Automated Analysis of Sleep Study Parameters Using Signal Processing and Artificial Intelligence
Abstract
1. Introduction
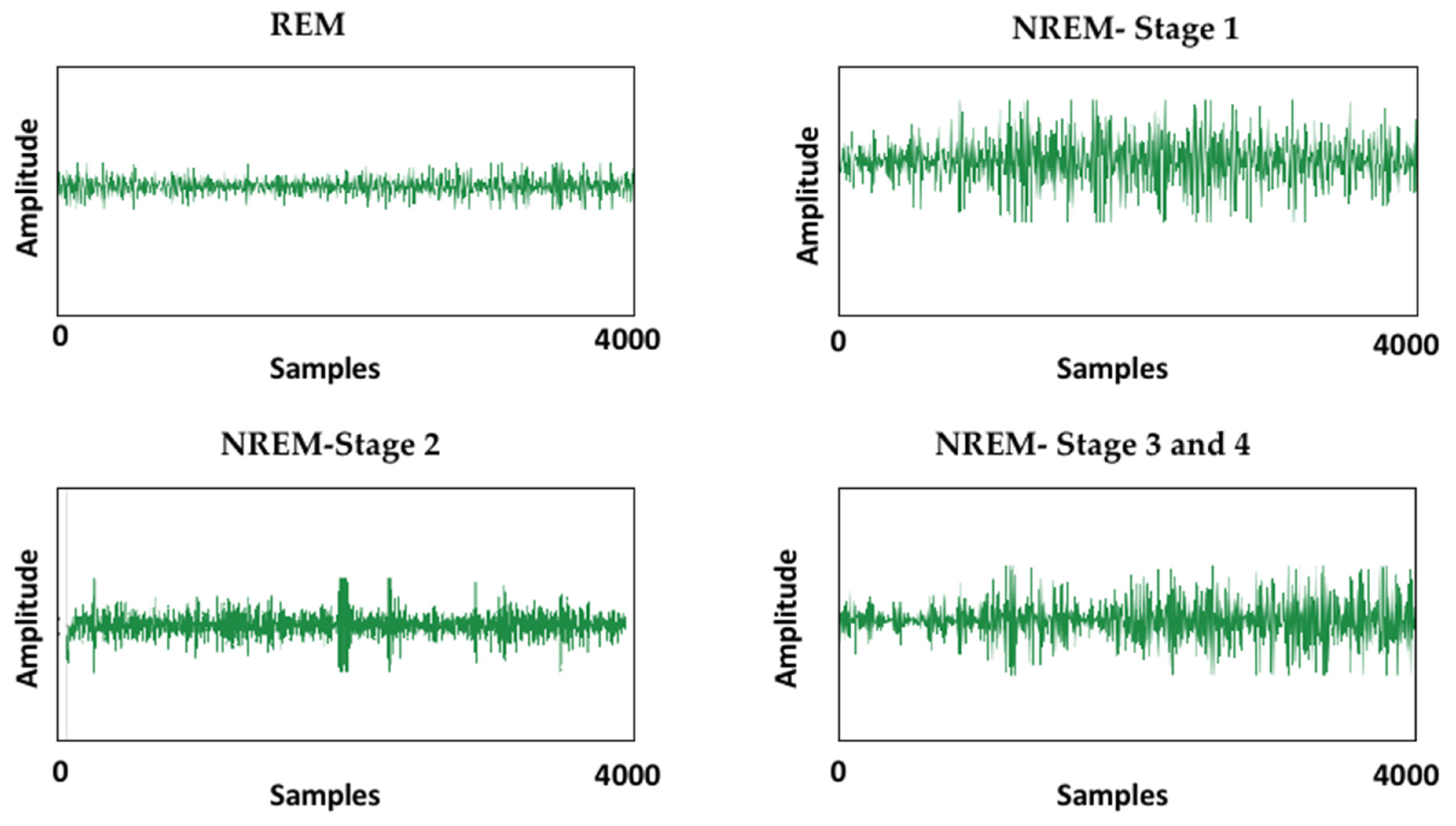
- The decomposition of complex EEG signals, also known as noise-contaminated signals, as they may contain an abundance of unwanted information into different intrinsic modes so that signal processing at later stages becomes easy.
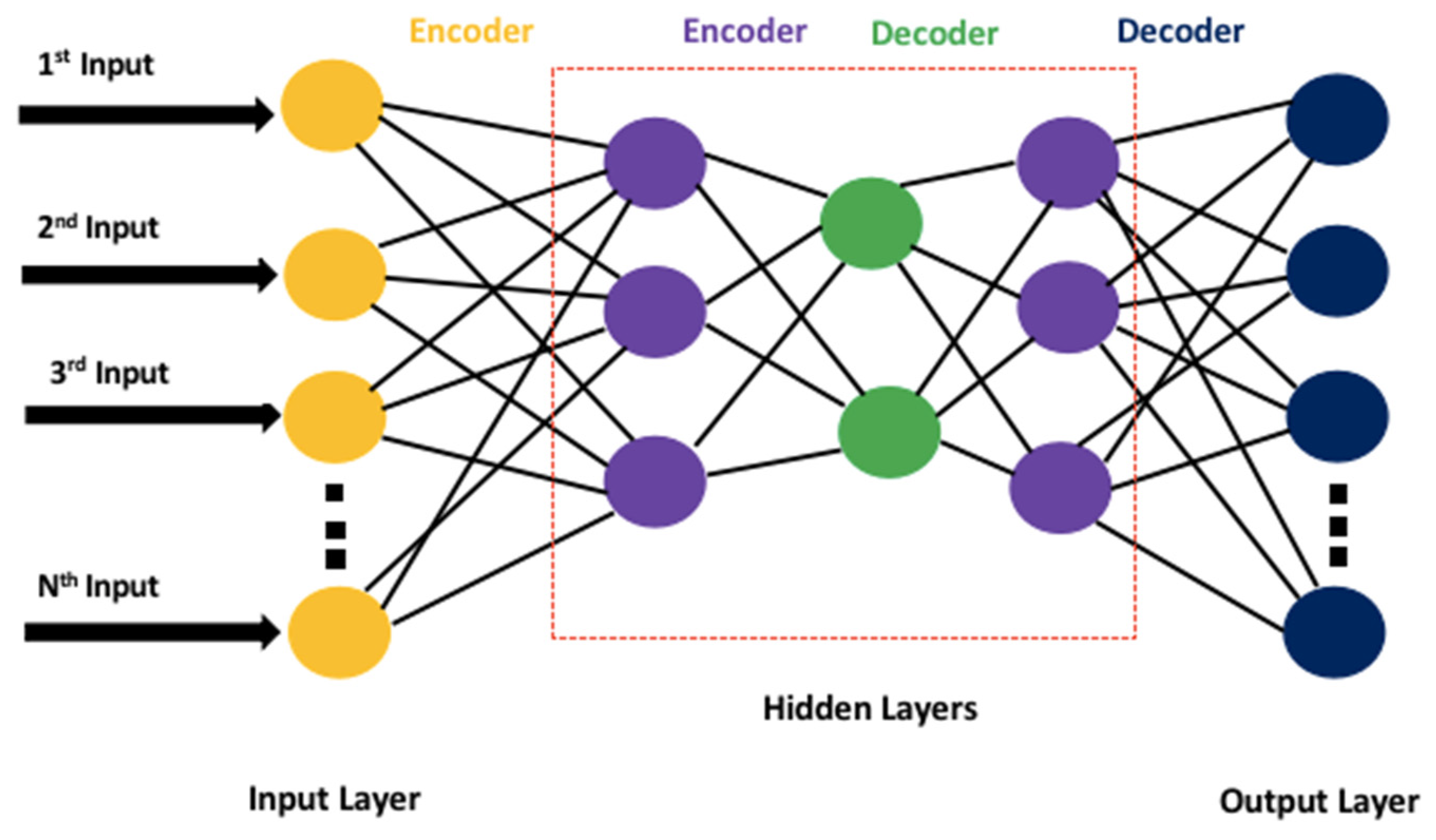
- The application of an autoencoder-based deep neural network to extract nonoverlapping reduced feature pools to improve the classification accuracy of the proposed model.
- The elimination of the use of a separate feature selection step as it can be performed through the proposed autoencoder-based DNN automatically.
2. Literature Review
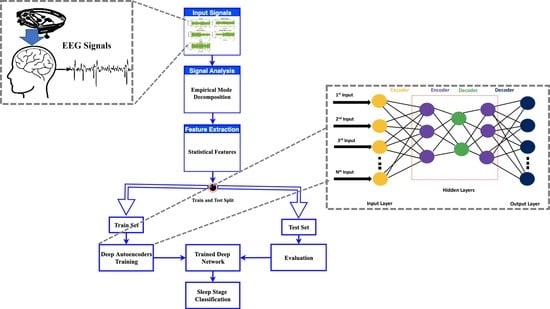
3. Material and Methods
3.1. Dataset Description
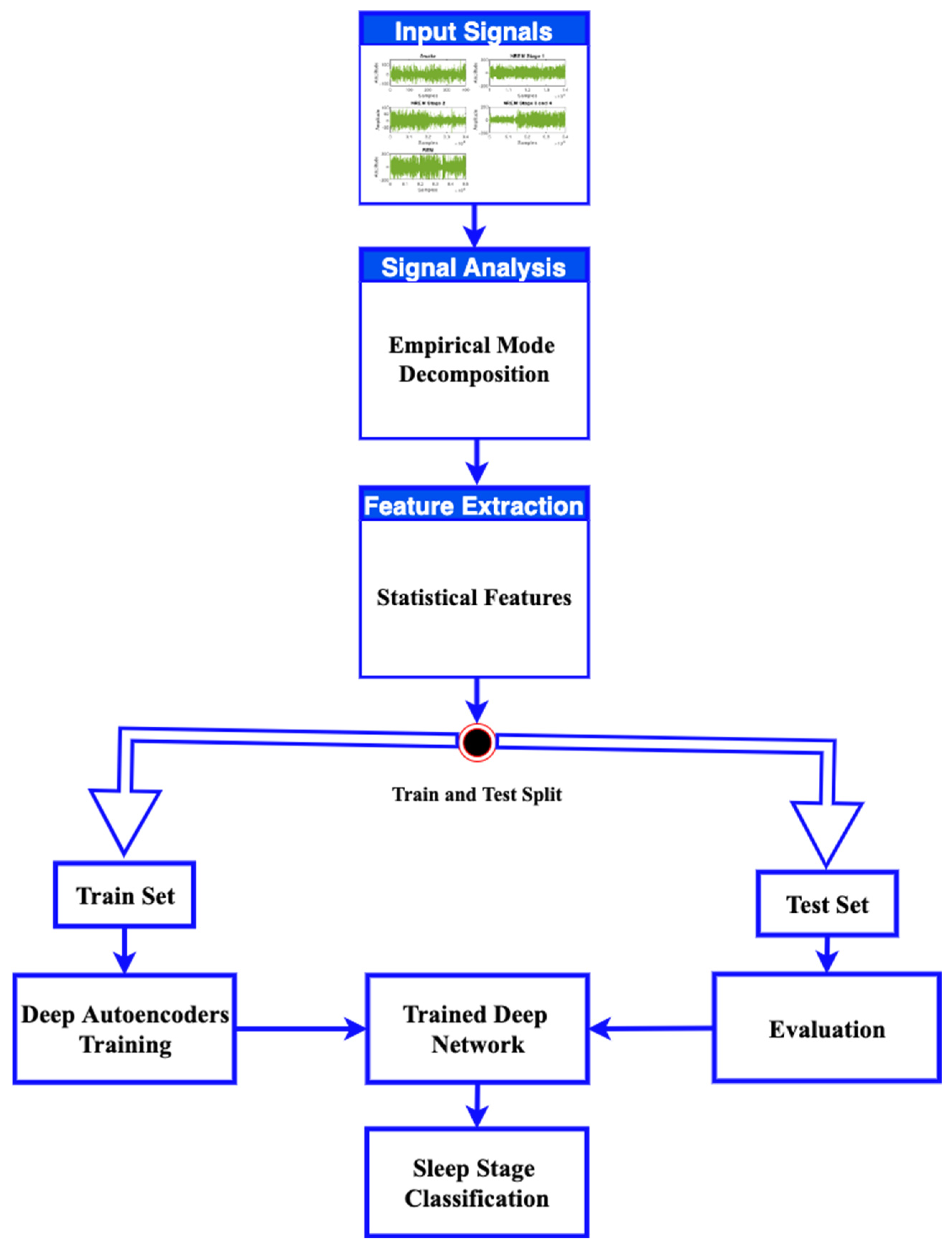
3.2. Methodology
3.3. Evaluation Matrices
4. Results Analysis
5. Conclusions
- This study presents a mechanism to easily denoise a contaminated EEG signal so that it can be effectively utilized for further analysis and complete the sleep stage classification task.
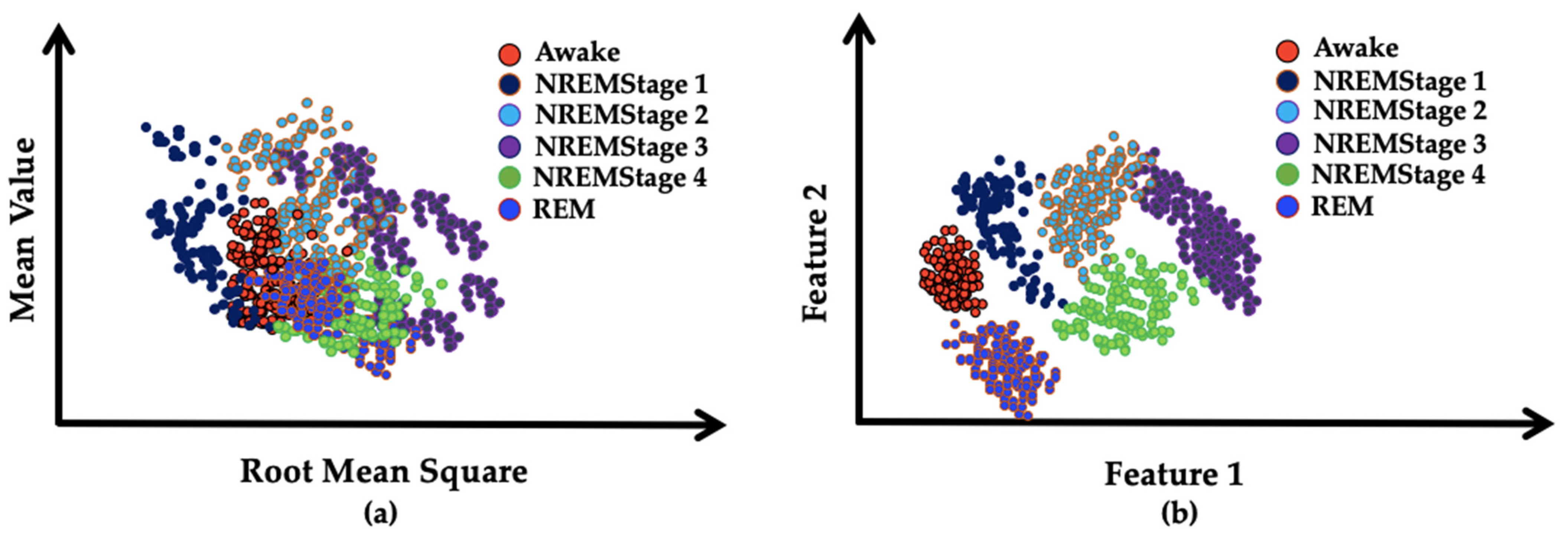
- In traditional data-driven classification tasks, a separate feature selection step is required after feature engineering and extraction. It is an important step because it eliminates redundant data from the inputs, hence, reducing the dimension of the data. So, the use of a separate feature selection algorithm in the classification pipeline is considered overhead. It not just increases the time complexity of an algorithm but also enhances the implementation and debugging complexity of the algorithm. Therefore, in this work, a specialized deep neural network based on autoencoders was used for automatic input compression that learned a reduced pool of abstract features. Apart from the dimensionality reduction of the data, the same deep neural network could be used to classify the data. Therefore, this algorithm eliminates the need for separate feature selection and classification steps. Moreover, the abstract feature pool significantly enhanced the classification performance of the deep neural network as the features that belonged to different classes had distinct distribution patterns that reduced the overlapping of the features to a minimalistic level.
- This study also highlights the need for a non-redundant and non-overlapping feature pool in classification tasks. As it is evident from the results that using features with overlapping patterns deteriorates the final classification accuracy of a classifier. On the other hand, a non-redundant feature pool with distinct distribution patterns that do not overlap each other significantly enhances the performance. The effect of the features as selected by the feature selection algorithm on performance is found to be more positive and significant when compared to the use of all features.
- The current research study focused on a single parameter, i.e., EEG signals. However, this methodology can be applied to multimodal data. For this purpose, a tweaking of the current model will be required according to the data used. Moreover, this study relied on statistical features. The values of these features can abruptly change with a change in the characteristics of the signals. The future prospects of this work could be the introduction of a sophisticated pattern recognition mechanism to eliminate the dependency on the statistical features set and a more generalized network that can be directly adopted by multimodal data.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mindell, J.A.; Meltzer, L.J.; Carskadon, M.A.; Chervin, R.D. Developmental Aspects of Sleep Hygiene: Findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009, 10, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Rodenbeck, A.; Binder, R.; Geisler, P.; Danker-Hopfe, H.; Lund, R.; Raschke, F.; Weeß, H.; Schulz, H. A Review of Sleep EEG Patterns. Part I: A Compilation of Amended Rules for Their Visual Recognition According to Rechtschaffen and Kales. Somnologie 2006, 10, 159–175. [Google Scholar] [CrossRef]
- Tarokh, L.; Carskadon, M.A. Developmental Changes in the Human Sleep EEG during Early Adolescence. Sleep 2010, 33, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; Mcleland, J.S.; Toedebusch, C.D.; Boyd, J.; Morris, J.C.; Landsness, E.C.; Yamada, K.; Holtzman, D.M. Comparison of a Single-channel EEG Sleep Study to Polysomnography. J. Sleep Res. 2016, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Chriskos, P.; Frantzidis, C.A.; Nday, C.M.; Gkivogkli, P.T.; Bamidis, P.D.; Kourtidou-Papadeli, C. A Review on Current Trends in Automatic Sleep Staging through Bio-Signal Recordings and Future Challenges. Sleep Med. Rev. 2021, 55, 101377. [Google Scholar] [CrossRef]
- Mohebbi, M.; Ghassemian, H. Prediction of Paroxysmal Atrial Fibrillation Using Recurrence Plot-Based Features of the RR-Interval Signal. Physiol. Meas. 2011, 32, 1147. [Google Scholar] [CrossRef]
- Senthilpari, C.; Yong, W.H. Epileptic EEG Signal Classifications Based on DT-CWT and SVM Classifier. J. Eng. Res. 2021, 10, N0 2A. [Google Scholar] [CrossRef]
- IEEE Transmitter. Improving the Quality of Sleep with AI and Machine Learning. 21 May 2021. Available online: https://transmitter.ieee.org/improving-the-quality-of-sleep-with-ai-and-machine-learning (accessed on 10 October 2022).
- Imtiaz, S.A. A Systematic Review of Sensing Technologies for Wearable Sleep Staging. Sensors 2021, 21, 1562. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.A.; Rodriguez-Villegas, E. Automatic Sleep Staging Using State Machine-Controlled Decision Trees. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: Manhattan, NY, USA, 2015; pp. 378–381. [Google Scholar]
- Şen, B.; Peker, M.; Çavuşoğlu, A.; Çelebi, F.V. A Comparative Study on Classification of Sleep Stage Based on EEG Signals Using Feature Selection and Classification Algorithms. J. Med. Syst. 2014, 38, 18. [Google Scholar] [CrossRef]
- Memar, P.; Faradji, F. A Novel Multi-Class EEG-Based Sleep Stage Classification System. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 26, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Santaji, S.; Desai, V. Analysis of EEG Signal to Classify Sleep Stages Using Machine Learning. Sleep Vigil. 2020, 4, 145–152. [Google Scholar] [CrossRef]
- Bhusal, A.; Alsadoon, A.; Prasad, P.W.C.; Alsalami, N.; Rashid, T.A. Deep Learning for Sleep Stages Classification: Modified Rectified Linear Unit Activation Function and Modified Orthogonal Weight Initialisation. Multimed. Tools Appl. 2022, 81, 9855–9874. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, Y.; Yang, P.; Nan, F.; Zhang, Y.; Rao, Y.; Du, F. A Novel Feature Relearning Method for Automatic Sleep Staging Based on Single-Channel EEG. Complex Intell. Syst. 2022. [Google Scholar] [CrossRef]
- Yulita, I.N.; Fanany, M.I.; Arymurthy, A.M. Sleep Stage Classification Using Convolutional Neural Networks and Bidirectional Long Short-Term Memory. In Proceedings of the 2017 International Conference on Advanced Computer Science and Information Systems (ICACSIS), Bali, Indonesia, 5 June 2017; IEEE: Manhattan, NY, USA, 2017; pp. 303–308. [Google Scholar]
- Islam, M.R.; Rahim, M.A.; Akter, H.; Kabir, R.; Shin, J. Optimal IMF Selection of EMD for Sleep Disorder Diagnosis Using EEG Signals. In Proceedings of the 3rd International Conference on Applications in Information Technology, Aizu-Wakamatsu, Japan, 1–3 November 2018; pp. 96–101. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A. A Manual for Standardized Terminology, Techniques and Scoring System for Sleep Stages in Human Subjects. Brain Inf. Serv. 1968. [Google Scholar] [CrossRef]
- Hassan, A.R.; Bhuiyan, M.I.H. Computer-Aided Sleep Staging Using Complete Ensemble Empirical Mode Decomposition with Adaptive Noise and Bootstrap Aggregating. Biomed. Signal Process. Control 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Hasan, M.J.; Shon, D.; Im, K.; Choi, H.K.; Yoo, D.S.; Kim, J.M. Sleep state classification using power spectral density and residual neural network with multichannel EEG signals. Appl. Sci. 2020, 10, 7639. [Google Scholar] [CrossRef]


| No. of EEG Recordings (20 Hours Each) | Period of Segmentation (Seconds) | Total Number of Samples after Segmentation | No. of Samples in Train and Test Subsets after Splitting | |
|---|---|---|---|---|
| Train (60%) | Test (40%) | |||
| 153 | 30 | 367,200 | 220,320 | 146,880 |
| Features | Equations | Features | Equations | Features | Equations |
|---|---|---|---|---|---|
| Mean value | Standard deviation | Root mean square (RMS) | |||
| Peak-to-peak value (PPV) | Skewness value (SV) | Margin factor (MF) | |||
| Crest factor | Impulse factor | Square root of the magnitude (SRM) | |||
| Kurtosis value (KV) | Kurtosis factor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohaib, M.; Ghaffar, A.; Shin, J.; Hasan, M.J.; Suleman, M.T. Automated Analysis of Sleep Study Parameters Using Signal Processing and Artificial Intelligence. Int. J. Environ. Res. Public Health 2022, 19, 13256. https://doi.org/10.3390/ijerph192013256
Sohaib M, Ghaffar A, Shin J, Hasan MJ, Suleman MT. Automated Analysis of Sleep Study Parameters Using Signal Processing and Artificial Intelligence. International Journal of Environmental Research and Public Health. 2022; 19(20):13256. https://doi.org/10.3390/ijerph192013256
Chicago/Turabian StyleSohaib, Muhammad, Ayesha Ghaffar, Jungpil Shin, Md Junayed Hasan, and Muhammad Taseer Suleman. 2022. "Automated Analysis of Sleep Study Parameters Using Signal Processing and Artificial Intelligence" International Journal of Environmental Research and Public Health 19, no. 20: 13256. https://doi.org/10.3390/ijerph192013256
APA StyleSohaib, M., Ghaffar, A., Shin, J., Hasan, M. J., & Suleman, M. T. (2022). Automated Analysis of Sleep Study Parameters Using Signal Processing and Artificial Intelligence. International Journal of Environmental Research and Public Health, 19(20), 13256. https://doi.org/10.3390/ijerph192013256