1. Introduction
The COVID-19 pandemic has had a significant impact on both physical health and psychological well-being of communities and is gripping the world through its devastating pandemic [
1].
Conversely, it has also contributed to reinforce the awareness of the importance of defending us against the spread of infectious diseases and effectively tackling their occurrence [
2]. While it has been dominating the media since 2020, COVID-19 is not the only outbreak that has been causing concern worldwide, especially in low-income countries. In fact, besides COVID-19, newly emerging or re-emerging viruses and microbial infections are now the top causes of death worldwide, especially in young populations [
3]. Deaths from infectious diseases are common—and in some cases dominant—across low- and middle-income nations. On the other hand, healthcare-associated infections have been becoming a major problem in industrialized countries, with 1.7 million cases, and an estimated 100,000 deaths, per annum reported in the US alone [
4].
COVID-19 has undoubtedly placed hygiene at the center of disease prevention and has challenged basic hygiene behavior as hand washing and surfaces disinfection in the daily routine. As described by Enyoh and colleagues [
5] the virus persistence time on different inanimate surfaces differs from 24 h (aluminum or surgical gloves) to more than 5 days (ceramic, Teflon, silicon, or paper) depending on the type of surface, but in any case, it represents one of the main issues in the spreading of COVID-19 infection. For this reason, during the COVID-19 pandemic, in all the high-risk public spaces, such as schools, healthcare facilities and workspaces, the global consumption of sanitizers and disinfectants has unprecedently been raising [
6], to the extent that the global disinfection market size has been estimated to be worth USD 105 billion by 2028, growing at a CAGR of 20.8% from now on [
7].
Otherwise, a substantial concern about the possible adverse effects of disinfectants has also been raised [
8]. Indeed, when abused or misused, disinfectants can also be hazardous to humans as well as to the environment and may drive to worldwide secondary global noxious consequences for human health and ecosystems [
9,
10]. Common disinfectants, such as sodium hypochlorite, ozone, oxygen peroxide, and nanomaterials, are also known to cause irritation of the skin, eyes and to the respiratory tract [
11,
12].
Currently, an eco-friendly disinfectant acting as effective biocide and virucide at a broad spectrum of activity, while causing minimal or no harm to the environment, prepared in a way to avoid waste, with a very low toxicity, safe and easy to handle and store, would be strongly needed [
13]. Moreover, disinfectants and disinfection techniques used in the fight against infectious agents should be safe for a long exposure time on personnel, equipment, and surfaces. Disinfection technologies for indoors are important to reduce transmission, especially in crowded settings [
14].
Hypochlorous acid (HOCl) could be proposed as a promising candidate to fulfill that role. There is wide literature demonstrating its efficacy against a broad range of microorganisms, including spores and viruses [
15]. HOCl is an endogenous substance in all mammals, usually produced by white blood cells to surround pathogens, e.g., when the skin is injured, and pathogens begin to invade [
16]. Its mechanism of action relates to the destruction of the cytoplasmic membrane and the cell wall of the bacteria, due to its small molecular dimensions, such as the water molecule, and neutral charge, not repelled by bacteria [
17]. For decades, chlorination has been securing the microbiological quality of drinking water [
18]. One of the recent advancements in chlorine-based disinfection is its utilization in the form of electrolyzed water (EW). This method became attractive due to its proven simplicity, biocidal efficiency, and convenience of in situ production, which reduces the hazard of handling and transporting concentrated chlorine reagents [
19]. EW is usually produced by passing a dilute salt solution (NaCl) through an electrolytic cell, subjected to a DC voltage. The spontaneous reduction of hypochlorous acid to chloride, after disinfection process via oxidation, accelerated if exposed to light and air, assures the absence of residual effects or damages on environment [
20].
In recent years, while EW liquid solution has been widely accepted for disinfection by different sectors, such as medical, agricultural, food processing and sanitary industries [
21], its application as aerosolized dry fog is still barely known and few studies have investigated its potentiality for indoor environments and surfaces disinfection [
22]. The exposure of individuals to microbes can take place via inhalation or through contact with contaminated surfaces and objects and is allowed by the transport of the species, in the form of bioaerosol, either by binding to dust or particles present in the air, or through individuals themselves. In particular, the contamination of surfaces, which act as reservoirs for microorganisms, is a crucial point in the indirect transmission of infections [
23]. Starting from this evidence, here we describe the experimental proof of antimicrobial and antiviral activity of neutral HOCl solution aerosolized on different surfaces (flat, porous, and semi-porous) and at different times of indoor treatments. We have started this study on the development of a smart prefabricated disinfection chamber (SPSC) by way of aerosolized hypochlorous acid at 300–10,000 ppm concentration interval. Then, by means of a 2D model of human keratinocytes cell line (HaCaT cells), we assessed the optimal dose of aerosolized HOCl to be used in the next experiments.
The choice to use different surfaces for our study is therefore due to the different ability of microorganisms (bacteria and viruses) to adhere to the surface, but also to the different nature of the materials. At this point we considered it important to evaluate the capacity and effectiveness of HOCl in nebulized form on different surfaces (semi-porose, flat and porose), contaminated by bacteria and viruses. To be considered a good disinfectant, the product must have a broad-spectrum action [
24,
25].
HOCl was tested in
E. Coli,
S. Aureus and
P. Aeruginosa as a bacterial model, as
E. coli and
P. aeruginosa bacteria can survive in conditions of high humidity, unlike the
S. aureus bacterium which prefers drier environments, as well as being able to survive even at low temperatures, from 4 °C to 6 °C [
26]. Viruses also can resist various environmental factors and disinfection techniques, even those that are prolonged over time. The absence of an envelope (Adenovirus V) gives them greater resistance to drying, making them more stable in the environment. While enveloped viruses, such as respiratory viruses (coronavirus), usually remain infectious on surfaces for hours or days [
27]. Finally, the toxicity of the selected dose of HOCl on human cell-based 3D tissues was evaluated; in particular, we selected two of the principal target tissues of nebulized drugs: the cutaneous and respiratory tissues.
This study proposes the use of an in situ HOCl-based disinfectant production unit. The ecological consequences of this new system are limited and HOCl does not leave residue on the environmental surface nor in the indoor air and is not corrosive to the equipment and furniture. Even from this limited demonstration study, we proved its efficacy and safety as biocide even in aerosolized form. Important general advantages on its potential application in fighting against the COVID-19 pandemic and other infectious diseases can be extracted by performing a relative comparison with other different disinfection methods.
2. Materials and Methods
2.1. Preparation of HOCl Solution
HOCl solution was prepared by electrolysis of a mixture of aqueous dilute solutions of NaCl at 30,000 ppm and deionized water added with a salt-based buffer (Na2SO4, 7000 ppm) for maintaining pH at slightly acid value (about 6.5). The EW generator works at 12.0 V and 50.0 A for 3 h, consuming 0.06 kWh/L. It basically consists of an electrolytic cell with anode and cathode electrodes, without a separating membrane between them. The two electrodes are composed of a titanium core coated with iridium or platinum oxide, without polarity inversion. During electrolysis Na+ ions will be directed to the cathode, while Cl− ions will approach the anode, where hypochlorous acid (HOCl) is formed and released in solution as the prevalent species (>95%), when pH is maintained within the 6.0–6.7 interval. HOCl concentration was determined using the DPD colorimetric test method.
HOCl solution has been produced at 10,000 ± 250 ppm of HOCl and subsequently diluted with deionized water at 1:2 (5000 ppm), 1:5 (2000 ppm), 1:10 (1000 ppm), 1:33 (300 ppm) and 1:100 (100 ppm). HOCl solutions have been stored in the dark at 4 °C.
2.2. HOCl Solutions Aerosolization
HOCl solutions have been transferred into a nebulizer, equipped with a tank (24 L of working volume) connected with two stainless steel nozzles by means of a HDPE pipe (length, 3 m). Nozzles have been designed to ensure a constant release in the environment of a dry fog of 1 mm of droplet diameter. Hydraulic pump pressure was 0.4 bar and the volumetric flowrate 100 ± 20 mL/min. The aerosolization has been performed using a specific plexiglass system specifically designed for this experiment (patent 102022000002699 pending). Chamber saturation was reached in 3 min, aerosolizing 300 mL of HOCl solutions at different concentrations.
2.3. 2D Human Keratinocytes and Cell Viability
The spontaneously immortalized human keratinocyte HaCaT cell line was used for the cellular experiments. HaCaT cells were cultured as previously described [
28] and exposed to different doses of HOCl (from 50 ppm to 10,000 ppm) for 30 min. At the end of the exposure, the cells were harvested and analyzed at different time points; in particular, cell viability was evaluated by Trypan blue staining. After the exposure to HOCl cells were trypsinized and resuspended in 1 mL of culture media. Next, 10 µL of Trypan blue solution (Sigma, Milan, Italy) was added onto 10 µL of cell suspension and viable cells were counted in a Bürker cell counting chamber at each time point. After the addiction of Trypan blue solution, the non-viable cells appeared blue. Viable and non-viable cells were counted separately, and the cell viability was expressed as percentage of living cells with respect to the total number of cells:
viable cells (%) = (total number of viable cells per mL/total number of cells per mL) × 100 as previously demonstrated [
29].
2.4. MTT Assay on 2D Human Keratinocytes
HaCaT cells were plated (2.0 × 10
4 cells/well) in 96 well multiplates in DMEM with 10% FBS. After 24 h, the medium was removed, and cells were incubated for 4 h with fresh medium in the presence of 1.2 mM MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, St. Louis, MO, USA). After that, the MTT solution was removed and 100 µL of DMSO was added to each well to dissolve the blue formazan crystals. The absorbance of the formazan dye was measured at 570 nm with a microplate reader (Spectramax M5 multimode microplate reader, Molecolar Device, San Jose, CA, USA). Data were expressed as a percentage of the control value as previously demonstrated [
30].
2.5. Scanning Electron Microscopy (SEM) Methods on 2D Human Keratinocytes
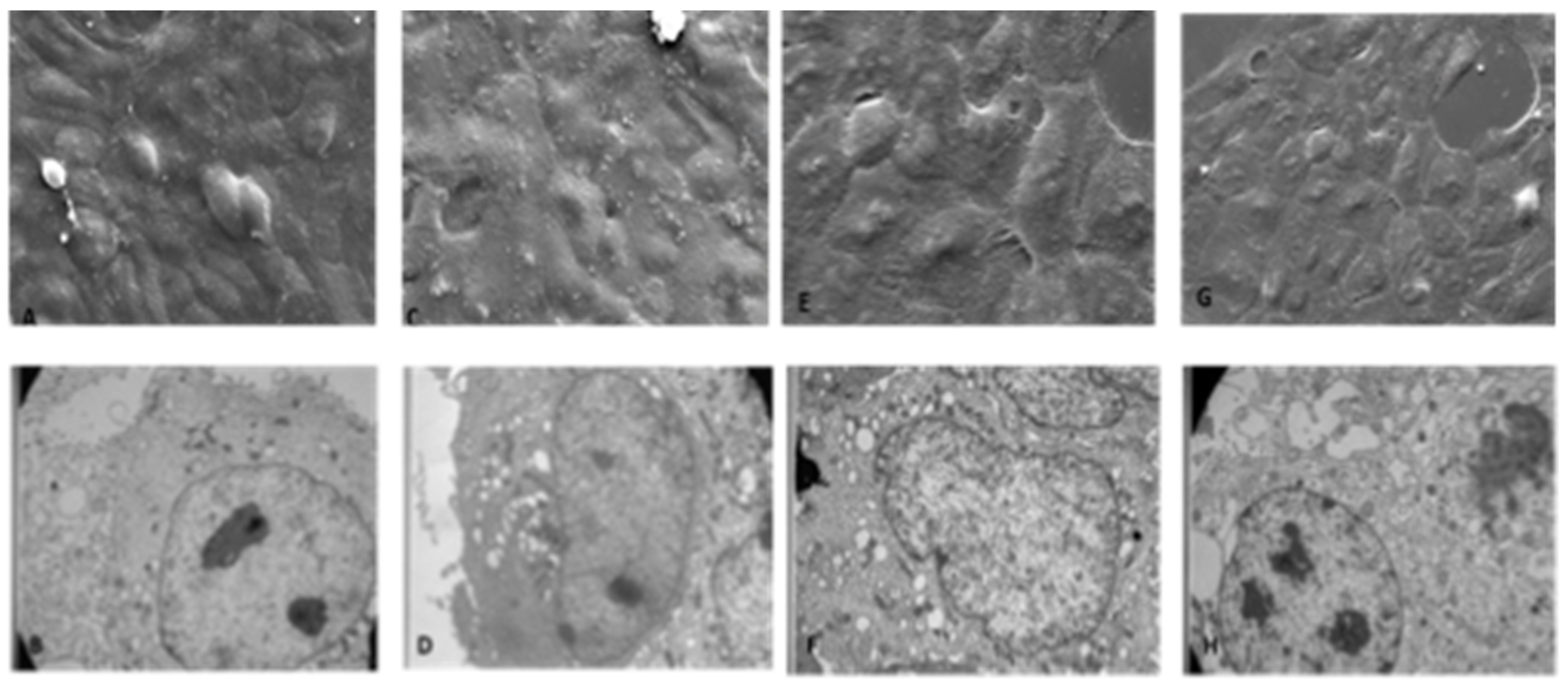
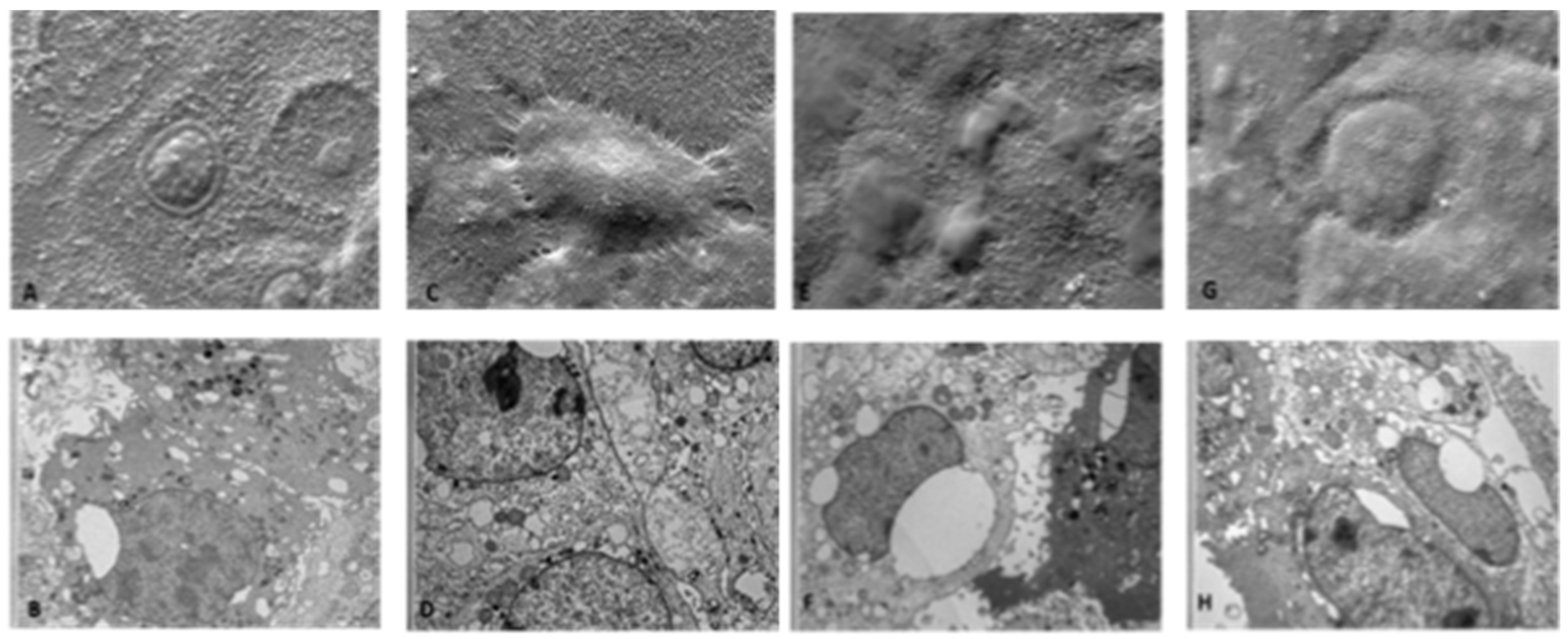
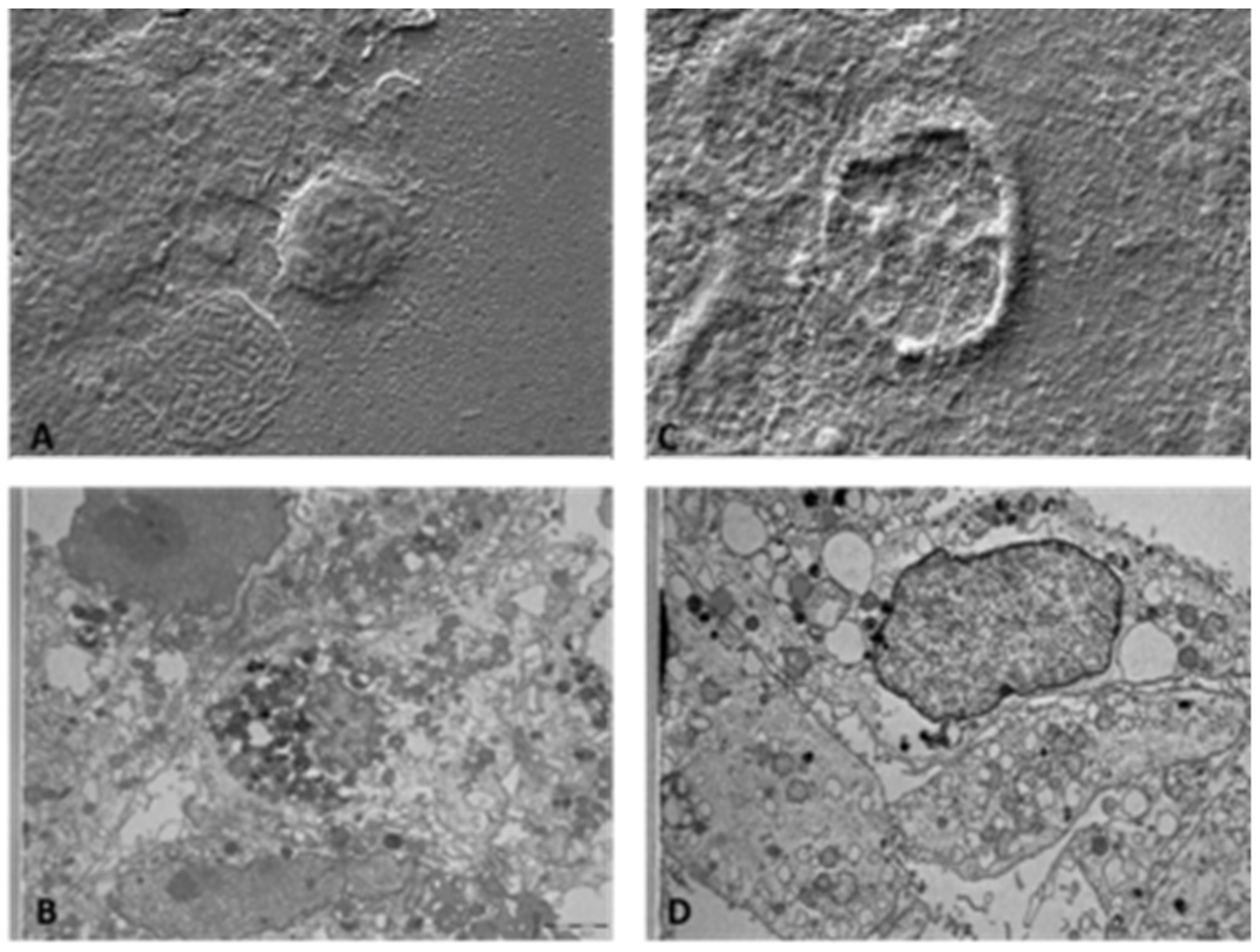
Sample morphology, size and distributions were evaluated by observation by optical and electron microscopy. To analyze the external morphology, the cells were fixed in 2.5% glutaraldehyde in cacodylate buffer for 3 h at 4 °C. Then, cells were post-fixed in 1% osmium tetroxide for 2 h at 4 °C and dried in a graded series of alcohol; samples were then metalized by gold coating (Edwards Sputter Coating S150) and analyzed at 15–20 Kv by a scanning electron microscope (Zeiss EVO 40) source LaB6.
2.6. Transmission Electron Microscopy (TEM) Methods on 2D Human Keratinocytes
For ultrastructural analysis, treated HaCaT cells were fixed in 2.5% glutaraldehyde in cacodylate buffer for 3 h at 4 °C. Then, cells were post-fixed in 1% osmium tetroxide for 2 h at 4 °C, dehydrated in a graded series of alcohol, embedded in Araldite resins, and polymerized in oven for 48 h at 60 °C. Ultrathin sections of 60 nm were cut with an ultramicrotome (Ultratome Reichert SuperNova Leica, Wien, Austria), stained with uranyl acetate and lead citrate and examined in a Philips CM100 transmission electron microscope.
2.7. 3D Skin Model and Epiairway Model
A 3D skin model “EpiDerm” (reconstructed human epidermis (RHE)) and 3D Epiairway model were purchased from MatTek corporation (MatTek In Vitro Life Science Laboratories, Bratislava, Slovak Republic). Upon arrival, the 24 inserts containing 3D tissues were transferred into 6-well plates prefilled with 1 mL of specific MatTek Assay medium (provided by MatTek Corporation, Ashland, MA, USA), according to the manufacturer’s instructions.
The tissues were kept at 37 °C, 5% CO
2 atmosphere overnight. On the day of the experiment, fresh media were added, and the tissues were exposed to 300 ppm HOCL for 30′ in the aforementioned box. For the Epiairway tissues, the apical surface was rinsed with PBS to eliminate accumulated mucus, then fresh medium was added immediately before the experiment [
31]. Control tissues were left in the incubator. After exposure, tissues were immediately processed (time point T0) or after 24 h (time point T24).
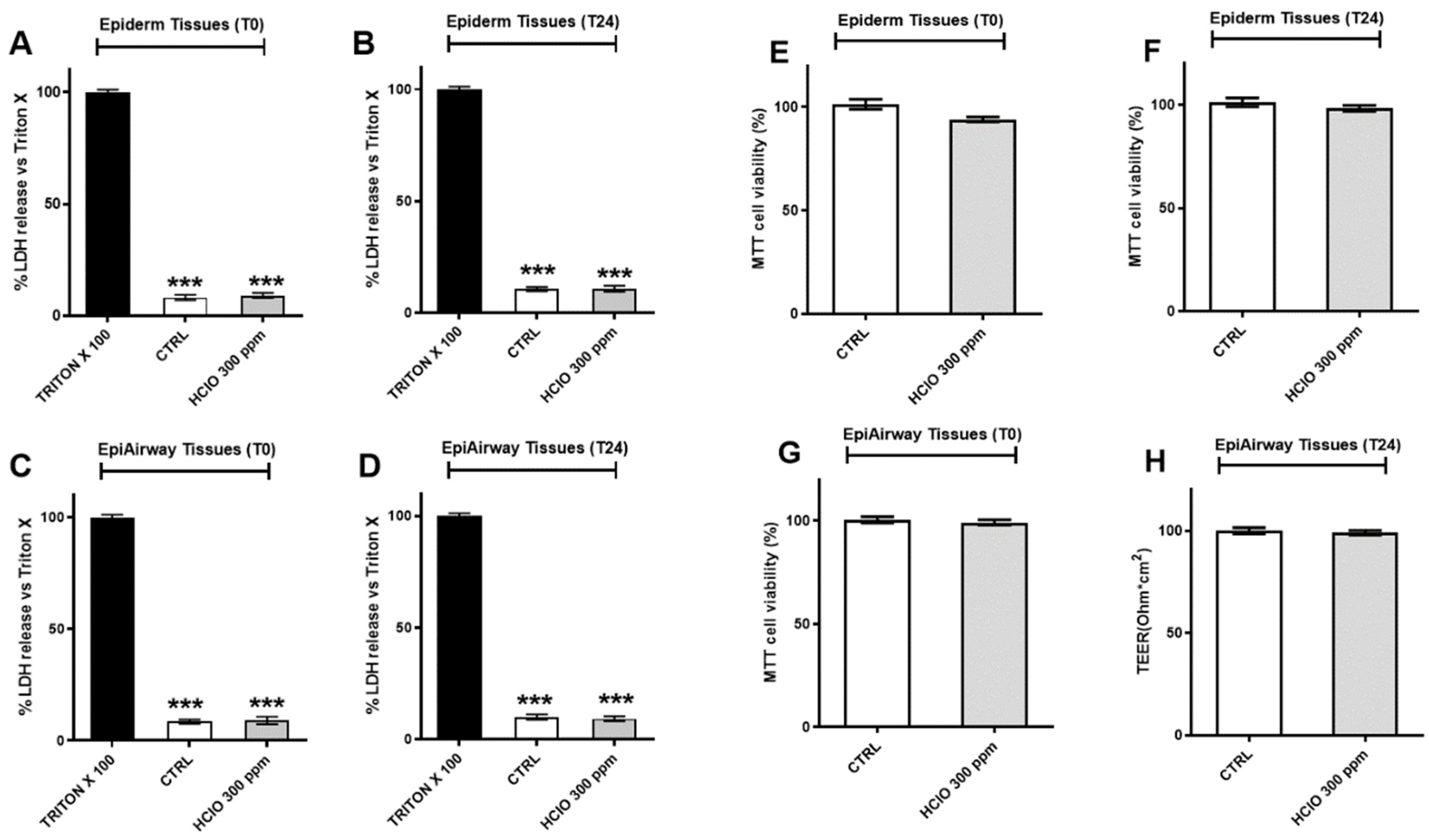
2.8. Cytotoxicity Determination (LDH Assay)
Cytotoxicity was assessed by lactate dehydrogenase (LDH) leakage in the culture medium, consequent to loss of the plasma membrane integrity. LDH activity was determined by measuring the conversion of pyruvate to lactate via NADH oxidation (the rate of NADH consumption is proportional to LDH activity). Cell-free culture medium (40 µL) was collected for each sample and added to 160 μL of the assay mixture (substrate buffer:NADH buffer, 9:1). The substrate buffer was 1.2 mM sodium pyruvate, 5 mM ethylenediaminetetraacetic acid (EDTA), 13.8 mM sodium azide, in 50 mM Tris buffer, pH 7.4; the NADH buffer was 1.8 mM NADH, 13.8 mM sodium azide, in 50 mM Tris buffer, pH 10.2. LDH activity was measured at room temperature in a microplate reader (Victor3 Multilabel Counter; Perkin-Elmer, Waltham, MA, USA); the absorbance decrease (measuring NADH oxidation) was recorded at 340 nm over a period of 8–10 min. In order to obtain a representative maximal LDH release as the positive control with 100% toxicity, a triplicate set of samples were lysed with 2% (v/v) Triton X-100 in culture media for 30 min at 37 °C.
2.9. MTT Assay on 3D Tissues
Tissue viability was measured by reduction of the tetrazolium salt Methyl Thiazoyl Tetrazolium (MTT). Briefly, a 1 mg/mL of MTT stock solution was prepared in maintenance media (provided with tissues) just prior to use and warmed to 37 °C.
Tissue samples were transferred to 24-well plates containing 300 µL MTT reagent per well and placed in the 37 °C, 5% CO2 incubator for 3 h. After that, the tissues were submerged in 2 mL of MTT extract and maintained overnight at room temperature protected from light. The day after, 200 µL of extraction solution was transferred to a clear 96-well plate and the optical density was measured with a spectrophotometer at 570 nm. Finally, the cell viability was calculated as percent of the mean of the control tissue.
2.10. Measurement of TEER
Tissues barrier integrity was evaluated by TEER measurements using a Millicell-ERS 2 V-Ohmmeter (Millipore Co., Bedford, MA, USA) at T0 and T24 after 300 ppm HOCL nebulization. For each well, 3 measures were performed. Briefly, the tissues were equilibrated for about 20 min at room temperature before the measurements. Blank measurements were performed on an empty insert. TEER value was then calculated using the following equation: TEER (Ωcm2) = (Rsample—Rblank) × membrane area (cm2).
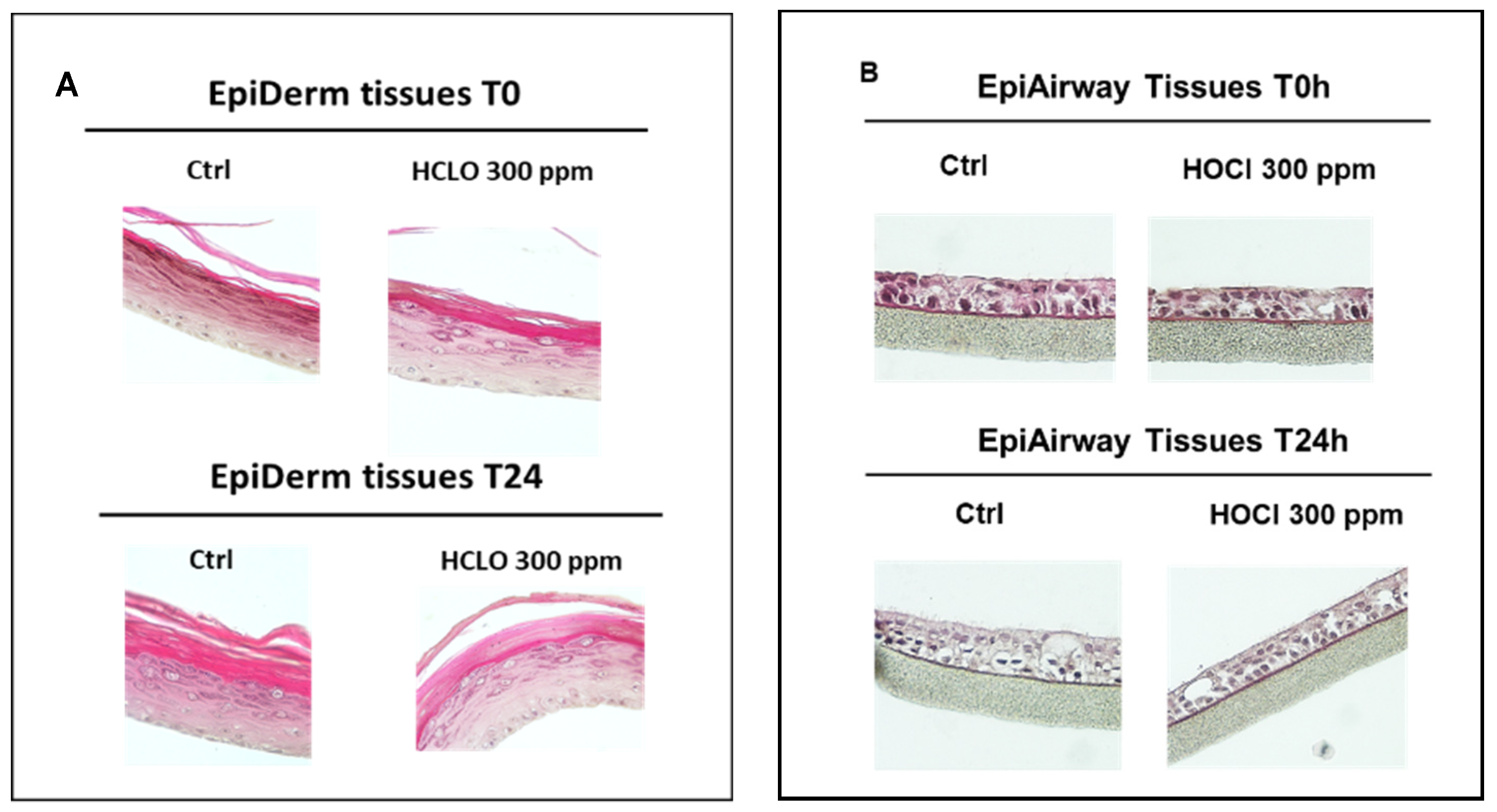
2.11. Histological Analysis
For this step, 3D tissues, exposed or un-exposed to 300 ppm HOCL, were immersion-fixed in 10% NBF (neutral-buffered formalin) for 24 h at room temperature, then dehydrated in alcohol gradients and embedded in paraffin. For histological observation, the sections (4 µm thickness) were deparaffinized in xylene and rehydrated in alcohol gradients (100%, 90%, 80% 70%). The sections were stained with hematoxylin for 3–5 min, washed in running tap water for 5 min, and stained with Eosin Y for 2 min. The sections were then washed in tap water for 1–5 min, dehydrated in increasing concentrations of alcohols and cleared in xylene. The sections were then mounted with a rapid non aqueous mounting medium, containing toluene (Entellan, Merck KGaA, Darmstadt, Germany) and observed under Nikon Microphot FXA microscope (Nikon Instruments, Amsterdam, The Netherlands).
2.12. Bacterial Culture
Bacteria used for this study were purchased from the ATCC company, Escherichia Coli (ATCC 8739); Pseudomonas Aeruginosa (ATCC 9027); Stafilococco Aureus (ATCC 6538). Supplied in dry pellet and subsequently resuspended in tryptic soy broth. Bacterial cultures and experimental procedures were executed in a biosafety level 2 laboratory, designed and managed in accordance with safety regulations. Liquid cultures were grown for 18 h in Tryptic soy broth at 37 °C.
2.13. Cell Culture Used for Virucidal Assay
The human lung fibroblast cell line MRC-5 was supply by (LGC Standard-ATCC CCL171). MRC-5 cells were maintained in Eagle’s Minimum Essential Medium (EMEM) ATCC 30-2003, supplemented with 15% FBS (Euroclone), 1% penicillin-streptomycin solution 100X (Euroclone) and 1% non-essential-amino acid (Euroclone).
For the human cancer cell line HeLa (LGC Standard-ATCC CCL-2), cells were cultured in RPMI-1640 medium (Euroclone), with 10% FBS, 1% penicillin–streptomycin solution 100X (Euroclone). Cells were incubated at 37 °C in 5% CO2.
2.14. Growth of Virus
Human coronavirus strain 229E and Human Adenovirus V were supplied by (LGC Standard-ATCC Coronavirus 229E VR-740; Adenovirus V VR-5). MRC-5 or Hela monolayers were infected with 229E or Adenovirus V at a multiplicity of 0.1 MOI and were incubated in medium with 2% serum, supplemented with glutamine, antibiotics and nonessential amino acid at 33 °C—5% CO2 incubator for until complete cytopathic effect (CPE) is reached. Cells and supernatant were collected, cells removed by low-speed centrifugation and the supernatant was centrifugated a 20,000 rpm for 30 min. The pellet obtained was resuspended in small aliquots and kept at −80 °C until use.
2.15. Bactericidal Activity by Nebulization
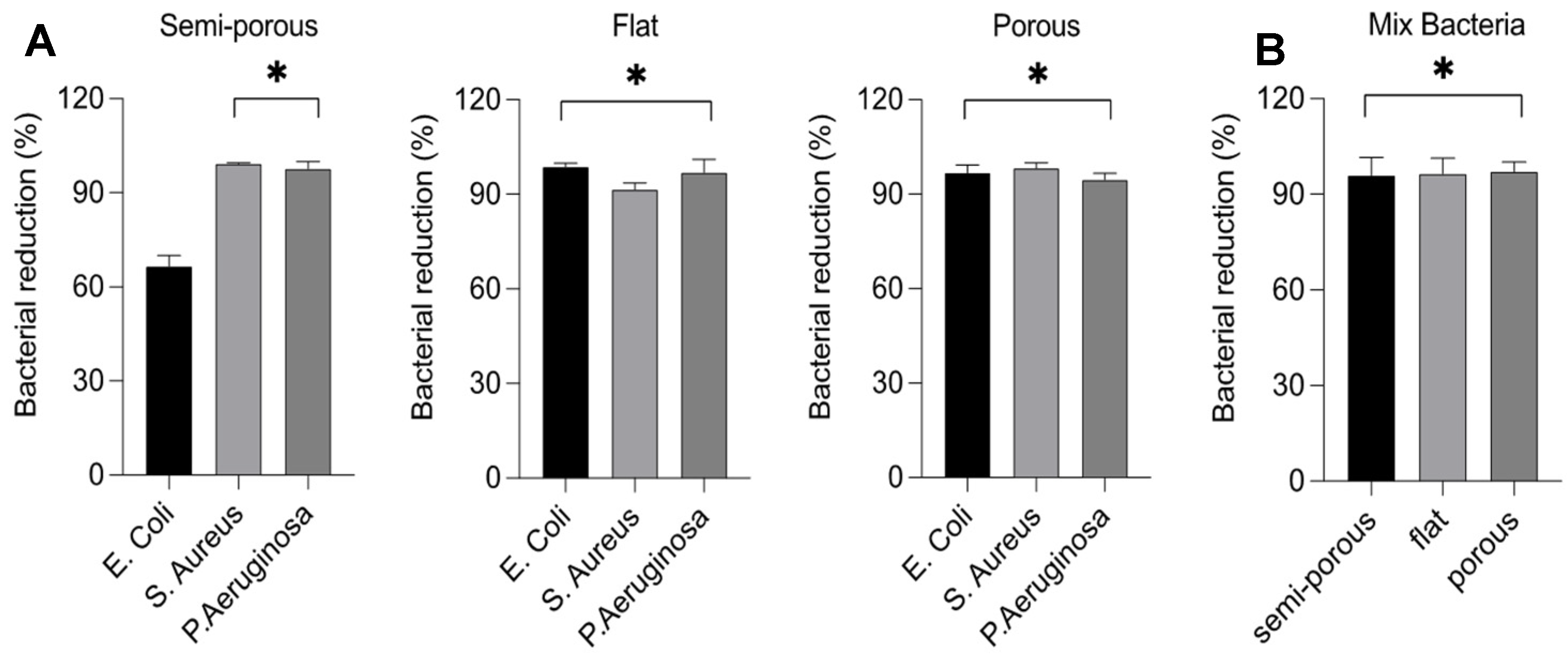
This test involves the nebulization of the EW solution (300 ppm) on three different surfaces (semi-porous-flat and porous) previously contaminated with the bacterial suspension. On the surfaces, areas to be contaminated were indicated, all of the same size, in order to minimize variability. After contamination, the surfaces were placed inside the plexiglass chamber and subjected 10 min of exposition of nebulization The test includes a negative control represented by the sample contaminated with the bacterial culture and not subjected to nebulization. After treatment with EW solution, swabs were carried out on the surfaces to evaluate the residual bacterial colonies. These swabs were resuspended in medium and subsequently serial dilutions were made and seeded on agar plates. The plates were incubated at 37 °C for 24 h and checked to assess the presence or absence of bacterial colonies.
The calculation of antibacterial activity was carried out according to the formula:
where:
R is the antibacterial activity;
U0 is the average of the common logarithm of the number of viable bacteria, in cells/cm2, recovered from the untreated test specimens immediately after inoculation;
Ut is the average of the common logarithm of the number of viable bacteria, in cells/cm2, recovered from the untreated test specimens after 24 h;
At is the average of the common logarithm of the number of viable bacteria, in cells/cm2, recovered from the treated test specimens after 24 h.
2.16. Virucidal Activity by Nebulization
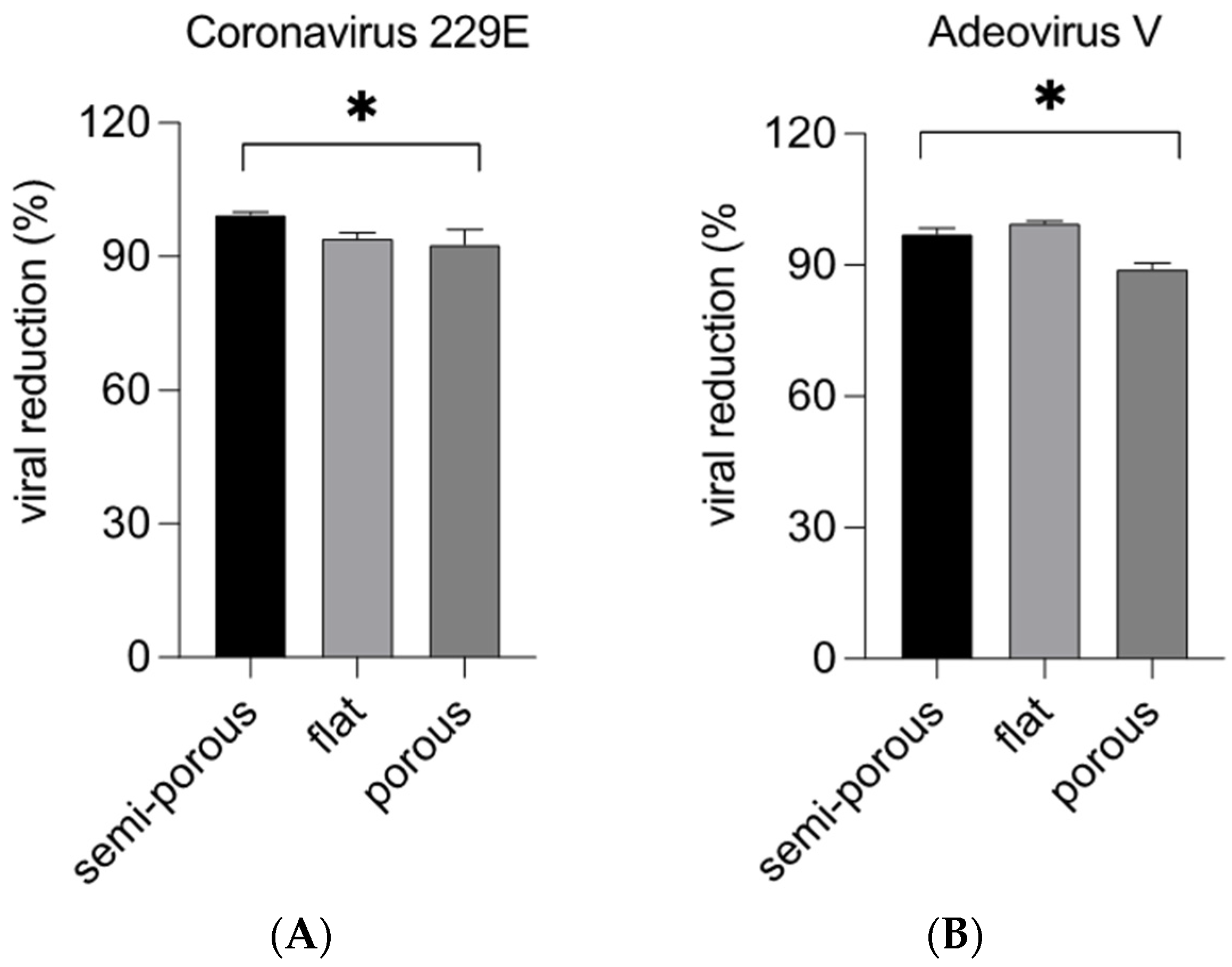
Three different surfaces (semi-porous, flat and porous) were used to evaluate the virucidal activity of hypochlorous acid in nebulized form. These surfaces were contaminated with Coronavirus 229E (virus a RNA with envelope) or Adenovirus V (virus a DNA without envelope) and then sprayed for 10 min with hypochlorous acid. After treatment, swabs were carried out on the surfaces to check for the possible presence of viruses after nebulization. Serial dilutions were then carried out on plates previously seeded with the MRC-5 or Hela cell lines. As a control, infection was carried out on the three surfaces not subjected to treatment. The plates were transferred to a 37 °C incubator with 5% CO2. In the following days, the plates were checked under a microscope to verify the presence or absence of the virus. The number of infectious viral particles is often quantified using the Median Tissue Culture Infectious Dose (TCID50) assay.
The amount of virus is expressed as TCID50/mL:
where:
D = −log10 of the last dilution which has 100% virus positivity (for example 3 wells/3 wells);
d = −log10 of the dilution factor;
S = number of wells in which the virus is present, including the last dilution with 100% positivity.
2.17. Statistical Analysis
All experiments were repeated three times and statistical values were expressed as the mean ± standard deviation (SD). A Student’s t-test or One-way ANOVA were used. Values of p < 0.05 were considered statistically significant. For all data analysis, GraphPad Prism 9 software (GraphPad Software Inc., San Diego, CA, USA) was used.
4. Discussion
HOCl is classified within a group of molecules known as reactive oxygen species with an extremely powerful oxidation potential, which can participate in subsequent non-enzymatic reactions, such as the oxidation and chlorination of cellular components. The usual chemical behavior of HOCl with the carbon and nitrogen of the amino group is as an electrophilic agent, in which the chlorine atom combines with a pair of electrons from the substrate, while the hydroxide ion is simultaneously or subsequently separated, often assisted by a proton from the solvent or from a reactive center elsewhere in the substrate. This feature mainly occurs in reactions with ammonia nitrogen, amides, amino acids, and peptides, with phenolic and other aromatic compounds. Chloride ion transfer is favored by the negative charge, basicity, and nucleophilicity of the receiving agent. HOCl is known to rapidly react with biomolecules, and no specific enzymatic mechanism is known to remove these oxidants. Phospholipids, fatty acids, sterols, and sphingolipids are also susceptible to HOCl oxidation. Another lipid target of HOCl is the primary amino groups present in ethanolamine and serine glycerophospholipids [
34].
The aim of our work was to provide information regarding disinfection of indoor environments using EW solution, a relatively well-studied compound, largely used as liquid biocide in many industries, from the food sector to healthcare applications, including chronic wound care disinfection. Although less studied, the use of HOCl as dry fog has shown potential benefit to disinfect indoor spaces where aerosols can be airborne for extended periods. Additionally, the COVID-19 virus can persist on inanimate surfaces for up to 9 days, and the periodical disinfection of indoor spaces could reflect the benefits on all surfaces in the room, contributing to reduce transmission [
35]. Aside from an extensive selection of disinfectants against coronaviruses being certified by the EPA, there has not been an ideal disinfectant until now [
36].
As recently reported, the World Health Organization (WHO) has requested to add HOCl to the core Essential Medicines List as a disinfectant, antiseptic and wound care agent because it has emerged in the current pandemic as the most potent and environmentally safe disinfectant available and with a wide range of efficacy against many human pathogens, in the range 180–460 ppm [
37].
Starting from this evidence, in the present work, we have first established the optimal dose of HOCl to be used in the experimental protocols; the experimental results obtained on a well-studied 2D cellular model demonstrated that 24 h of exposure to 300 ppm of aerosolized HOCl did not affect cell viability, suggesting that 30-min nebulization is not toxic to cells. To expand our analysis, we evaluated the effects of aerosolization on cell morphology by scanning electron microscope (SEM) and transmission (TEM) analysis. Based on the experimental evidence also in this case, we can conclude that HOCl at 300 ppm does not cause damage to cell morphology, neither immediately after nebulization, nor after 24 h.
In consideration of the potential capacity of microorganisms to survive on surfaces, it is good practice to proceed frequently and to sanitize (cleaning and/or disinfect) the surfaces, operations which must be more accurate and regular for surfaces with high contact frequency. In the choice of multipurpose detergents and disinfectants it is necessary to consider a series of requirements, such as rapid action and long persistence of activity, biocidal activity, broadest possible spectrum of action, less danger at the concentrations of use for humans and on the materials to be treated, ease of application, quality and safety, cost-effectiveness of management, and non-induction to resistances. The WHO recommends “to ensure that environmental cleaning and disinfection procedures are followed consistently and correctly” [
38]. Contamination of frequent touch surfaces in healthcare settings are therefore a potential source of viral transmission.
The air and surfaces of equipment, work surfaces, and work clothes, as well as hands, can represent important vehicles of microbiological contamination and potential sources of transmission of infectious agents. Numerous studies have highlighted the role of the inanimate environment in the epidemiology of infections caused by pathogenic bacteria, such as methicillin-resistant
Staphylococcus aureus (MRSA),
Enterococcus spp. vancomycin resistant (VRE), etc. Studies carried out on the persistence of microbes on surfaces have shown that these microorganisms are able to survive on dry surfaces for hours or days and in some cases for months [
39]. Infections caused by Gram-negative and Gram-positive bacteria are frequently occurring.
Escherichia coli is a well-studied model organism for Gram-negative bacteria. Survival analyses of
E. coli on several inanimate surface have been published in numerous amounts. Different authors show
E. coli survival on different inanimate surfaces. It is assumed that
E. coli persistence is dependent on several factors. Next to strain specific characteristics and material nature, environmental conditions, such as temperature and humidity, have been shown to exhibit a huge impact on survivability duration. In contrast to
S. aureus, where all bacterial strains survived on ceramic floors, synthetic fiber, cotton, and mattresses for more than eight weeks,
E. coli was not so stable on all materials.
Pseudomonas aeruginosa, Gram-negative bacteria, is associated with hospital acquired infections due to its capability to survive under challenging environmental conditions; it can survive on different inanimate surfaces in a material, species, and strain dependent manner.
As with bacteria, viral species also exhibit a different resistance on inanimate surfaces. The danger of viruses has been confirmed both by epidemiological evidence and by microbiological evidence that detect the presence of them on a large variety of surfaces. Viruses are able to resist various environmental factors and disinfection techniques, even those that are prolonged over time. The most important characteristic to keep in mind when evaluating the environmental resistance of viruses is of the “biological” type. The absence of an envelope gives them greater resistance to drying, making them more stable in the environment. It possesses a high genetic variability and a high recombination capacity that induce the development of dangerous viral agents that can stress unexpected health emergencies, such as that of the SARS-CoV-2 virus.
Coronaviridae consist of positive single strand RNA viruses with an envelope, contributing to its name (from lat. corona—crown). There are seven human pathogenic strains of coronaviruses (CoV)—four endemic strains (229E, OC43, NL63, and HKU1) and three epidemic strains (severe acute respiratory syndrome coronavirus (SARS-1-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2)). The mode of transmission is mainly by respiratory droplets and aerosols, but a certain contribution of contact transmission, e.g., via fomites, seems likely [
40]. The family
Adenoviridae comprises nonenveloped viruses with an icosahedral nucleocapsid containing a double stranded DNA genome, causing a wide range of illnesses, from mild respiratory tract infections or conjunctivitis to life-threatening multi-organ disease in patients with a weakened immune system. In addition, the survival of viruses on surfaces depends on many factors, the type of surface and its composition, the environment, the conditions of analysis as well as the period [
41].
The efficiency of the transfer of a pathogen from surfaces to the skin is an important parameter for understanding transmission capacity and implementing effective hygiene measures. In the current study, we were able to demonstrate that aerosolized 300 ppm of HOCl had powerful and rapid killing properties on common bacteria (E. coli, S. aureus and P. aeruginosa) and viruses (Coronavirus 229E and Adenovirus V) on various surfaces (semi-porous, flat and porous).
Our study focuses on the use of HOCl solution in nebulized form for disinfection against microorganisms and surfaces of different nature to best estimate the bactericidal and virucidal activity of the HOCl. Nebulization time of 3 min and exposure time of 10 min showed excellent bacterial and viral growth reduction values on all microorganisms used.
It must be taken into account that these tests have been carried out starting from a stock of HCoV-229E and Adenovirus V with a high titer, and that, on average, the viral load found in aerosols or on surfaces may be significantly similar.
The results obtained show that the diversity of the materials after treatment does not affect the ability of the product to neutralize microorganisms. The values obtained as percentages of bacterial or viral growth reduction are in the range of 90–99.9%, values considered significant.
While most disinfectants are tested or studied in their liquid form on several types of surfaces, the only studies of viricidal/bactericidal efficacy of nebulized HOCl have been carried out when used as wet spray. Hakim et al. [
42] present a comprehensive insight into the efficacy of HOCl in spray form to inactivate
Escherichia coli and
Salmonella with an effective concentration of 50–100 ppm on rayon fabric. Naka et al. [
43] found that HOCl exhibited higher bactericidal activity compared with NaOCl with the same concentration of 0.5 ppm. Okamoto et al. [
44] also showed that 20-min exposure to sprayed HOCl of 0.01% was sufficient to reduce 99.5% of
Staphylococcus epidermidis.Block and Rowan [
15] also found the effective concentration of HOCl to be 200 ppm in decontaminating inert surfaces carrying noroviruses and other enteric viruses in a 1-min contact time. When diluted 10-fold, HOCl solutions at 20 ppm were still effective in decontaminating environmental surfaces carrying viruses in a 10-min contact time.
Air disinfection and hand hygiene are the major preventive measure in containing infections. High-risk exposed surface areas need to be cleaned frequently with a suitable disinfectant product [
45]. The characteristic features of an ideal disinfectant are a low contact time with significant bactericidal and viricidal activity with it being safe for humans, the environment, surfaces, and equipment.
The amount of active chlorine declined markedly in aerosolized HOCl droplets, due to air and light exposure, falling by up to 60% by the time they cover the distance between the nozzle and the space around [
46]. Despite fairly low concentration of HOCl used in the present work, we were able to clearly demonstrate antimicrobial and antivirus activities, making our discoveries particularly relevant to human daily life [
47]. Solutions of HOCl have been shown to rapidly inactivate pathogens and viruses in test systems comparable to those used in this study [
48]. Based on those results, commercial products containing HOCl have been recently placed on commerce and approved by the US Environmental Protection Agency (EPA), EU Chemical Agency (ECHA) and Australian Therapeutic Goods Administration (TGA), as a disinfectant against COVID-19.
More broadly, we note that widespread, high-volume use of disinfectants has come about as a response to the current pandemic, and it is becoming increasingly apparent that many of these, namely ozone or hydrogen peroxide, are inappropriate for such use patterns, never having been designed or intended for intensive applications around—and sometimes upon—humans. Toxicological evidence of serious untoward effects of repeated exposures to commonplace disinfectants, especially by inhalation of aerosols, are emerging [
10]. Formaldehyde- and glutaraldehyde-based disinfectants are noxious and cytotoxic and may pose a serious health risk in addition to adversely affecting the environment [
49]. Sodium hypochlorite-based disinfectants have severe adverse effects and cause skin irritation, membrane irritation, and acute toxicity [
50]. Quaternary ammonium-based disinfectants irritate the skin and mucous membranes and are related to the development of airway allergies as well as induce occupational asthma and contact dermatitis [
51]. For alcohol-based disinfectants and hand sanitizers, frequent use of high-concentration formulations may lead to skin damage or contact dermatitis with skin irritation, dryness, redness, and cracking [
52]. Additionally, all of them may negatively impact the environment, soil, aquatic organisms, and wildlife, leading to serious and dramatic future consequences. In our study, the usage of 3D models helped us to further validate the 2D cell results concerning the lack of harmful effects of HOCl nebulization for human health. Indeed, 3D cell culture represents a more physiological approach closer to the morphology and physiology of the human tissues and therefore better mimicking the in vivo conditions.
Epiderm® and
Epiairway®, resembling cutaneous and respiratory tract tissues, respectively, were not affected by 300 ppm of HOCl exposure at the morphologically, metabolic rate and viability analyses. In addition, transepithelial electrical resistance (TEER) measurement confirmed that the nebulization did not induce any alteration of the cellular junctions, in either of the tissues.
Moreover, HOCl does not lead to the development of antimicrobial resistance, unlike genotoxic chemicals [
53].
For its nature, HOCl is unstable because it rapidly decays to chloride, unless it is stored in dark and not exposed to air, preferably at 4 °C [
54], ensuring a relatively short shelf-life, but it can be easily and inexpensively produced in situ combining an electrolysis and aerosolizing machine. A liter of HOCl can be manufactured only from sodium chloride, water and electricity, with an average cost of about EUR 0.25, considering the 2020 average European electric energy cost of 21.92 €cent/kWh [
1]. Moreover, it avoids transportation of harmful products, such as chlorine gas and concentrated bulk hypochlorite, and it has been shown to hold significant economic advantage. In situ generation is of particular interest for developing countries, where the supply chain of bulk chemical commodities can be unreliable and accessing many locations is often a hurdle. Having cost-effective devices that could work in a standalone, solar-driven fashion, and generate chlorinated products on-site at low cost could help to improve the life of those communities [
55,
56].