Heterotrophic Bioleaching of Vanadium from Low-Grade Stone Coal by Aerobic Microbial Consortium
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and the Inoculum
2.2. Bioreactor Setting-Up and Experimental Operations
2.3. Analytical Methods
2.4. Microbiological Analysis
3. Results and Discussion
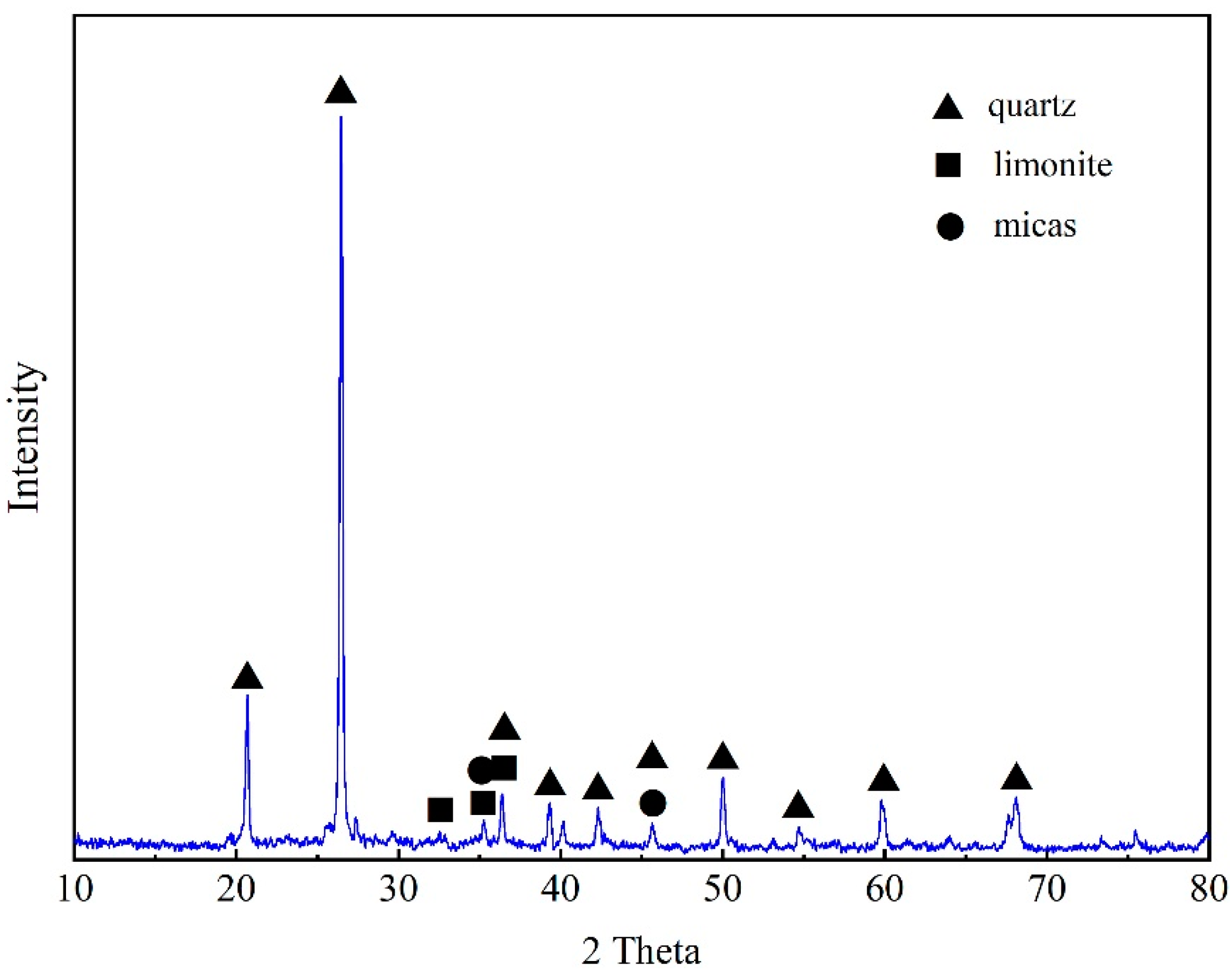
3.1. Characteristics of the Stone Coal
3.2. Bioleaching Performance
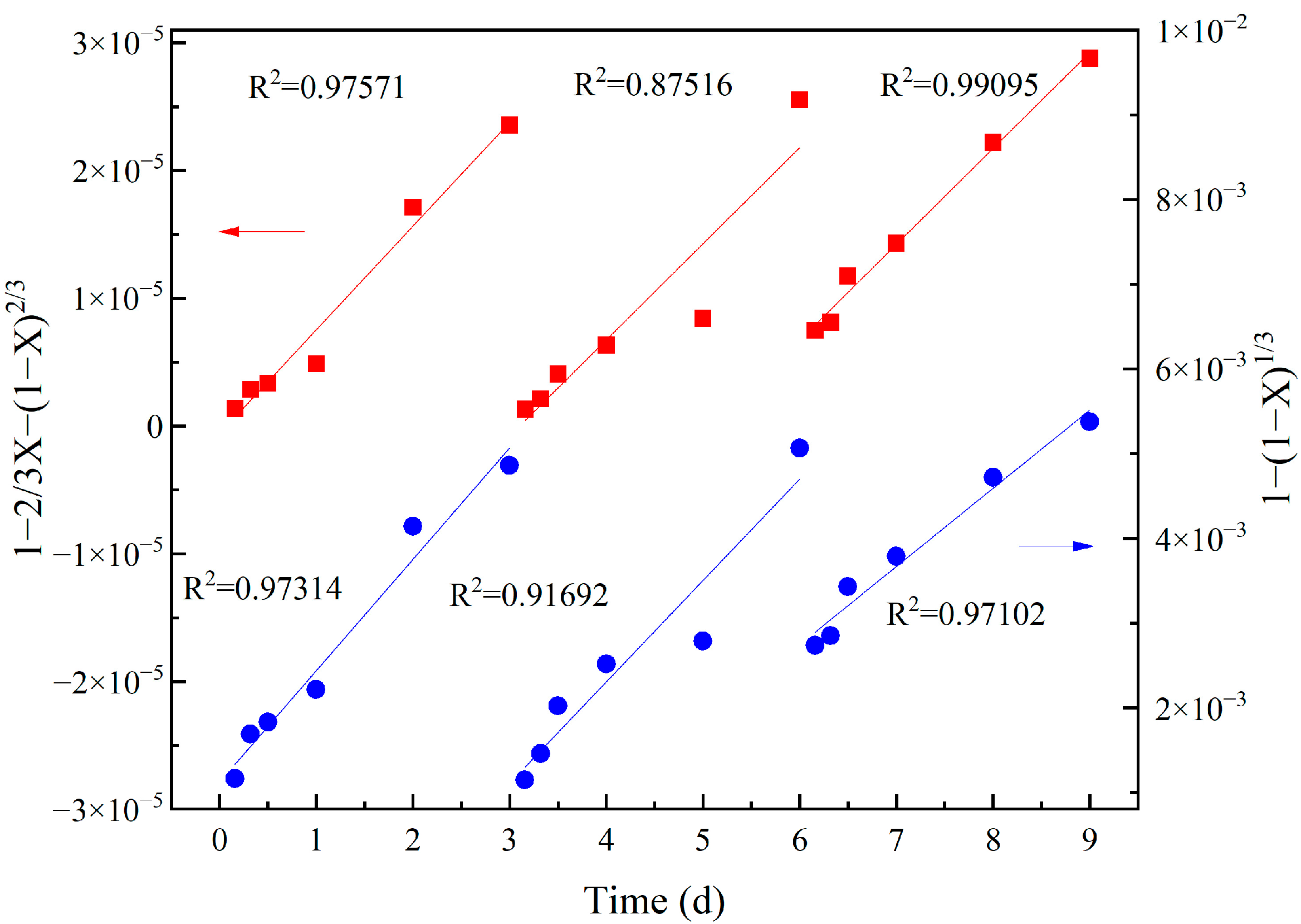
3.3. Vanadium Leaching Kinetics
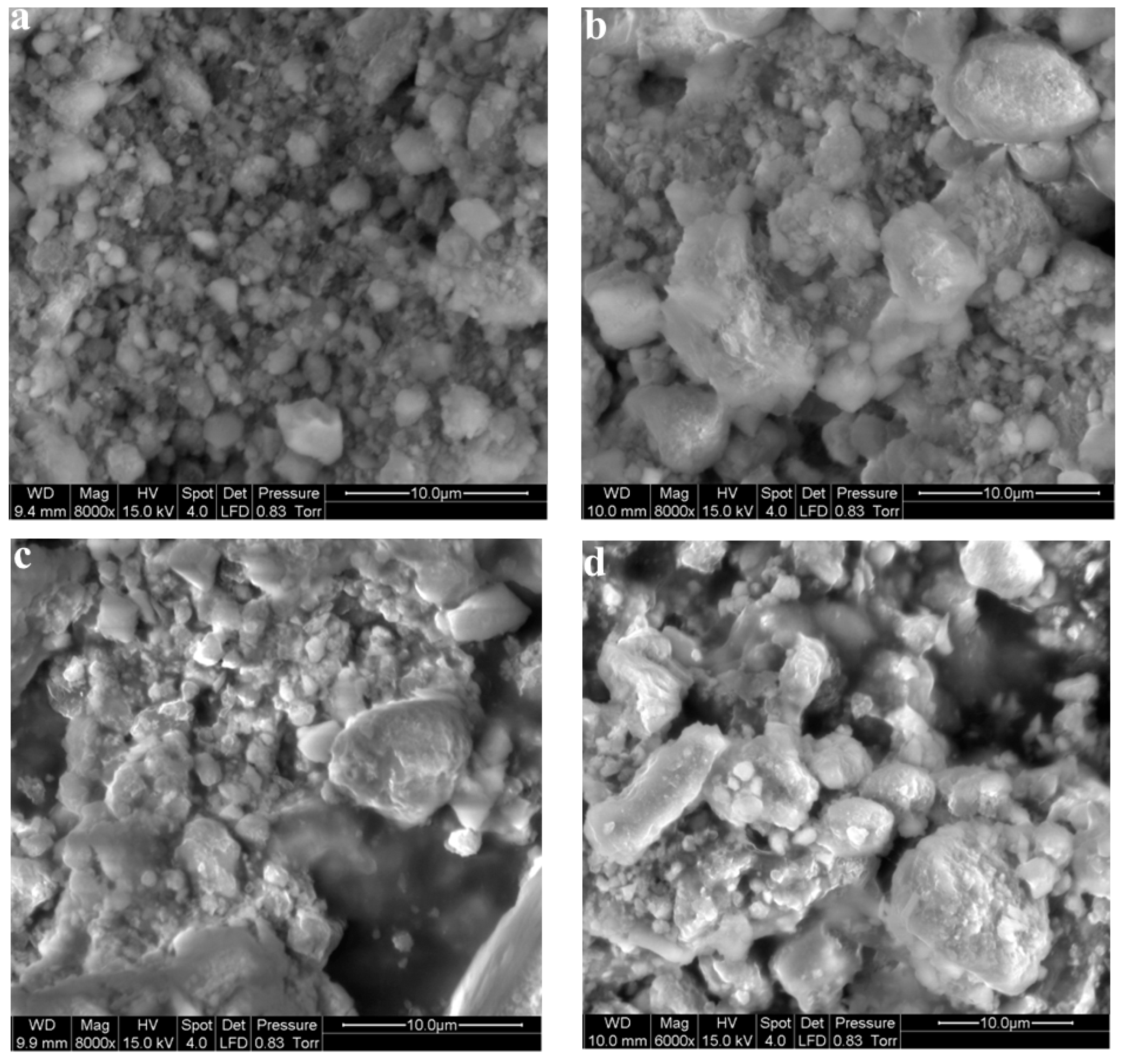
3.4. Morphological Characterization
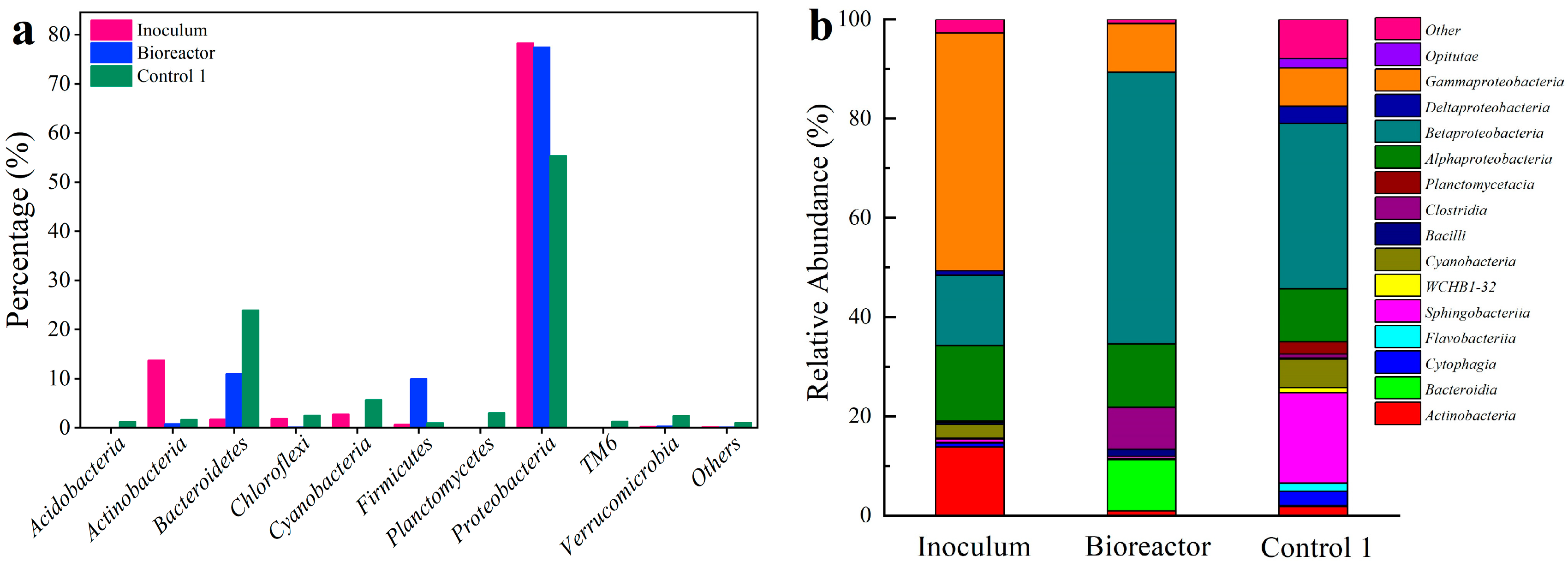
3.5. Microbial Community Characteristics
3.6. Evaluation of Practical Implication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fei, Y.; Zhang, B.; He, J.; Chen, C.; Liu, H. Dynamics of vertical vanadium migration in soil and interactions with indigenous microorganisms adjacent to tailing reservoir. J. Hazard. Mater. 2022, 424, 127608. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, L.; Tian, C.; Yuan, S.; Feng, C.; Ni, J.; Borthwick, A.G.L. Microbial reduction and precipitation of vanadium (V) in groundwater by immobilized mixed anaerobic culture. Bioresour. Technol. 2015, 192, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kurniawan; Kim, E.Y.; Chung, K.W.; Kim, R.; Jeon, H.S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Zhang, C. Extraction and removal of vanadium by adsorption with resin 201*7 from vanadium waste liquid. Environ. Res. 2020, 180, 108865. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Alessi, D.S.; Tack, F.M.G.; Ok, Y.S.; Kim, K.H.; Gustafsson, J.P.; Sparks, D.L.; Rinklebe, J. Redox chemistry of vanadium in soils and sediments: Interactions with colloidal materials, mobilization, speciation, and relevant environmental implications—A review. Adv. Colloid Interface Sci. 2019, 265, 1–13. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Li, L.; Graff, G.; Liu, J.; Zhang, H.; Yang, Z.; Hu, J.A. Towards understanding the poor thermal stability of V5+ electrolyte solution in vanadium redox flow batteries. J. Power Sources 2011, 196, 3669–3672. [Google Scholar] [CrossRef]
- He, Z.; Lv, Y.; Zhang, T.; Zhu, Y.; Dai, L.; Yao, S.; Zhu, W.; Wang, L. Electrode materials for vanadium redox flow batteries: Intrinsic treatment and introducing catalyst. Chem. Eng. J. 2022, 427, 131680. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Li, J.; Liu, H.; Wu, S. Leaching of vanadium from carbonaceous shale. Hydrometallurgy 2009, 99, 97–99. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Li, P.; Li, S. A new process of extracting vanadium from stone coal. Int. J. Miner. Metall. Mater. 2010, 17, 381–388. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Jin, J.; Zhou, Z. New Insights into the Penetration Depth of Sulfuric Acid and Leaching Effect in the Sulfuric Acid Curing-Leaching Process of Vanadium-Bearing Stone Coal. ACS Omega 2021, 6, 17599–17608. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, S.; Liu, T.; Chen, T.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, T.; Chen, T.; Bian, Y.; Bao, S. Pre-concentration of vanadium from stone coal by gravity separation. J. Miner. Process. 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.; Huang, J.; Liu, T.; Wang, L. Separation of impurities aluminum and iron during pressure acid leaching of vanadium from stone. J. Cleaner Prod. 2017, 166, 1265–1273. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Zhang, Y.; Song, S.; Bao, S. In-situ investigation on mineral phase transition during roasting of vanadium-bearing stone coal. Adv. Powder Technol. 2017, 28, 1103–1107. [Google Scholar] [CrossRef]
- Xin, B.; Jiang, W.; Aslam, H.; Zhang, K.; Liu, C.; Wang, R.; Wang, Y. Bioleaching of zinc and manganese from spent Zn-Mn batteries and mechanism exploration. Bioresour. Technol. 2012, 106, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hocheng, H.; Chang, J.H.; Jadhav, U.U. Micromachining of various metals by using Acidithiobacillus ferrooxidans 13820 culture supernatant experiments. J. Cleaner Prod. 2012, 20, 180–185. [Google Scholar] [CrossRef]
- Rastegar, S.O.; Mousavi, S.M.; Shojaosadati, S.A.; Mamoory, R.S. Bioleaching of V, Ni, and Cu from residual produced in oil fired furnaces using Acidithiobacillus ferrooxidans. Hydrometallurgy 2015, 157, 50–59. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Liu, Y.; Zhao, Y. Blank roasting and bioleaching of stone coal for vanadium recycling. J. Cleaner Prod. 2020, 243, 118625. [Google Scholar] [CrossRef]
- Dong, Y.; Chong, S.; Lin, H. Enhanced effect of biochar on leaching vanadium and copper from stone coal tailings by Thiobacillus ferrooxidans. Environ. Sci. Pollut. Res. 2022, 29, 20398–20408. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Mishra, D.; Kim, D.J.; Ahn, J.G.; Chaudhury, G.R.; Lee, S.W. Bioleaching kinetics and multivariate analysis of spent petroleum catalyst dissolution using two acidophiles. J. Hazard. Mater. 2010, 175, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bredberg, K.; Karlsson, H.T.; Holst, O. Reduction of vanadium (V) with Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Bioresour. Technol. 2004, 92, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, T.; Zhang, Y.; Cai, Z.; He, J.; Xu, C. Vanadium bioleaching behavior by Acidithiobacillus ferrooxidans from a vanadium-bearing shale. Minerals 2018, 8, 24. [Google Scholar] [CrossRef]
- Mirazimi, S.M.J.; Abbasalipour, Z.; Rashchi, F. Vanadium removal from LD converter slag using bacteria and fungi. J. Environ. Manag. 2015, 153, 144–151. [Google Scholar] [CrossRef]
- Varia, J.C.; Snellings, R.; Hennebel, T. Sustainable Metal Recovery from Secondary Resources: Screening and Kinetic Studies Using Analogue Heterotrophic Metabolites. Waste Biomass Valorization 2021, 12, 2703–2721. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Xu, H.; Jiang, Z. Co-applying biochar and manganese ore can improve the formation and stability of humic acid during co-composting of sewage sludge and corn straw. Bioresour. Technol. 2022, 358, 127297. [Google Scholar] [CrossRef]
- Sajjad, W.; Zheng, G.; Din, G.; Ma, X.; Rafiq, M.; Xu, W. Metals Extraction from Sulfide Ores with Microorganisms: The Bioleaching Technology and Recent Developments. Trans. Indian Inst. Met. 2019, 72, 559–579. [Google Scholar] [CrossRef]
- Wan, Q.; Han, Q.; Luo, H.; He, T.; Xue, F.; Ye, Z.; Chen, C.; Huang, S. Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater. Int. J. Environ. Res. Public Health 2020, 17, 4692. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, J.; Chen, J.; Chen, D.; Deng, Q. The role of available phosphorous in vanadate decontamination by soil indigenous microbial consortia. Environ. Pollut. 2021, 289, 117839. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Xing, Y.; Hao, L. Behavior of dissolved organic carbon sources on the microbial reduction and precipitation of vanadium (V) in groundwater. RSC Adv. 2016, 6, 97253–97258. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, B.; Qiu, R.; Lai, C.; Jiang, Y.; He, C.; Guo, J. Microbial chromate reduction coupled to anaerobic oxidation of elemental sulfur or zerovalent iron. Environ. Sci. Technol. 2019, 53, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Z.; Zhang, B.; Li, L.; Sun, W. Synergy between pyridine anaerobic mineralization and vanadium (V) oxyanion bio-reduction for aquifer remediation. J. Hazard. Mater. 2021, 48, 126339. [Google Scholar] [CrossRef]
- Biswas, S.; Dey, R.; Mukherjee, S.; Banerjee, P.C. Bioleaching of nickel and cobalt from lateritic chromite overburden using the culture filtrate of Aspergillus niger. Appl. Biochem. Biotechnol. 2013, 170, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Mulaba-Bafubiandi, A.F. Advances in microbial leaching processes for nickel extraction from lateritic minerals—A review. Korean J. Chem. Eng. 2015, 32, 1447–1454. [Google Scholar] [CrossRef]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Nicholson, N.; Loudon, C.M.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; et al. Microbially-Enhanced Vanadium Mining and Bioremediation Under Micro- and Mars Gravity on the International Space Station. Front. Microbiol. 2021, 12, 641387. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Effect of silicate activator pH on the leaching and material characteristics of waste-based inorganic polymers. Miner. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M. Maximization of organic acids production by Aspergillus niger in a bubble column bioreactor for vanadium and Ni recovery enhancement from power plant residual ash in spent-medium bioleaching experiments. Bioresour. Technol. 2016, 216, 729–736. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of spent hydro-processing catalyst using acidophilic bacteria and its kinetics aspect. J. Hazard. Mater. 2008, 152, 1082–1091. [Google Scholar] [CrossRef]
- He, C.; Zhang, B.; Lu, J.; Qiu, R. A newly discovered function of nitrate reductase in chemoautotrophic vanadate transformation by natural mackinawite in aquifer. Water Res. 2021, 189, 116664. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, B.; Cheng, Y.; Peng, K.J. Microbial vanadate reduction coupled to co-metabolic phenanthrene biodegradation in groundwater. Water Res. 2020, 186, 116354. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, J.; Chen, S.; Wang, J.; Zhang, B. Synchronous bio-reduction of Uranium(VI) and Vanadium(V) in aquifer: Performance and mechanisms. Chemosphere 2022, 288, 132539. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Cheng, Y.; Tang, C.; Chen, Z. Bioleaching of heavy metals from sewage sludge using indigenous iron-oxidizing microorganisms. J. Soils Sediments 2013, 13, 166–175. [Google Scholar] [CrossRef]
- Tabak, O.; Mete, B.; Aydin, S.; Mandel, N.M.; Otlu, B.; Ozaras, R.; Tabak, F. Port-related Delftia Tsuruhatensis bacteremia in a patient with breast cancer. New Microbiol. 2013, 36, 199–201. [Google Scholar] [PubMed]
- Brandl, H.; Lehman, S.; Faramazi, M.A.; Martinelli, D. Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy 2008, 94, 14–17. [Google Scholar] [CrossRef]
- Mishra, D.; Rhee, Y.H. Microbial leaching of metals from solid industrial wastes. J. Microbiol. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, B.; Liu, J.; Fang, Y.; Wang, A. Spatiotemporal dynamics in microbial communities mediating biogeochemical cycling of nutrients across the Xiaowan Reservoir in Lancang River. Sci. Total Environ. 2022, 813, 151862. [Google Scholar] [CrossRef]
- Brereton, N.J.B.; Gonzalez, E.; Desjardins, D.; Labrecque, M.; Pitre, F.E. Co-cropping with three phytoremediation crops influences rhizosphere microbiome community in contaminated soil. Sci. Total Environ. 2020, 711, 135067. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Jiang, Z.; Li, Q. Molecular understanding of autotrophic CO2-fixing bacterial communities in composting based on RuBisCO genes analysis. J. Biotechnol. 2020, 320, 36–43. [Google Scholar] [CrossRef]
- She, J.; Wang, J.; Wei, X.; Zhang, Q.; Xie, Z.; Beiyuan, J.; Xiao, E.; Yang, X.; Liu, J.; Zhou, Y.; et al. Survival strategies and dominant phylotypes of maize-rhizosphere microorganisms under metal(loid)s contamination. Sci. Total Environ. 2021, 774, 145143. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Liu, R.; Gu, X. Flotation recovery of vanadium from low-grade stone coal. Trans. Nonferrous Met. Soc. China 2014, 24, 1145–1151. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Z.; Liu, H.; Wang, W.; Cao, Y. Direct Acid Leaching of Vanadium from Stone Coal. Hign Temp. Mater. Process. 2016, 36, 877–883. [Google Scholar] [CrossRef]
- Long, S.; Feng, Q.; Zhang, G.; He, D. Recovery of vanadium from alkaline leaching solution from roasted stone coal. Sci. Asia. 2014, 40, 69–72. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Zhang, B.; Yang, M.; Lin, H. Bioleaching of vanadium by Acidithiobacillus ferrooxidans from vanadium-bearing resources: Performance and mechanisms. J. Hazard. Mater. 2021, 416, 125843. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.; Funari, V.; Mayes, W.; Rogerson, M.; Prior, T. Recovery of Al, Cr and V from steel slag by bioleaching: Batch and column experiments. J. Environ. Manag. 2018, 222, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Kim, D.; Ralph, D.; Ahn, J.; Rhee, Y. Bioleaching of vanadium rich spent refinery catalysts using sulfur oxidizing lithotrophs. Hydrometallurgy 2007, 88, 202–209. [Google Scholar] [CrossRef]
- Dong, Y.; Chong, S.; Lin, H. Bioleaching and biosorption behavior of vanadium-bearing stone coal by Bacillus mucilaginosus. Int. J. Miner. Metall. Mater. 2022, 30, 1–10. [Google Scholar]
- Bellenberg, S.; Turner, S.; Seidel, L.; Wyk, N.; Zhang, R.; Sachpazidou, V.; Embile, R.; Walder, I.; Leiviska, T.; Dopson, M. Towards Bioleaching of a Vanadium Containing Magnetite for Metal Recovery. Front. Microbiol. 2021, 12, 693615. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shi, J.; Chen, C.; Yang, M.; Lu, J.; Zhang, B. Heterotrophic Bioleaching of Vanadium from Low-Grade Stone Coal by Aerobic Microbial Consortium. Int. J. Environ. Res. Public Health 2022, 19, 13375. https://doi.org/10.3390/ijerph192013375
Zhang H, Shi J, Chen C, Yang M, Lu J, Zhang B. Heterotrophic Bioleaching of Vanadium from Low-Grade Stone Coal by Aerobic Microbial Consortium. International Journal of Environmental Research and Public Health. 2022; 19(20):13375. https://doi.org/10.3390/ijerph192013375
Chicago/Turabian StyleZhang, Han, Jiaxin Shi, Cuibai Chen, Meng Yang, Jianping Lu, and Baogang Zhang. 2022. "Heterotrophic Bioleaching of Vanadium from Low-Grade Stone Coal by Aerobic Microbial Consortium" International Journal of Environmental Research and Public Health 19, no. 20: 13375. https://doi.org/10.3390/ijerph192013375
APA StyleZhang, H., Shi, J., Chen, C., Yang, M., Lu, J., & Zhang, B. (2022). Heterotrophic Bioleaching of Vanadium from Low-Grade Stone Coal by Aerobic Microbial Consortium. International Journal of Environmental Research and Public Health, 19(20), 13375. https://doi.org/10.3390/ijerph192013375





