Do Loneliness and Per Capita Income Combine to Increase the Pace of Biological Aging for Black Adults across Late Middle Age?
Abstract
1. Introduction
1.1. The Need for Examination of Within-Person Change
1.2. Epigenetic Measurement Is a Muti-Purpose Tool Enhancing the Study of Health Outcomes
1.2.1. Advances in the Measurement of Health Impacts: Epigenetic Clocks
1.2.2. The Development of the DunedinPACE
1.2.3. Advances in the Measurement of Health Behavior: Smoking
1.3. Intrinsic vs. Extrinsic Indices of Aging to Examine System Effects
1.4. Control Variables
2. Materials and Methods
2.1. Sample
2.2. Procedures and Measures
2.2.1. Primary Predictors
Loneliness
Per Capita Income
DNA Methylation-Based Measures
DunedinPACE
Cigarette Smoking
Alcohol Index
Cell Type Variation
Control Variables
2.3. Analytic Strategy
3. Results
3.1. Descriptive Findings
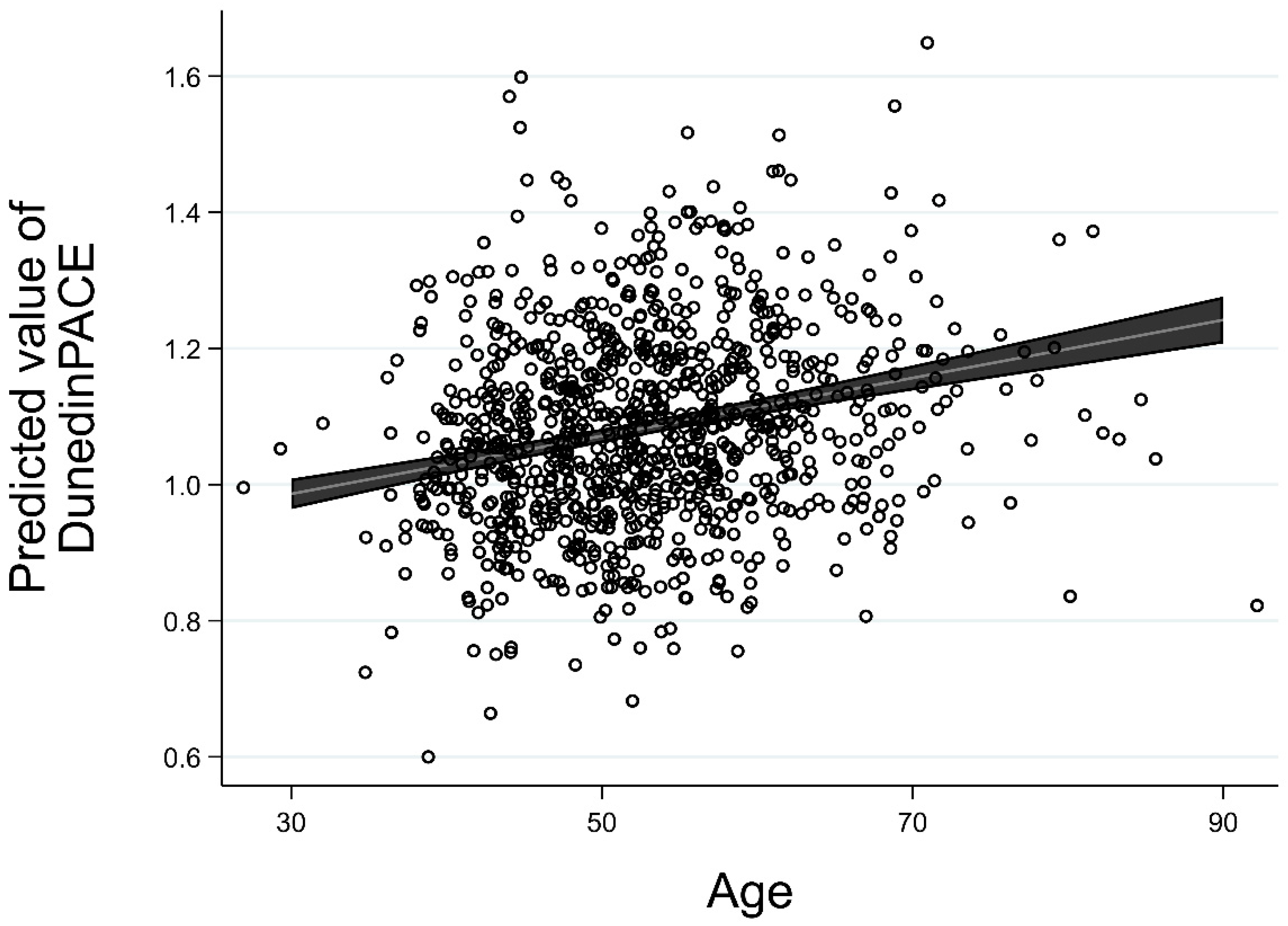
3.2. Test of Invariance of Change in PACE Relative to Baseline Age
3.3. Examination of Loneliness and Per Capita Income
3.4. Examination of Effects Using Intrinsic DunedinPACE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumeister, R.F.; Leary, M.R. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 1995, 117, 497–529. [Google Scholar] [CrossRef]
- Martire, L.M.; Schulz, R.; Mittelmark, M.B.; Newsom, J.T. Stability and change in older adults’ social contact and social support: The Cardiovascular Health Study. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 1999, 54, S302–S311. [Google Scholar] [CrossRef]
- Cacioppo, S.; Capitanio, J.P.; Cacioppo, J.T. Toward a neurology of loneliness. Psychol. Bull. 2014, 140, 1464–1504. [Google Scholar] [CrossRef] [PubMed]
- Hawkley, L.C.; Wroblewski, K.; Kaiser, T.; Luhmann, M.; Schumm, L.P. Are US older adults getting lonelier? Age, period, and cohort differences. Psychol. Aging 2019, 34, 1144–1157. [Google Scholar] [CrossRef]
- Cudjoe, T.K.; Roth, D.L.; Szanton, S.L.; Wolff, J.L.; Boyd, C.M.; Thorpe, R.J., Jr. The epidemiology of social isolation: National health and aging trends study. J. Gerontol. Ser. B 2020, 75, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gerst-Emerson, K.; Jayawardhana, J. Loneliness as a public health issue: The impact of loneliness on health care utilization among older adults. Am. J. Public Health 2015, 105, 1013–1019. [Google Scholar] [CrossRef]
- Coyle, C.E.; Dugan, E. Social isolation, loneliness and health among older adults. J. Aging Health 2012, 24, 1346–1363. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Thisted, R.A.; Masi, C.M.; Cacioppo, J.T. Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychol. Aging 2010, 25, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hawkley, L.C.; Waite, L.J.; Cacioppo, J.T. Loneliness, health, and mortality in old age: A national longitudinal study. Soc. Sci. Med. 2012, 74, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Wippold, G.M.; Tucker, C.M.; Roncoroni, J.; Henry, M.A. Impact of stress and loneliness on health-related quality of life among low income senior African Americans. J. Racial Ethn. Health Disparities 2021, 8, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Jaremka, L.M.; Fagundes, C.P.; Peng, J.; Bennett, J.M.; Glaser, R.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Loneliness promotes inflammation during acute stress. Psychol. Sci. 2013, 24, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Raymo, J.M.; Wang, J. Loneliness at oder ages in the United States: Lonely life expectancy and the role of loneliness in health disparities. Demography 2022, 59, 921–947. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Thisted, R.A. Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol. Aging 2006, 21, 140–151. [Google Scholar] [CrossRef]
- Taylor, H.O.; Nguyen, A.W. Depressive symptoms and loneliness among Black and White older adults: The moderating effects of race. Innov. Aging 2020, 4, igaa048. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H. Social isolation, loneliness, and physical and mental health among black older adults. Annu. Rev. Gerontol. Geriatr. 2022, 41, 123–144. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.K.; Beach, S.R.H.; Philibert, R.A.; Cutrona, C.E.; Gibbons, F.X.; Barr, A. Economic hardship and biological weathering: The epigenetics of aging in a US sample of black women. Soc. Sci. Med. 2016, 150, 192–200. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.-K.; Klopack, E.; Beach, S.R.H.; Gibbons, F.X.; Philibert, R.A. The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Soc. Sci. Med. 2021, 282, 113169. [Google Scholar] [CrossRef] [PubMed]
- American Psychological Association. Stress in America. Available online: https://www.apa.org/news/press/releases/stress/index (accessed on 9 September 2022).
- Thoits, P.A. Stress and health: Major findings and policy implications. J. Health Soc. Behav. 2010, 51, S41–S53. [Google Scholar] [CrossRef]
- Umberson, D.; Williams, K.; Thomas, P.A.; Liu, H.; Thomeer, M.B. Race, gender, and chains of disadvantage: Childhood adversity, social relationships, and health. J. Health Soc. Behav. 2014, 55, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ghose, B.; Tang, S. Effect of financial stress on self-rereported health and quality of life among older adults in five developing countries: A cross sectional analysis of WHO-SAGE survey. BMC Geriatr. 2020, 20, 288. [Google Scholar] [CrossRef]
- Lei, M.-K.; Gibbons, F.X.; Gerrard, M.; Beach, S.R.H.; Dawes, K.; Philibert, R. Digital methylation assessments of alcohol and cigarette consumption account for common variance in accelerated epigenetic ageing. Epigenetics 2022, 1–15. [Google Scholar] [CrossRef]
- Beach, S.R.H.; Ong, M.L.; Gibbons, F.X.; Gerrard, M.; Lei, M.K.; Dawes, K.; Philibert, R. Epigenetic and Proteomic Biomarkers of Elevated Alcohol Use Predict Epigenetic Aging and Cell-Type variation Better than Self-Report. Genes 2022. under review. [Google Scholar]
- Shaw, B.A.; Agahi, N.; Krause, N. Are changes in financial strain associated with changes in alcohol use and smoking among older adults? J. Stud. Alcohol Drugs 2011, 72, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W. Human social genomics. PLoS Genet. 2014, 10, e1004601. [Google Scholar] [CrossRef]
- Klopack, E.T.; Crimmins, E.M.; Cole, S.W.; Seeman, T.E.; Carroll, J.E. Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the Health and Retirement Study. PNAS 2022, 119, e2202780119. [Google Scholar] [CrossRef]
- Ryan, C.P. “Epigenetic clocks”: Theory and applications in human biology. Am. J. Hum. Biol. 2021, 33, e23488. [Google Scholar] [CrossRef]
- Geronimus, A.T. The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethn. Dis. 1992, 2, 207–221. [Google Scholar]
- Hillary, R.F.; Stevenson, A.J.; McCartney, D.L.; Campbell, A.; Walker, R.M.; Howard, D.M.; Ritchie, C.W.; Horvath, S.; Hayward, C.; McIntosh, A.M. Epigenetic measures of ageing predict the prevalence and incidence of leading causes of death and disease burden. Clin. Epigenetics 2020, 12, 115. [Google Scholar] [CrossRef]
- Li, X.; Ploner, A.; Wang, Y.; Magnusson, P.K.; Reynolds, C.; Finkel, D.; Pedersen, N.L.; Jylhävä, J.; Hägg, S. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife 2020, 9, e51507. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- McCrory, C.; Fiorito, G.; Hernandez, B.; Polidoro, S.; O’Halloran, A.M.; Hever, A.; Ni Cheallaigh, C.; Lu, A.T.; Horvath, S.; Vineis, P. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J. Gerontol. Ser. A 2021, 76, 741–749. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Baccarelli, A.; Corcoran, D.L.; Gao, X.; Hannon, E.; Harrington, H.L.; Rasmussen, L.J.; Houts, R. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife 2020, 9, e54870. [Google Scholar] [CrossRef]
- Ellis, K.R.; Hecht, H.K.; Young, T.L.; Oh, S.; Thomas, S.; Hoggard, L.S.; Ali, Z.; Olawale, R.; Carthron, D.; Corbie-Smith, G. Peer reviewed: Chronic disease among African American families: A systematic scoping review. Prev. Chronic Dis. 2020, 17, E167. [Google Scholar] [CrossRef]
- Elliott, M.L.; Caspi, A.; Houts, R.M.; Ambler, A.; Broadbent, J.M.; Hancox, R.J.; Harrington, H.; Hogan, S.; Keenan, R.; Knodt, A. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat. Aging 2021, 1, 295–308. [Google Scholar] [CrossRef]
- Sugden, K.; Hannon, E.J.; Arseneault, L.; Belsky, D.W.; Corcoran, D.L.; Fisher, H.L.; Houts, R.M.; Kandaswamy, R.; Moffitt, T.E.; Poulton, R. Patterns of reliability: Assessing the reproducibility and integrity of DNA methylation measurement. Patterns 2020, 1, 100014. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Corcoran, D.L.; Sugden, K.; Poulton, R.; Arseneault, L.; Baccarelli, A.; Chamarti, K.; Gao, X.; Hannon, E. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife 2022, 11, e73420. [Google Scholar] [CrossRef]
- Prevention, C.f.D.C. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1226–1228. [Google Scholar]
- Mokdad, A.H.; Marks, J.S.; Stroup, D.F.; Gerberding, J.L. Actual causes of death in the United States, 2000. JAMA 2004, 291, 1238–1245. [Google Scholar] [CrossRef]
- Kanny, D.; Brewer, R.D.; Mesnick, J.B.; Paulozzi, L.J.; Naimi, T.S.; Lu, H. Vital signs: Alcohol poisoning deaths—United States, 2010–2012. Morb. Mortal. Wkly. Rep. 2015, 63, 1238. [Google Scholar]
- Oblak, L.; Van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Aging Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Simons, R.L.; Ong, M.L.; Lei, M.-K.; Klopach, E.; Berg, M.; Zhang, Y.; Philibert, R.; Gibbons, F.X.; Beach, S.R.H. Shifts in lifestyle and socioeconomic circumstances predict change—For better or worse—In speed of epigenetic aging: A study of middle-aged black women. Soc. Sci. Med. 2022, 307, 115175. [Google Scholar] [CrossRef]
- Dogan, M.V.; Grumbach, I.M.; Michaelson, J.J.; Philibert, R.A. Integrated genetic and epigenetic prediction of coronary heart disease in the Framingham Heart Study. PLoS ONE 2018, 13, e0190549. [Google Scholar] [CrossRef]
- Dogan, M.V.; Beach, S.R.; Simons, R.L.; Lendasse, A.; Penaluna, B.; Philibert, R.A. Blood-based biomarkers for predicting the risk for five-year incident coronary heart disease in the Framingham Heart Study via machine learning. Genes 2018, 9, 641. [Google Scholar] [CrossRef]
- Lei, M.-K.; Simons, R.L.; Beach, S.R.H.; Philibert, R.A. Neighborhood disadvantage and biological aging: Using marginal structural models to assess the link between neighborhood census variables and epigenetic aging. J. Gerontol. Ser. B 2019, 74, e50–e59. [Google Scholar] [CrossRef]
- Lei, M.-K.; Berg, M.T.; Simons, R.L.; Beach, S.R.H. Neighborhood structural disadvantage and biological aging in a sample of Black middle age and young adults. Soc. Sci. Med. 2022, 293, 114654. [Google Scholar] [CrossRef]
- Beach, S.R.H.; Ong, M.L.; Lei, M.-K.; Carter, S.E.; Simons, R.L.; Gibbons, F.X.; Philibert, R.A. Methylation of FKBP5 is associated with accelerated DNA methylation ageing and cardiometabolic risk: Replication in young-adult and middle-aged Black Americans. Epigenetics 2021, 17, 982–1002. [Google Scholar] [CrossRef]
- Simons, R.L.; Ong, M.L.; Lei, M.-K.; Klopack, E.; Berg, M.; Zhang, Y.; Philibert, R.; Beach, S.R.H. Unstable childhood, adult adversity, and smoking accelerate biological aging among middle-age African Americans: Similar findings for GrimAge and PoAm. J. Aging Health 2021, 34, 08982643211043668. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Chen, B.H.; Marioni, R.E.; Colicino, E.; Peters, M.J.; Ward-Caviness, C.K.; Tsai, P.-C.; Roetker, N.S.; Just, A.C.; Demerath, E.W.; Guan, W. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging 2016, 8, 1844–1859. [Google Scholar] [CrossRef]
- Carr, D. Golden Years?: Social Inequality in Later Life; Russell Sage Foundation: New York, NY, USA, 2019. [Google Scholar]
- Jia, H.; Lubetkin, E.I. Life expectancy and active life expectancy by marital status among older US adults: Results from the US Medicare Health Outcome Survey (HOS). SSM-Popul. Health 2020, 12, 100642. [Google Scholar] [CrossRef]
- Lei, M.-K.; Gibbons, F.X.; Simons, R.L.; Philibert, R.A.; Beach, S.R.H. The effect of tobacco smoking differs across indices of DNA methylation-based aging in an African American sample: DNA methylation-based indices of smoking capture these effects. Genes 2020, 11, 311. [Google Scholar] [CrossRef]
- Gibbons, F.X.; Gerrard, M.; Cleveland, M.J.; Wills, T.A.; Brody, G. Perceived discrimination and substance use in African American parents and their children: A panel study. J. Personal. Soc. Psychol. 2004, 86, 517. [Google Scholar] [CrossRef]
- Russell, D.W. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. J. Personal. Assess. 1996, 66, 20–40. [Google Scholar] [CrossRef]
- Russell, D.; Peplau, L.A.; Cutrona, C.E. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J. Personal. Soc. Psychol. 1980, 39, 472–480. [Google Scholar] [CrossRef]
- Davis, S.; Bilke, S. An Introduction to the Methylumi Package. Bioconductor Package. 2010. Available online: https://bioconductor.statistik.tu-dortmund.de/packages/3.8/bioc/vignettes/methylumi/inst/doc/methylumi.pdf (accessed on 9 September 2022).
- Wong, C.C.; Pidsley, R.; Schalkwyk, L.C. The WateRmelon Package. 2013. Available online: https://rdrr.io/bioc/wateRmelon/f/inst/doc/wateRmelon.pdf (accessed on 9 September 2022).
- Illumina Infinium MethylationEPIC Product Files. Available online: https://support.illumina.com/array/array_kits/infinium-methylationepic-beadchip-kit/downloads.html (accessed on 9 September 2022).
- Beach, S.R.H.; Gibbons, F.X.; Carter, S.E.; Ong, M.L.; Lavner, J.A.; Lei, M.-K.; Simons, R.L.; Gerrard, M.; Philibert, R.A. Childhood adversity predicts black young adults’ DNA methylation-based accelerated aging: A dual pathway model. Dev. Psychopathol. 2022, 34, 689–703. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Marabita, F.; Lechner, M.; Bartlett, T.; Tegner, J.; Gomez-Cabrero, D.; Beck, S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013, 29, 189–196. [Google Scholar] [CrossRef]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef]
- Triche, T.J., Jr.; Weisenberger, D.J.; Van Den Berg, D.; Laird, P.W.; Siegmund, K.D. Low-level processing of Illumina Infinium DNA methylation beadarrays. Nucleic Acids Res. 2013, 41, e90. [Google Scholar] [CrossRef]
- Philibert, R.; Miller, S.; Noel, A.; Dawes, K.; Papworth, E.; Black, D.W.; Beach, S.R.H.; Long, J.D.; Mills, J.A.; Dogan, M. A four marker digital PCR toolkit for detecting heavy alcohol consumption and the effectiveness of its treatment. J. Insur. Med. 2019, 48, 90–102. [Google Scholar] [CrossRef]
- Miller, S.; Mills, J.A.; Long, J.; Philibert, R. A comparison of the predictive power of DNA methylation with carbohydrate deficient transferrin for heavy alcohol consumption. Epigenetics 2021, 16, 969–979. [Google Scholar] [CrossRef]
- Dawes, K.; Andersen, A.; Reimer, R.; Mills, J.A.; Hoffman, E.; Long, J.D.; Miller, S.; Philibert, R. The relationship of smoking to cg05575921 methylation in blood and saliva DNA samples from several studies. Sci. Rep. 2021, 11, 21627. [Google Scholar] [CrossRef]
- Hillary, R.F.; Marioni, R.E. MethylDetectR: A software for methylation-based health profiling. Wellcome Open Res. 2020, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef]
- Curran, P.J.; Bollen, K.A. The best of both worlds: Combining autoregressive and latent curve models. In New Methods for the Analysis of Change; Collins, L.M., Sayer, A.G., Eds.; American Psychological Association: Washington, DC, USA, 2001; pp. 107–135. [Google Scholar] [CrossRef]
- Gabrio, A.; Plumpton, C.; Banerjee, S.; Leurent, B. Linear mixed models to handle missing at random data in trial-based economic evaluations. Health Econ. 2022, 31, 1276–1287. [Google Scholar] [CrossRef]
- Graham, J.W. Missing data analysis: Making it work in the real world. Annu. Rev. Psychol. 2009, 60, 549–576. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.L.; Lei, M.K.; Stewart, E.A.; Beach, S.R.H.; Brody, G.H.; Philibert, R.A.; Gibbons, F.X. Social adversity, genetic variation, street code, and aggression: A genetically informed model of violent behavior. Youth Violence Juv. Justice 2012, 10, 3–24. [Google Scholar] [CrossRef]
- Luchman, J.N. Determining relative importance in Stata using dominance analysis: Domin and domme. Stata J. 2021, 21, 510–538. [Google Scholar] [CrossRef]
- Barr, A.B.; Simons, L.G.; Simons, R.L.; Beach, S.R.H.; Philibert, R.A. Sharing the burden of the transition to adulthood: African American young adults’ transition challenges and their mothers’ health risk. J. Am. Sociol. Rev. 2018, 83, 143–172. [Google Scholar] [CrossRef]
| 2008 (n = 495) | 2019 (n = 478) | |||
|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD |
| DunedinPACE | 1.057 | 0.143 | 1.111 | 0.142 |
| Age | 48.765 | 8.354 | 57.143 | 6.781 |
| Loneliness | 3.420 | 1.486 | 6.730 | 1.636 |
| Per capita income | 13,019.750 | 13,494.020 | 19,463.940 | 17,763.060 |
| Female | 0.745 | 0.436 | 0.736 | 0.441 |
| Married/cohabiting | 0.578 | 0.494 | 0.554 | 0.498 |
| cg05575921 | 0.802 | 0.129 | 0.801 | 0.126 |
| Alcohol use | −12.371 | 0.463 | −12.191 | 0.447 |
| CD8+ T cells | 0.090 | 0.052 | 0.091 | 0.052 |
| CD4+ T cells | 0.205 | 0.081 | 0.180 | 0.073 |
| Natural killer cells | 0.032 | 0.042 | 0.031 | 0.039 |
| B cells | 0.085 | 0.064 | 0.078 | 0.055 |
| Monocytes | 0.055 | 0.026 | 0.064 | 0.030 |
| Model 1 | Model 2 | |
|---|---|---|
| Growth factor means | ||
| Initial status (age 30) | 0.986 ** | 0.963 ** |
| Linear growth rate (per year of age) | 0.004 ** | 0.006 ** |
| Quadratic growth rate | −0.001 | |
| Random variances | ||
| Initial status (age 30) | 0.007 | 0.007 |
| Linear growth rate (per year of age) | 2.45 × 10−6 | 4.25 × 10−18 |
| Quadratic growth rate | 8.78 × 10−10 | |
| Couple variance | 0.007 | 0.008 |
| Residual variance | 0.005 | 0.005 |
| DunedinPACE | ||||||
|---|---|---|---|---|---|---|
| Model 1A | Model 1B | Model 2A | Model 2B | Model 3A | Model 3B | |
| Variables | b/(SE) | b/(SE) | b/(SE) | b/(SE) | b/(SE) | b/(SE) |
| Fixed effects | ||||||
| Initial status | 1.014 ** (0.019) | 1.136 ** (0.115) | 0.981 ** (0.017) | 1.146 ** (0.114) | 1.006 ** (0.019) | 1.099 ** (0.115) |
| Linear growth rate | 0.003 ** (0.001) | 0.003 ** (0.001) | 0.005 ** (0.001) | 0.004 ** (0.001) | 0.004 ** (0.001) | 0.004 ** (0.001) |
| Time-varying covariates | ||||||
| Loneliness | 0.009 * (0.004) | 0.010 ** (0.004) | 0.012 ** (0.004) | 0.012 ** (0.004) | ||
| Per capita income | −0.016 ** (0.004) | −0.011 * (0.004) | −0.017 ** (0.004) | −0.013 ** (0.004) | ||
| Married/cohabiting | −0.011 (0.009) | −0.010 (0.009) | −0.006 (0.009) | −0.005 (0.009) | −0.008 (0.009) | −0.006 (0.009) |
| cg05575921 | −0.375 ** (0.039) | −0.358 ** (0.040) | −0.359 ** (0.039) | |||
| Alcohol use | −0.013 (0.010) | −0.008 (0.009) | −0.014 (0.010) | |||
| Time-invariant covariates | ||||||
| Female | 0.001 (0.013) | 0.030 * (0.012) | −0.001 (0.013) | 0.029 * (0.012) | −0.001 (0.012) | 0.028 * (0.012) |
| Random effects | ||||||
| 0.016 * | 0.015 * | 0.018 * | 0.017 * | 0.017 * | 0.016 * | |
| 4.31 × 10−6 | 3.25 × 10−6 | 9.26 × 10−6 | 7.00 × 10−6 | 6.72 × 10−6 | 4.75 × 10−6 | |
| −0.001 | −0.001 | −0.001 | −0.001 | −0.001 | −0.001 | |
| 0.005 * | 0.005 * | 0.005 * | 0.005 * | 0.005 * | 0.005 * | |
| DunedinPACE | ||||
|---|---|---|---|---|
| Model | Standardized Dominance Weight for within Individual | Standardized Dominance Weight for between Individual | ||
| Variables | b | SE | ||
| Fixed effects | ||||
| Initial status | 1.273 ** | 0.106 | ||
| Linear growth rate | 0.003 ** | 0.001 | 0.059 | 0.035 |
| Time-varying covariates | ||||
| Loneliness | 0.008 ** | 0.003 | 0.040 | 0.030 |
| Per capita income | −0.013 ** | 0.004 | 0.044 | 0.051 |
| Married/cohabiting | −0.003 | 0.008 | 0.009 | 0.010 |
| cg05575921 | −0.326 ** | 0.036 | 0.286 | 0.317 |
| Alcohol use | −0.013 | 0.009 | 0.003 | 0.002 |
| CD8+ T cells | −0.520 ** | 0.078 | 0.185 | 0.193 |
| CD4+ T cells | −0.481 ** | 0.053 | 0.272 | 0.268 |
| Natural killer cells | −0.302 ** | 0.096 | 0.027 | 0.027 |
| B cells | −0.170 * | 0.066 | 0.024 | 0.020 |
| Monocytes | 0.265 † | 0.140 | 0.039 | 0.032 |
| Time-invariant covariates | ||||
| Female | 0.028 * | 0.011 | 0.004 | 0.015 |
| Random effects | ||||
| 0.010 * | ||||
| 5.07 × 10−7 | ||||
| 0.001 | ||||
| 0.004 * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beach, S.R.H.; Klopack, E.T.; Carter, S.E.; Philibert, R.A.; Simons, R.L.; Gibbons, F.X.; Ong, M.L.; Gerrard, M.; Lei, M.-K. Do Loneliness and Per Capita Income Combine to Increase the Pace of Biological Aging for Black Adults across Late Middle Age? Int. J. Environ. Res. Public Health 2022, 19, 13421. https://doi.org/10.3390/ijerph192013421
Beach SRH, Klopack ET, Carter SE, Philibert RA, Simons RL, Gibbons FX, Ong ML, Gerrard M, Lei M-K. Do Loneliness and Per Capita Income Combine to Increase the Pace of Biological Aging for Black Adults across Late Middle Age? International Journal of Environmental Research and Public Health. 2022; 19(20):13421. https://doi.org/10.3390/ijerph192013421
Chicago/Turabian StyleBeach, Steven R. H., Eric T. Klopack, Sierra E. Carter, Robert A. Philibert, Ronald L. Simons, Frederick X. Gibbons, Mei Ling Ong, Meg Gerrard, and Man-Kit Lei. 2022. "Do Loneliness and Per Capita Income Combine to Increase the Pace of Biological Aging for Black Adults across Late Middle Age?" International Journal of Environmental Research and Public Health 19, no. 20: 13421. https://doi.org/10.3390/ijerph192013421
APA StyleBeach, S. R. H., Klopack, E. T., Carter, S. E., Philibert, R. A., Simons, R. L., Gibbons, F. X., Ong, M. L., Gerrard, M., & Lei, M.-K. (2022). Do Loneliness and Per Capita Income Combine to Increase the Pace of Biological Aging for Black Adults across Late Middle Age? International Journal of Environmental Research and Public Health, 19(20), 13421. https://doi.org/10.3390/ijerph192013421







