Setting: Systematic Review and Economic Evaluation—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326920/ (accessed on 20 August 2022).
- Lee, L.; Saleem, A.; Landry, T.; Latimer, E.; Chaudhury, P.; Feldman, L.S. Cost Effectiveness of Mesh Prophylaxis to Prevent Parastomal Hernia in Patients Undergoing Permanent Colostomy for Rectal Cancer. J. Am. Coll. Surg. 2014, 218, 82–91. [Google Scholar] [CrossRef]
- van den Hil, L.C.L.; van Steensel, S.; Schreinemacher, M.H.F.; Bouvy, N.D. Prophylactic Mesh Placement to Avoid Incisional Hernias after Stoma Reversal: A Systematic Review and Meta-Analysis. Hernia 2019, 23, 733. [Google Scholar] [CrossRef]
- Liu, D.S.H.; Banham, E.; Yellapu, S. Prophylactic Mesh Reinforcement Reduces Stomal Site Incisional Hernia after Ileostomy Closure. World J. Surg. 2013, 37, 2039–2045. [Google Scholar] [CrossRef]
- Warren, J.A.; Beffa, L.R.; Carbonell, A.M.; Cull, J.; Sinopoli, B.; Ewing, J.A.; McFadden, C.; Crockett, J.; Cobb, W.S. Prophylactic Placement of Permanent Synthetic Mesh at the Time of Ostomy Closure Prevents Formation of Incisional Hernias. Surgery 2018, 163, 839–846. [Google Scholar] [CrossRef]
- Maggiori, L.; Moszkowicz, D.; Zappa, M.; Mongin, C.; Panis, Y. Bioprosthetic Mesh Reinforcement during Temporary Stoma Closure Decreases the Rate of Incisional Hernia: A Blinded, Case-Matched Study in 94 Patients with Rectal Cancer. Surgery 2015, 158, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.M.; Tosteson, A.N.A. Measuring Preferences for Cost-Utility Analysis. Pharmacoeconomics 2012, 25, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.J.; Ali, S. Health Outcomes in Economic Evaluation: The QALY and Utilities. Br. Med. Bull. 2010, 96, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Assessing Cost Effectiveness|The Guidelines Manual|Guidance|NICE. Available online: https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness (accessed on 21 August 2022).
- Thorlby, R. Spending Review 2020—The Health Foundation. Available online: https://www.health.org.uk/publications/long-reads/spending-review-2020 (accessed on 21 August 2022).
- Horesh, N.; Lessing, Y.; Rudnicki, Y.; Kent, I.; Kammar, H.; Ben-Yaacov, A.; Dreznik, Y.; Tulchinsky, H.; Avital, S.; Mavor, E.; et al. Considerations for Hartmann’s Reversal and Hartmann’s Reversal Outcomes—A Multicenter Study. Int. J. Colorectal. Dis. 2017, 32, 1577–1582. [Google Scholar] [CrossRef]
- Bhangu, A.; Futaba, K.; Patel, A.; Pinkney, T.; Morton, D. Reinforcement of Closure of Stoma Site Using a Biological Mesh. Tech. Coloproctol. 2014, 18, 305–308. [Google Scholar] [CrossRef]
- Findlay, J.M.; Wood, C.P.J.; Cunningham, C. Prophylactic Mesh Reinforcement of Stomas: A Cost-Effectiveness Meta-Analysis of Randomised Controlled Trials. Tech. Coloproctol. 2018, 22, 265–270. [Google Scholar] [CrossRef]
- Rognoni, C.; Cuccurullo, D.; Borsoi, L.; Bonavina, L.; Asti, E.; Crovella, F.; Bassi, U.A.; Carbone, G.; Guerini, F.; de Paolis, P.; et al. Clinical Outcomes and Quality of Life Associated with the Use of a Biosynthetic Mesh for Complex Ventral Hernia Repair: Analysis of the “Italian Hernia Club” Registry. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- BNF (British National Formulary)|NICE. Available online: https://bnf.nice.org.uk/ (accessed on 21 August 2022).
- Colostomy—Reversal—NHS. Available online: https://www.nhs.uk/conditions/colostomy/reversal/ (accessed on 21 August 2022).
- Bambha, K.; Kim, W.R. Cost-Effectiveness Analysis and Incremental Cost-Effectiveness Ratios: Uses and Pitfalls. Eur. J. Gastroenterol. Hepatol. 2004, 16, 519–526. [Google Scholar] [CrossRef]
- Khorgami, Z.; Hui, B.Y.; Mushtaq, N.; Chow, G.S.; Sclabas, G.M. Predictors of Mortality after Elective Ventral Hernia Repair: An Analysis of National Inpatient Sample. Hernia 2018, 23, 979–985. [Google Scholar] [CrossRef]
- Kingsnorth, A. The Management of Incisional Hernia. Ann. R Coll. Surg. Engl. 2006, 88, 252–260. [Google Scholar] [CrossRef]
- Fitzgerald, J.F.; Kumar, A.S. Biologic versus Synthetic Mesh Reinforcement: What Are the Pros and Cons? Clin. Colon. Rectal. Surg. 2014, 27, 140–148. [Google Scholar] [CrossRef]
- CADTH. Biological mesh: A review of clinical indications, clinical effectiveness, cost-effectiveness, and clinical practice guidelines. 2015. Available online: https://www.cadth.ca/sites/default/files/pdf/L0229_Biological_Mesh_final.pdf (accessed on 20 August 2022).
- Montgomery, A. The Battle between Biological and Synthetic Meshes in Ventral Hernia Repair. Hernia 2013, 17, 3–11. [Google Scholar] [CrossRef]
- Fischer, J.P.; Basta, M.N.; Mirzabeigi, M.N.; Bauder, A.R.; Fox, J.P.; Drebin, J.A.; Serletti, J.M.; Kovach, S.J. A Risk Model and Cost Analysis of Incisional Hernia after Elective Abdominal Surgery Based on 12,373 Cases. the Case for Targeted Prophylactic Intervention. Ann. Surg. 2016, 263, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- NHS Improvement National Tariff Payment System. 2019. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjO3q-v-rfuAhViShUIHVr-CMkQFjABegQIARAC&url=https%3A%2F%2Fimprovement.nhs.uk%2Fdocuments%2F479%2FAnnex_DtA_National_tariff_workbook.xlsx&usg=AOvVaw0rr7lbjT54BHw8iMC3HQsj (accessed on 20 August 2022).
- Abbott, T.; White, S.M.; Pandit, J.J. Factors Affecting the Profitability of Surgical Procedures under ‘Payment by Results. ’ Anaesthesia 2011, 66, 283–292. [Google Scholar] [CrossRef] [PubMed]
- The King’s Fund. Understanding NHS Financial Pressures. 2020. Available online: https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/Understanding%20NHS%20financial%20pressures%20-%20full%20report.pdf (accessed on 20 August 2022).
- NHS Reference Costs 2019/20: Highlights, Analysis and Introduction to the Data. 2020. Available online: https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed on 20 August 2022).
- Walming, S.; Angenete, E.; Block, M.; Bock, D.; Gessler, B.; Haglind, E. Retrospective Review of Risk Factors for Surgical Wound Dehiscence and Incisional Hernia. BMC Surg. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Bartlett, A.S.J.R.; Gilkison, W.; Krishna, G. Quality of life assessment in patients with inguinal hernia. ANZ J. Surg. 2006, 76, 491–493. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, Y.; Asunramu, H.; Low, H.; Gakhar, D.; Muthukumar, K.; Yassin, H.; de Preux, L. A Cost-Utility Analysis of Mesh Prophylaxis in the Prevention of Incisional Hernias following Stoma Closure Surgery. Int. J. Environ. Res. Public Health 2022, 19, 13553. https://doi.org/10.3390/ijerph192013553
Sheikh Y, Asunramu H, Low H, Gakhar D, Muthukumar K, Yassin H, de Preux L. A Cost-Utility Analysis of Mesh Prophylaxis in the Prevention of Incisional Hernias following Stoma Closure Surgery. International Journal of Environmental Research and Public Health. 2022; 19(20):13553. https://doi.org/10.3390/ijerph192013553
Chicago/Turabian StyleSheikh, Yusuf, Hareef Asunramu, Heather Low, Dev Gakhar, Keerthi Muthukumar, Husam Yassin, and Laure de Preux. 2022. "A Cost-Utility Analysis of Mesh Prophylaxis in the Prevention of Incisional Hernias following Stoma Closure Surgery" International Journal of Environmental Research and Public Health 19, no. 20: 13553. https://doi.org/10.3390/ijerph192013553
APA StyleSheikh, Y., Asunramu, H., Low, H., Gakhar, D., Muthukumar, K., Yassin, H., & de Preux, L. (2022). A Cost-Utility Analysis of Mesh Prophylaxis in the Prevention of Incisional Hernias following Stoma Closure Surgery. International Journal of Environmental Research and Public Health, 19(20), 13553. https://doi.org/10.3390/ijerph192013553






