Nanoarchitectonics and Kinetics Insights into Fluoride Removal from Drinking Water Using Magnetic Tea Biochar
Abstract
1. Introduction
2. Material and Methods
2.1. Chemical and Reagents
2.2. Sorbent Preparation
2.3. Characterization
2.3.1. Ultimate and Proximate Analysis
2.3.2. Surface Area, Morphology, Magnetic and Thermal Analysis
2.3.3. Point of Zero Charge Analysis
2.4. Adsorption Experiment
2.4.1. Adsorption Kinetics Study
2.4.2. Adsorption Isotherm Study
2.5. Effect of Co-Exist Ions
2.6. Regeneration and Real Water Test
3. Results and Discussion
3.1. Proximate, Ultimate and BET Analysis
3.2. Morphology, Magnetic and Thermal Analysis
3.2.1. SEM Analysis
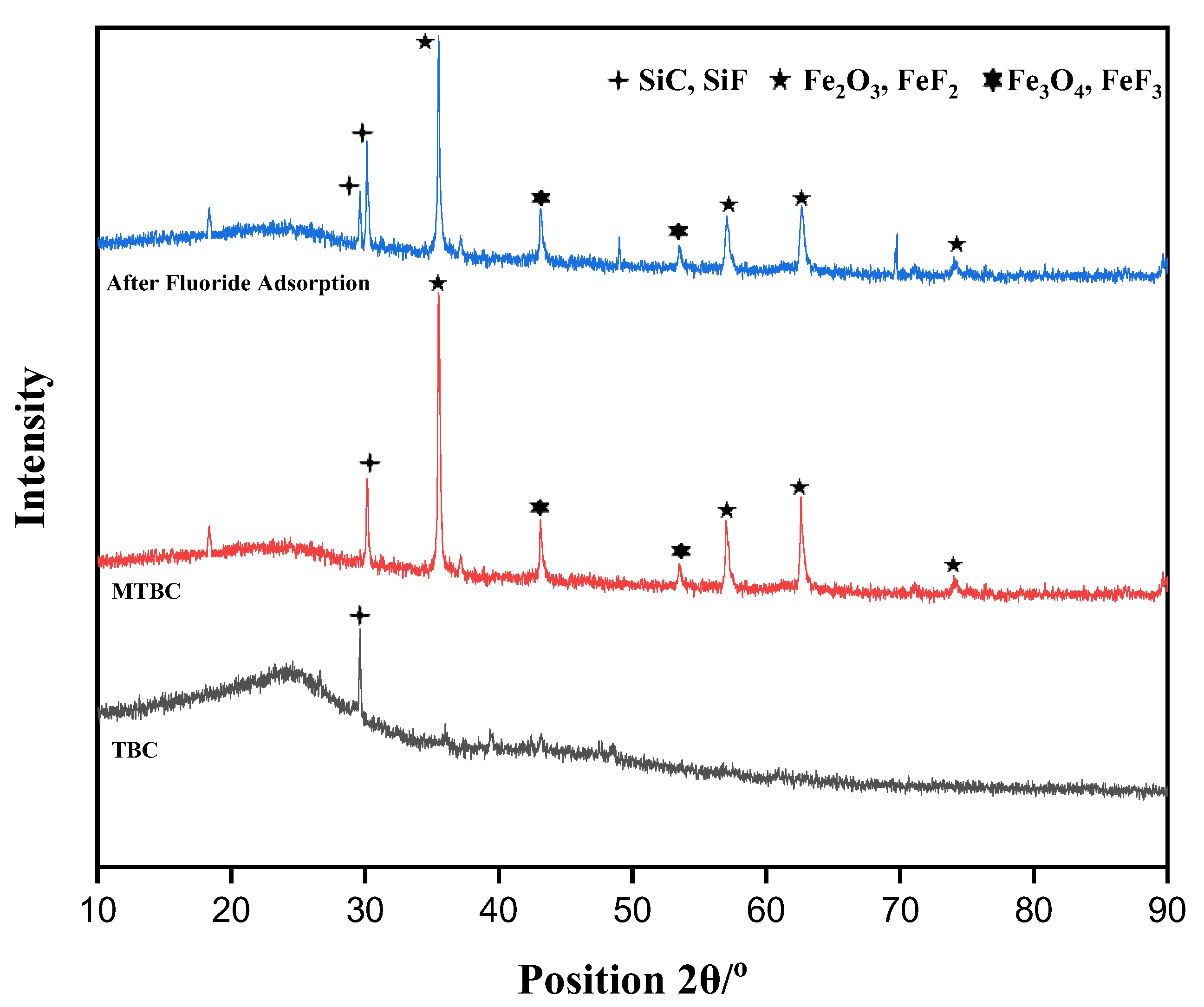
3.2.2. XRD Analysis
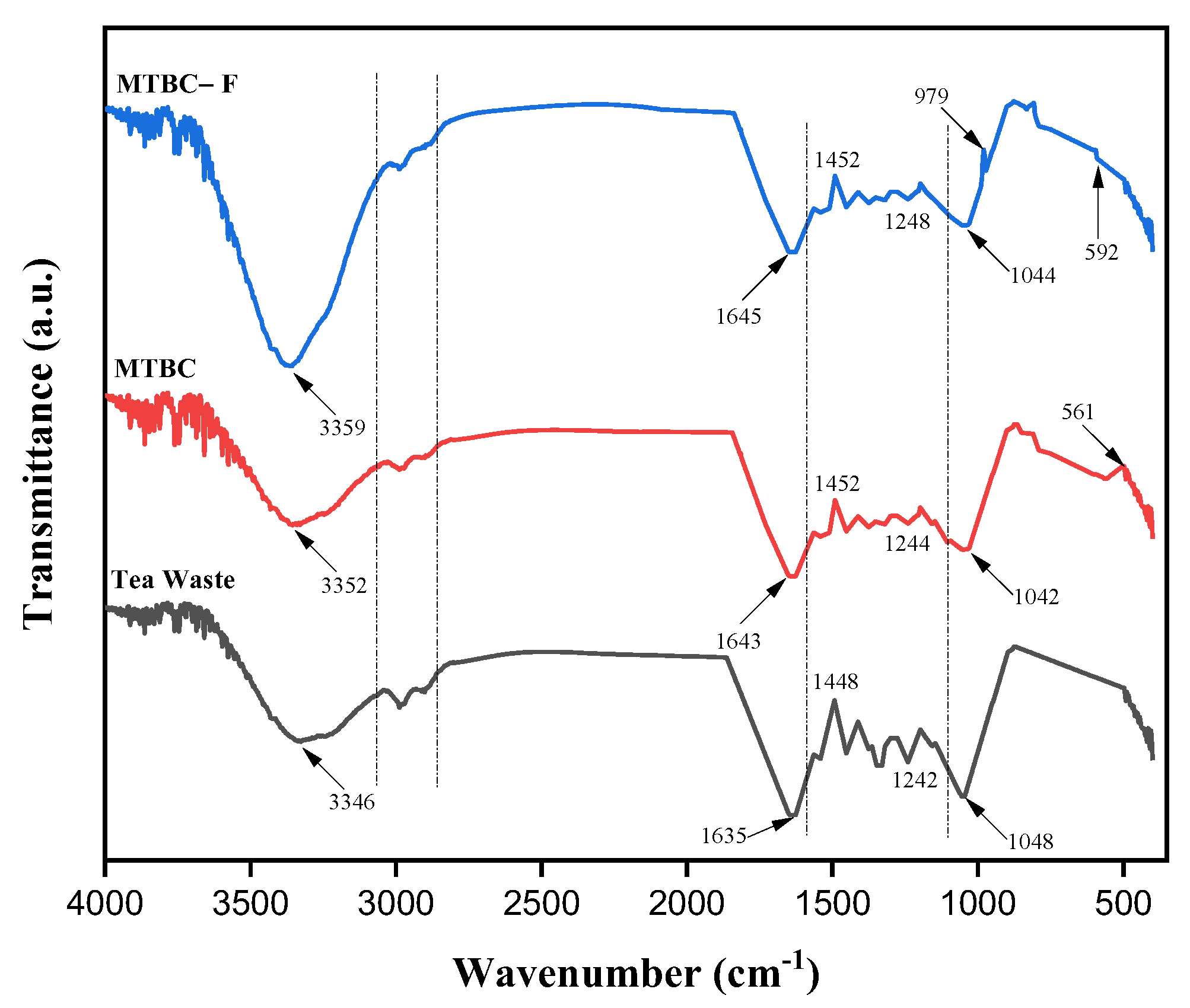
3.2.3. FTIR Analysis
3.2.4. VSM Analysis
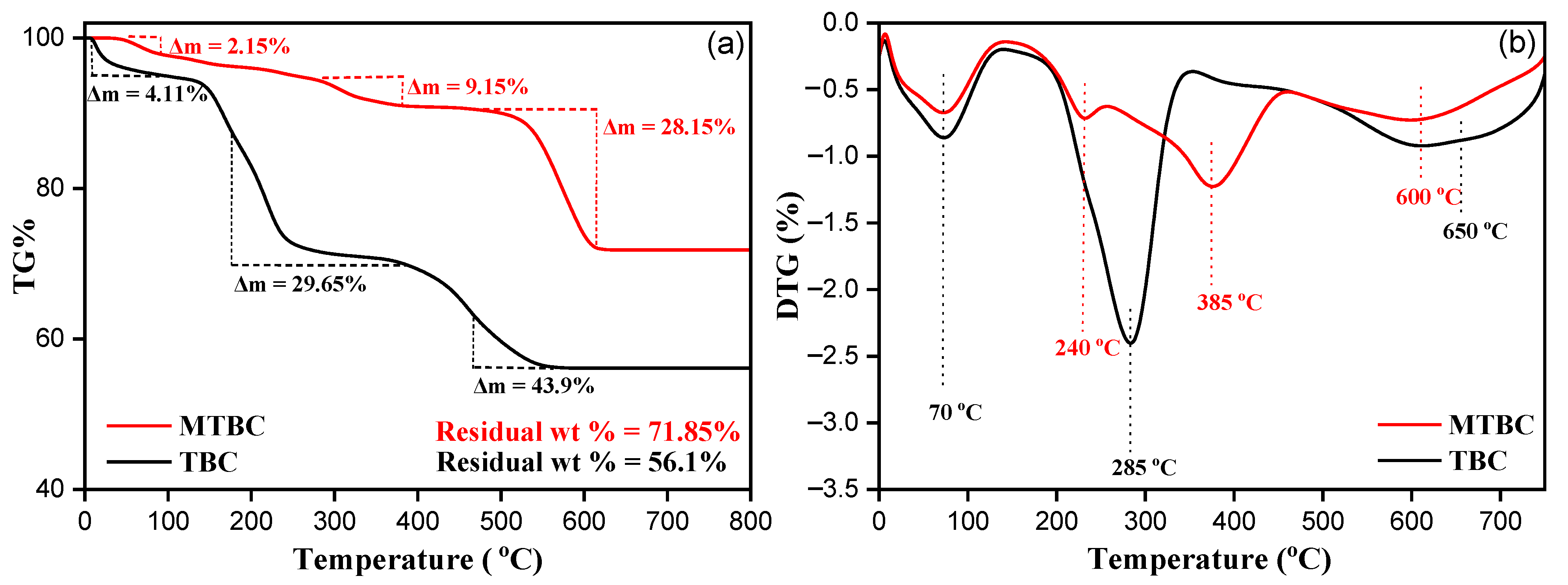
3.2.5. Thermogravimetric Analysis
3.3. Batch Adsorption Experiments
3.3.1. Effect of pH and Point to Zero Charges (pHpzc) Analysis
3.3.2. Effect of Adsorbent Dosage
3.3.3. Effect of Initial Fluoride Concentration
3.4. Adsorption Kinetics Study
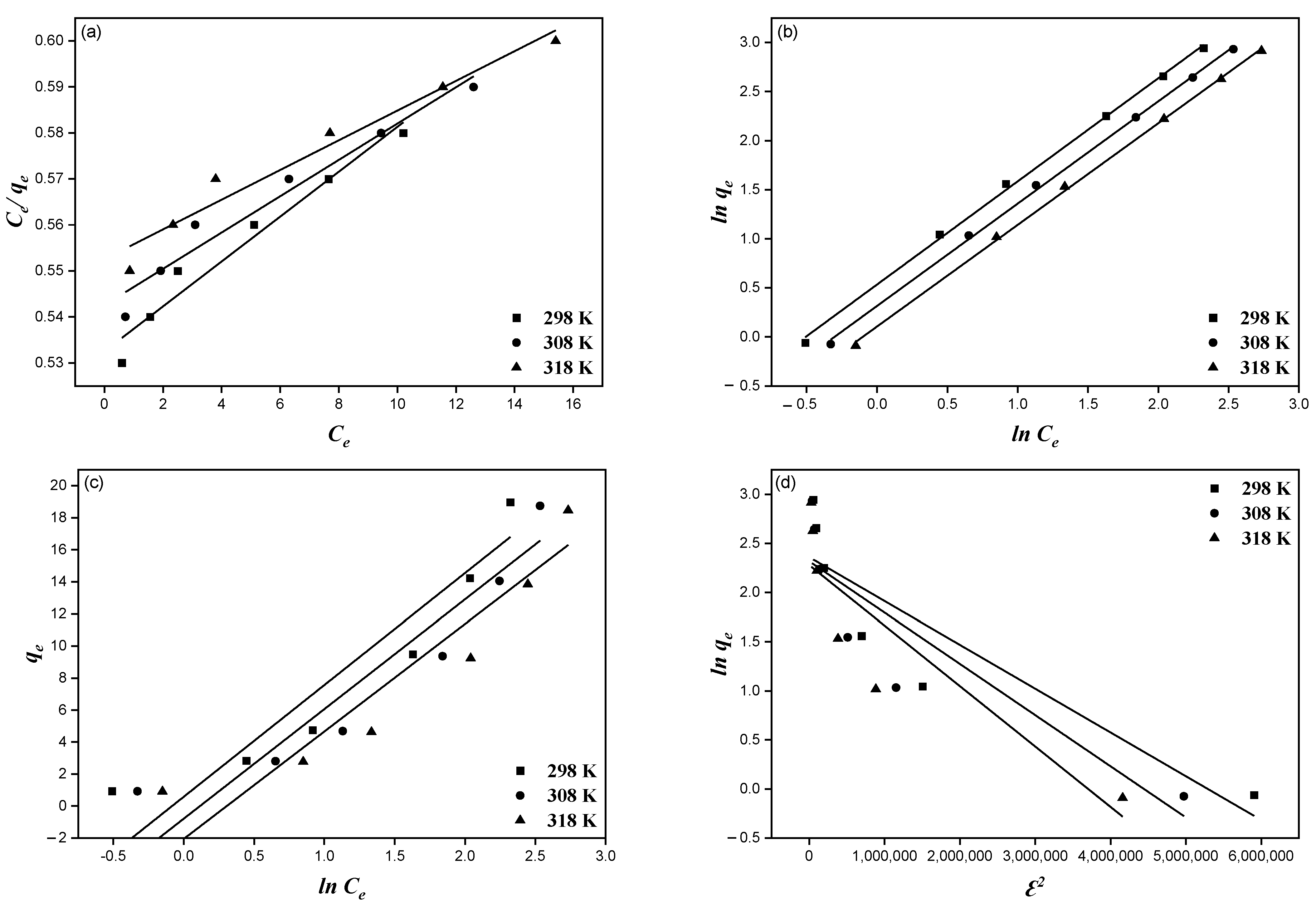
3.5. Adsorption Isotherms Study
3.6. Adsorption Thermodynamics Study
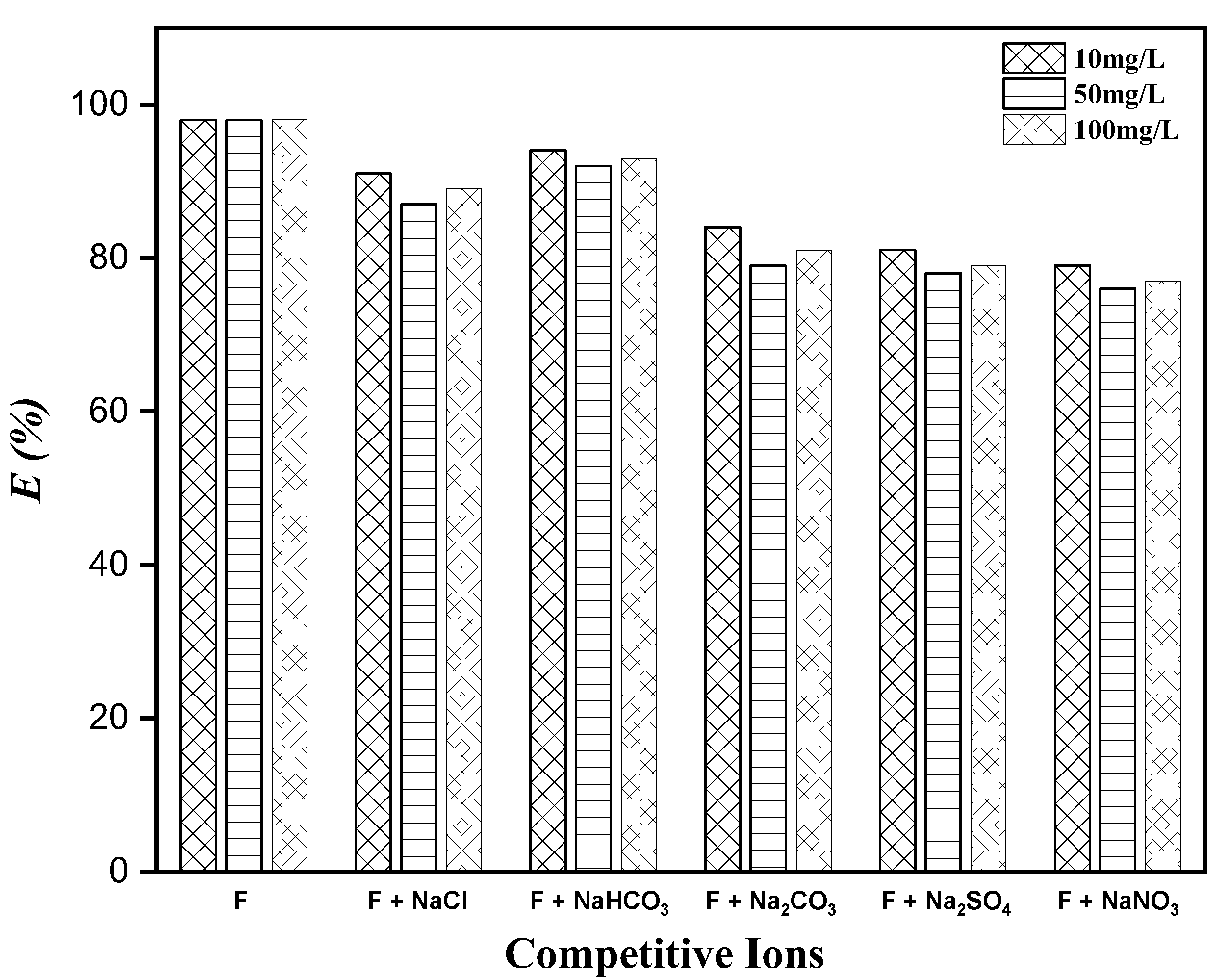
3.7. Effect of Co-Existing Anions
3.8. Regeneration Study and Application of Groundwater Samples
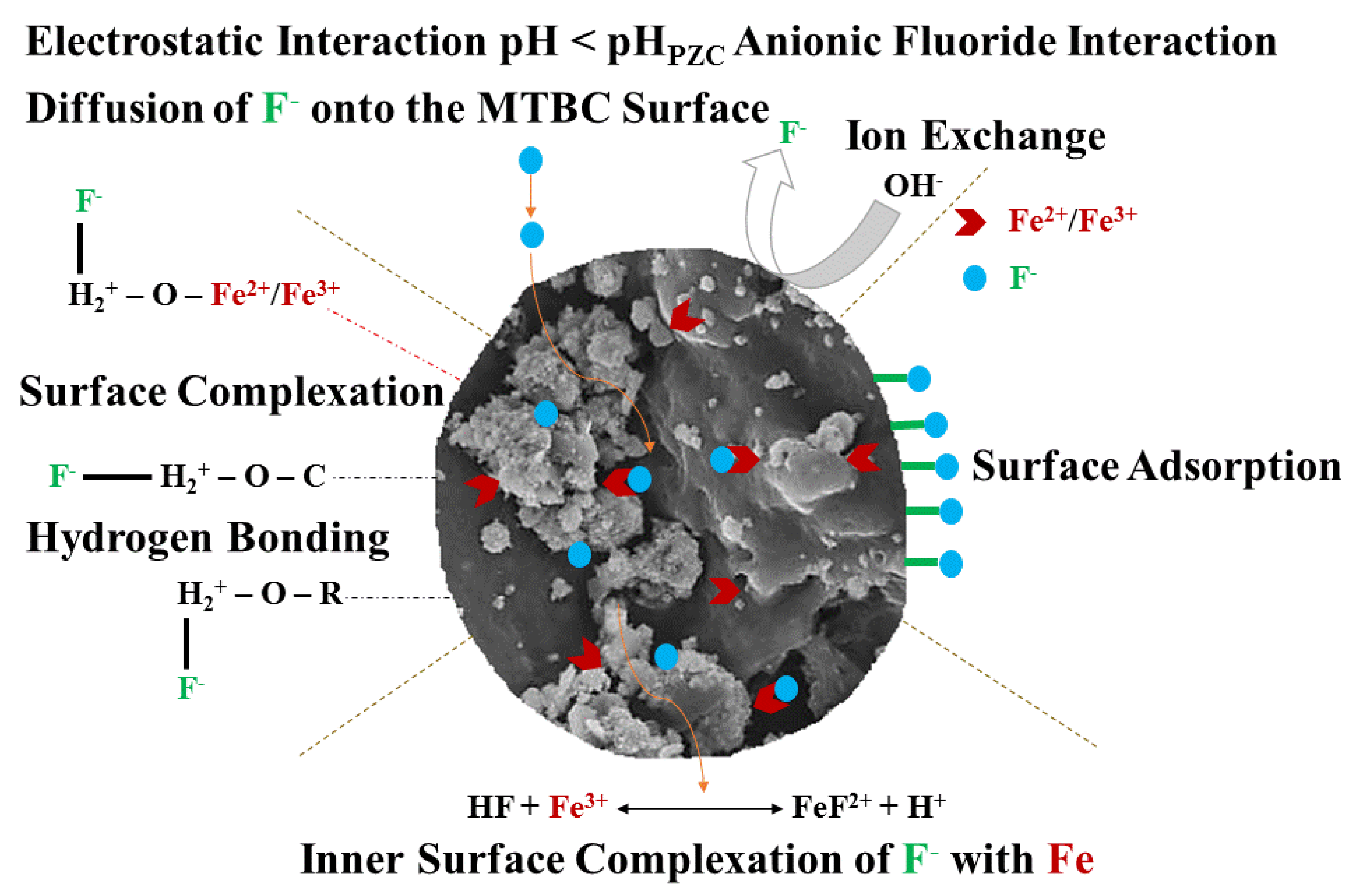
3.9. Possible Removal Mechanisms
3.10. Economic Implication for MTBC Usage in the Real World
- Raw material = 0 USD (collected from different cafes and teashops).
- Sample processing = 0.055 USD (tap water was used for initial wash. Distilled water was only used for rinsing [0.5 l × 0.11 USD per liter cost]).
- Fe (NO3)3 impregnation = 0.148 [5.5 g of Fe (NO3)3 was dissolved in 50 mL of distilled water for 10 g tea feedstock (5.5 × 0.027 cost per gram)].
- Pyrolysis = 0.516 USD (6 Units × 0.086 cost per unit).
- Total cost per gram = 0.0719 USD (0.719 ÷ 10).
- Total cost per Kg = 71.9 USD.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, R.; Tian, X.; Ashraf, I.; Chen, B. Fluoride removal using a chelating resin containing phosphonic-sulfonic acid bifunctional group. J. Chromatogr. A 2020, 1613, 460697. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; CCBY-NC-SA3.0IGO; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 5 March 2022).
- Chatterjee, A.; Pan, N.; Maji, T.K.; Pasha, S.S.; Singh, S.; Ahmed, S.A.; Al-Thakafy, J.T.; Pal, S.K. Highly Sensitive Optical Sensor for Selective Detection of Fluoride Level in Drinking Water: Methodology to Fabrication of Prototype Device. ACS Sustain. Chem. Eng. 2021, 9, 7160–7170. [Google Scholar] [CrossRef]
- Kumar, H.; Patel, M.; Mohan, D. Simplified Batch and Fixed-Bed Design System for Efficient and Sustainable Fluoride Removal from Water Using Slow Pyrolyzed Okra Stem and Black Gram Straw Biochars. ACS Omega 2019, 4, 19513–19525. [Google Scholar] [CrossRef]
- Goswami, R.; Kumar, M. Removal of fluoride from aqueous solution using nanoscale rice husk biochar. Groundw. Sustain. Dev. 2018, 7, 446–451. [Google Scholar] [CrossRef]
- Chandio, T.A.; Khan, M.N.; Muhammad, M.T.; Yalcinkaya, O.; Wasim, A.A.; Kayis, A.F. Fluoride and arsenic contamination in drinking water due to mining activities and its impact on local area population. Environ. Sci. Pollut. Res. 2021, 28, 2355–2368. [Google Scholar] [CrossRef]
- Sha, Q.; Xie, H.; Liu, W.; Yang, D.; Yang, C.; Wang, N.; Ge, C. Removal of fluoride using Platanus acerifoli leaves biochar—An efficient and low-cost application in wastewater treatment. Environ. Technol. 2021, 0, 1–15. [Google Scholar] [CrossRef]
- Borah, K.K.; Bhuyan, B.; Sarma, H.P. Lead, arsenic, fluoride, and iron contamination of drinking water in the tea garden belt of Darrang district, Assam, India. Environ. Monit. Assess. 2010, 169, 347–352. [Google Scholar] [CrossRef]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in drinking water and defluoridation of water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef]
- Farooqi, A.; Masuda, H.; Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan, and possible contaminant sources. Environ. Pollut. 2007, 145, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Naha, S.; Velmathi, S. Phenazine-Based Fluorescence “Turn-Off” Sensor for Fluoride: Application on Real Samples and to Cell and Zebrafish Imaging. Chem. Sel. 2019, 4, 2912–2917. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Liu, S.; Guo, H.; Meng, J.; Chang, M.; Wu, S. Highly sensitive detection of fluoride based on poly(3-aminophenylboronic acid)-reduced graphene oxide multilayer modified electrode. Food Chem. 2022, 400, 134042. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.; Saeed, A.; He, J.; Zhao, N.; Wang, J.; Xu, W.; Liu, J. Visual detection of fluoride in water by a dual-emitting, Eu-doped Sc-based metal organic framework. New J. Chem. 2022, 46, 13693–13699. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, F.; Li, Y.; Yan, M.; Cui, Y.; Sun, G. Three Indole Derived Azo-Azomethine Dyes as Effective Chemosensors for F− Ion and Trace Water Detection. Bull. Chem. Soc. Jap. 2020, 93, 870–879. [Google Scholar] [CrossRef]
- Yu, T.; Chen, Y.; Zhang, Y.; Tan, X.; Xie, T.; Shao, B.; Huang, X. Novel reusable sulfate-type zirconium alginate ion-exchanger for fluoride removal. Chin. Chem. Lett. 2021, 32, 3410–3415. [Google Scholar] [CrossRef]
- Elazhar, F.; Elazhar, M.; El-Ghzizel, S.; Tahaikt, M.; Zait, M.; Dhiba, D.; Elmidaoui, A.; Taky, M. Nanofiltration-reverse osmosis hybrid process for hardness removal in brackish water with higher recovery rate and minimization of brine discharges. Process Saf. Environ. Prot. 2021, 153, 376–383. [Google Scholar] [CrossRef]
- Boussouga, Y.A.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: System resilience under solar irradiance fluctuations during the treatment of fluoride-rich natural waters by different nanofiltration/reverse osmosis membranes. J. Memb. Sci. 2021, 617, 118452. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Ali, A.; Zhang, R.; Yang, W.; Xu, L.; Zhao, T. Microbially induced calcium precipitation based simultaneous removal of fluoride, nitrate, and calcium by Pseudomonas sp. WZ39: Mechanisms and nucleation pathways. J. Hazard. Mater. 2021, 416, 125914. [Google Scholar] [CrossRef]
- Issabayeva, G.; Wong, S.H.; Pang, C.Y.; Wong, M.C.; Aroua, M.K. Fluoride removal by low-cost palm shell activated carbon modified with prawn shell chitosan adsorbents. Int. J. Environ. Sci. Technol. 2021, 19, 3731–3740. [Google Scholar] [CrossRef]
- Cruz-Briano, S.A.; Medellín-Castillo, N.A.; Torres-Dosal, A.; Leyva-Ramos, R.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Díaz-Flores, P.E.; Reyes-López, S.Y.; Ocampo-Pérez, R. Bone Char from an Invasive Aquatic Specie as a Green Adsorbent for Fluoride Removal in Drinking Water. Water Air Soil Pollut. 2021, 232, 346. [Google Scholar] [CrossRef]
- Díaz-Flores, P.E.; Arcibar-Orozco, J.A.; Flores-Rojas, A.I.; Rangel-Méndez, J.R. Synthesis of a Chitosan-Zeolite Composite Modified with La(III): Characterization and its Application in the Removal of Fluoride from Aqueous Systems. Water Air Soil Pollut. 2021, 232, 235. [Google Scholar] [CrossRef]
- Banihashemi, A.; Zare, K.; Javanbakht, V.; Mohammadifard, H. Calcium carbonate nanoparticles fabricated by a facile method based on the colloidal gas aphrons for removal of fluoride ions from aqueous solutions. Mater. Chem. Phys. 2021, 258, 123934. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W. Removal of fluoride from groundwater using MnO2 bentonite-smectite rich clay soils composite. Groundw. Sustain. Dev. 2021, 14, 100623. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.J.C.; Hansima, M.A.C.K.; Nanayakkara, K.G.N. Adsorptive removal of fluoride from water by chemically modified coal fly ash: Synthesis, characterization, kinetics, and mechanisms. Groundw. Sustain. Dev. 2021, 16, 100699. [Google Scholar] [CrossRef]
- Srinivasulu, D. Senna auriculata L. flower petal biomass: An alternative green biosorbent for the removal of fluoride from aqueous solutions. Acta Ecol. Sin. 2021, in press. [Google Scholar] [CrossRef]
- Sadhu, M.; Bhattacharya, P.; Vithanage, M.; Padmaja Sudhakar, P. Adsorptive removal of fluoride using biochar—A potential application in drinking water treatment. Sep. Purif. Technol. 2021, 278, 119106. [Google Scholar] [CrossRef]
- Altaf, A.R.; Teng, H.; Zheng, M.; Ashraf, I.; Arslan, M.; Rehman, A.; Gang, L.; Pengjie, W.; Yongqiang, R.; Xiaoyu, L. One-step synthesis of renewable magnetic tea-biochar derived from waste tea leaves for the removal of Hg0 from coal-syngas. J. Environ. Chem. Eng. 2021, 9, 105313. [Google Scholar] [CrossRef]
- Akhayere, E.; Vaseashta, A.; Kavaz, D. Novel Magnetic Nano Silica Synthesis Using Barley Husk Waste for Removing Petroleum from Polluted Water for Environmental Sustainability. Sustainability 2020, 12, 10646. [Google Scholar] [CrossRef]
- Hussain, S.; Anjali, K.P.; Hassan, S.T.; Dwivedi, P.B. Waste tea as a novel adsorbent: A review. Appl. Water Sci. 2018, 8, 165. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, B.; Tyszczuk-Rotko, K.; Kończak, M.; Fakhrhoseini, S.M.; Yadav, R.; Naebe, M. Sustainable synthesis of rose flower-like magnetic biochar from tea waste for environmental applications. J. Adv. Res. 2021, 34, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, S.; Wu, R.; Zhang, L.; Wang, P.; Xiao, R. Magnetic biochars have lower adsorption but higher separation effectiveness for Cd2+ from aqueous solution compared to nonmagnetic biochars. Environ. Pollut. 2021, 275, 116485. [Google Scholar] [CrossRef] [PubMed]
- Altaf, A.R.; Teng, H.; Gang, L.; Adewuyi, Y.G.; Zheng, M. Effect of Sonochemical Treatment on Thermal Stability, Elemental Mercury (Hg0) Removal, and Regenerable Performance of Magnetic Tea Biochar. ACS Omega 2021, 6, 23913–23923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Xiong, Y. Synthesis and characterization of rice husk-based magnetic porous carbon by pyrolysis of pretreated rice husk with FeCl3 and ZnCl2. J. Anal. Appl. Pyrolysis 2020, 147, 104806. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. The role and mechanism of K2CO3 and Fe3O4 in the preparation of magnetic peanut shell based activated carbon. Powder Technol. 2016, 295, 152–160. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, G.; He, S.; Deng, F.; Pang, Q.; Xu, Y.; Yao, H. Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe(NO3)3-laden wood and polyvinyl chloride waste. Fuel 2019, 239, 982–990. [Google Scholar] [CrossRef]
- Altaf, A.R.; Adewuyi, Y.G.; Teng, H.; Liu, G.; Abid, F. Elemental mercury (Hg0) removal from coal syngas using magnetic tea-biochar: Experimental and theoretical insights. J. Environ. Sci. 2022, 122, 150–161. [Google Scholar] [CrossRef]
- Malakahmad, A.; Tan, S.; Yavari, S. Valorization of Wasted Black Tea as a Low-Cost Adsorbent for Nickel and Zinc Removal from Aqueous Solution. J. Chem. 2016, 2016, 5680983. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Shen, B.; Ren, K.; Wang, Y.; Luo, J. Enhanced elemental mercury removal via chlorine-based hierarchically porous biochar with CaCO3 as template. Chem. Eng. J. 2021, 46, 126828. [Google Scholar] [CrossRef]
- Li, C.; Chen, N.; Zhao, Y.; Li, R.; Feng, C. Polypyrrole-grafted peanut shell biological carbon as a potential sorbent for fluoride removal: Sorption capability and mechanism. Chemosphere 2016, 163, 81–89. [Google Scholar] [CrossRef]
- Kang, J.; Gou, X.; Hu, Y.; Sun, W.; Liu, R.; Gao, Z.; Guan, Q. Efficient utilisation of flue gas desulfurization gypsum as a potential material for fluoride removal. Sci. Total Environ. 2019, 649, 344–352. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Guo, X.; Chen, J.P. Modification of carbon derived from Sargassum sp. by lanthanum for enhanced adsorption of fluoride. J. Colloid Interface Sci. 2015, 441, 113–120. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Hu, Y.; Han, R. Preparation of Novel Magnetic Microspheres with the La and Ce-Bimetal Oxide Shell for Excellent Adsorption of Fluoride and Phosphate from Solution. J. Chem. Eng. Data 2019, 64, 3641–3651. [Google Scholar] [CrossRef]
- Tirkey, P.; Bhattacharya, T.; Chakraborty, S. Optimization of fluoride removal from aqueous solution using Jamun (Syzygium cumini) leaf ash. Process Saf. Environ. Prot. 2017, 115, 125–138. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Hu, X.; Ali, A.; Wu, Z. Isolation of biosynthetic crystals by microbially induced calcium carbonate precipitation and their utilization for fluoride removal from groundwater. J. Hazard. Mater. 2021, 406, 124748. [Google Scholar] [CrossRef] [PubMed]
- Gebrewold, B.D.; Kijjanapanich, P.; Rene, E.R.; Lens, P.N.L.; Annachhatre, A.P. Fluoride removal from groundwater using chemically modified rice husk and corn cob activated carbon. Environ. Technol. 2019, 40, 2913–2927. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Jagadevan, S. Influence of torrefaction and pyrolysis on engineered biochar and its applicability in defluoridation: Insight into adsorption mechanism, batch adsorber design and artificial neural network modelling. J. Anal. Appl. Pyrolysis 2021, 154, 105015. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Yakout, S.M.; Elreefy, S.A. Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J. Hazard. Mater. 2007, 147, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sharma, R.; Singh, V.K.; Steele, P.; Pittman, C.U. Fluoride Removal from Water using Bio-Char, a Green Waste, Low-Cost Adsorbent: Equilibrium Uptake and Sorption Dynamics Modeling. Ind. Eng. Chem. Res. 2012, 51, 900–914. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, S.; Srivastava, A. Fluoride removal from ground water using magnetic and nonmagnetic corn stover biochars. Ecol. Eng. 2014, 73, 798–808. [Google Scholar] [CrossRef]
- Dewage, N.B.; Liyanage, A.S.; Pittman, C.U.J.; Mohan, D.; Mlsna, T. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour. Technol. 2018, 263, 258–265. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Shah, M.; Sultania, R. Determination of fluoride removal using silica nano adsorbent modified by rice husk from water. Groundw. Sustain. Dev. 2020, 11, 100423. [Google Scholar] [CrossRef]
- Fadaei, A. Comparison of water defluoridation using different techniques. Int. J. Chem. Eng. 2021, 2021, 2023895. [Google Scholar] [CrossRef]






| Samples | Proximate Analysis | Ultimate Analysis | BET Analysis | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | AC | VM | FC | %C | %H | %N | %O | Other | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Width (nm) | ||
| TBC | 1.96 | 5.84 | 74.56 | 17.64 | 63.45 | 4.52 | 5.46 | 26.18 | 0.39 | 81.64 | 0.21 | 2.06 | 5.94 |
| MTBC | 1.86 | 5.34 | 79.73 | 13.07 | 63.75 | 4.65 | 5.63 | 25.56 | 0.41 | 115.65 | 0.40 | 5.42 | 5.78 |
| Co (mg/L) | qe,exp (mg/g) | Pseudo 1st Order | Pseudo 2nd Order | |||||
|---|---|---|---|---|---|---|---|---|
| qe, Cal (mg/g) | k1× 10−3 (h−1) | r12 | qe,cal (mg/g) | k2 (g/(mg·h) | h (mg/g·h) | r22 | ||
| 10 30 | 0.79 | 0.56 | −5.2 | 0.9912 | 0.88 | 0.30 | 0.23 | 0.9979 |
| 2.69 | 3.11 | −7.6 | 0.9564 | 3.21 | 0.06 | 0.64 | 0.9966 | |
| 50 | 4.90 | 5.24 | −8.0 | 0.9559 | 5.72 | 0.04 | 1.41 | 0.9999 |
| 100 | 9.60 | 5.43 | −3.8 | 0.9573 | 9.97 | 0.03 | 3.36 | 0.9999 |
| 150 | 14.25 | 6.84 | −3.4 | 0.8913 | 14.34 | 0.03 | 6.26 | 0.9994 |
| 200 | 18.79 | 7.55 | −3.0 | 0.7918 | 18.29 | 0.03 | 10.91 | 0.9989 |
| Elovich model | Intraparticle diffusion model | |||||||
| Co (mg/L) | (g/mg·h) | β (g/mg·h) | rE2 | kd1 (mg/g·h1/2) | kd2 (mg/g·h1/2) | C | ri2 | |
| 10 | 5.53 | −0.57 | 0.9802 | 0.20 | 0.07 | 0.23 | 0.9765 | |
| 30 | 1.47 | 0.38 | 0.9881 | 0.67 | 0.31 | 0.61 | 0.9980 | |
| 50 | 0.82 | 1.17 | 0.9932 | 1.40 | 0.46 | 1.47 | 0.9823 | |
| 100 | 0.50 | 2.14 | 0.9833 | 2.45 | 0.66 | 3.12 | 0.9683 | |
| 150 | 0.37 | 2.86 | 0.9622 | 3.53 | 0.58 | 5.81 | 0.9622 | |
| 200 | 0.31 | 3.57 | 0.9268 | 4.58 | 0.38 | 8.88 | 0.9544 | |
| Co (mg/L) | kd1 (mg/g·h1/2) | C | Ri | Ri Description | Adsorption Behavior | |
|---|---|---|---|---|---|---|
| Ri = 1 | No Initial Adsorption | |||||
| 10 | 0.20 | 0.02 | 0.97 | 0.9 < Ri < 1 | Weak initial adsorption | Weak initial adsorption |
| 30 | 0.67 | 0.02 | 0.99 | Weak initial adsorption | ||
| 50 | 1.40 | 0.26 | 0.90 | 0.5 < Ri < 0.9 | Intermediate initial adsorption | Intermediate initial adsorption |
| 100 | 2.45 | 0.50 | 0.89 | Intermediate initial adsorption | ||
| 150 | 3.53 | 1.41 | 0.87 | 0.1 < Ri < 0.5 | Strong initial adsorption | Intermediate initial adsorption |
| 200 | 4.58 | 2.67 | 0.86 | Intermediate initial adsorption | ||
| Ri < 0.1 | Adsorption completed in a short time | |||||
| Models | T (K) | Parameters | |||
|---|---|---|---|---|---|
| RL | KL (Lmg−1) | qmL (mg/g) | r2 | ||
| Langmuir | 298 | 0.89 | 1.09 | 18.78 | 0.9503 |
| 308 | 0.90 | 0.91 | 18.43 | 0.9513 | |
| 318 | 0.91 | 0.89 | 18.10 | 0.9507 | |
| KF (Lmg−1) | N | r2 | |||
| Freundlich | 298 | 1.71 | 2.30 | 0.9979 | |
| 308 | 1.37 | 2.34 | 0.9986 | ||
| 318 | 1.11 | 2.39 | 0.9981 | ||
| KT (Lmg−1) | b (kjmol−1) | r2 | |||
| Temkin | 298 | 1.09 | 6.98 | 0.8297 | |
| 308 | 0.89 | 6.85 | 0.8252 | ||
| 318 | 0.74 | 6.71 | 0.8183 | ||
| bDR× 10−7 | E (kjmol−1) | qmDR (mg/g) | r2 | ||
| Dubnin–Radushkevich | 298 | 4.46 | 1.06 | 10.59 | 0.7655 |
| 308 | 5.21 | 0.98 | 10.15 | 0.7438 | |
| 318 | 6.15 | 0.90 | 9.77 | 0.7256 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, I.; Li, R.; Chen, B.; Al-Ansari, N.; Rizwan Aslam, M.; Altaf, A.R.; Elbeltagi, A. Nanoarchitectonics and Kinetics Insights into Fluoride Removal from Drinking Water Using Magnetic Tea Biochar. Int. J. Environ. Res. Public Health 2022, 19, 13092. https://doi.org/10.3390/ijerph192013092
Ashraf I, Li R, Chen B, Al-Ansari N, Rizwan Aslam M, Altaf AR, Elbeltagi A. Nanoarchitectonics and Kinetics Insights into Fluoride Removal from Drinking Water Using Magnetic Tea Biochar. International Journal of Environmental Research and Public Health. 2022; 19(20):13092. https://doi.org/10.3390/ijerph192013092
Chicago/Turabian StyleAshraf, Imtiaz, Rong Li, Bin Chen, Nadhir Al-Ansari, Muhammad Rizwan Aslam, Adnan Raza Altaf, and Ahmed Elbeltagi. 2022. "Nanoarchitectonics and Kinetics Insights into Fluoride Removal from Drinking Water Using Magnetic Tea Biochar" International Journal of Environmental Research and Public Health 19, no. 20: 13092. https://doi.org/10.3390/ijerph192013092
APA StyleAshraf, I., Li, R., Chen, B., Al-Ansari, N., Rizwan Aslam, M., Altaf, A. R., & Elbeltagi, A. (2022). Nanoarchitectonics and Kinetics Insights into Fluoride Removal from Drinking Water Using Magnetic Tea Biochar. International Journal of Environmental Research and Public Health, 19(20), 13092. https://doi.org/10.3390/ijerph192013092









