Crocus Sativus for Insomnia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Searching Strategies
2.2. Inclusion and Exclusion Criteria
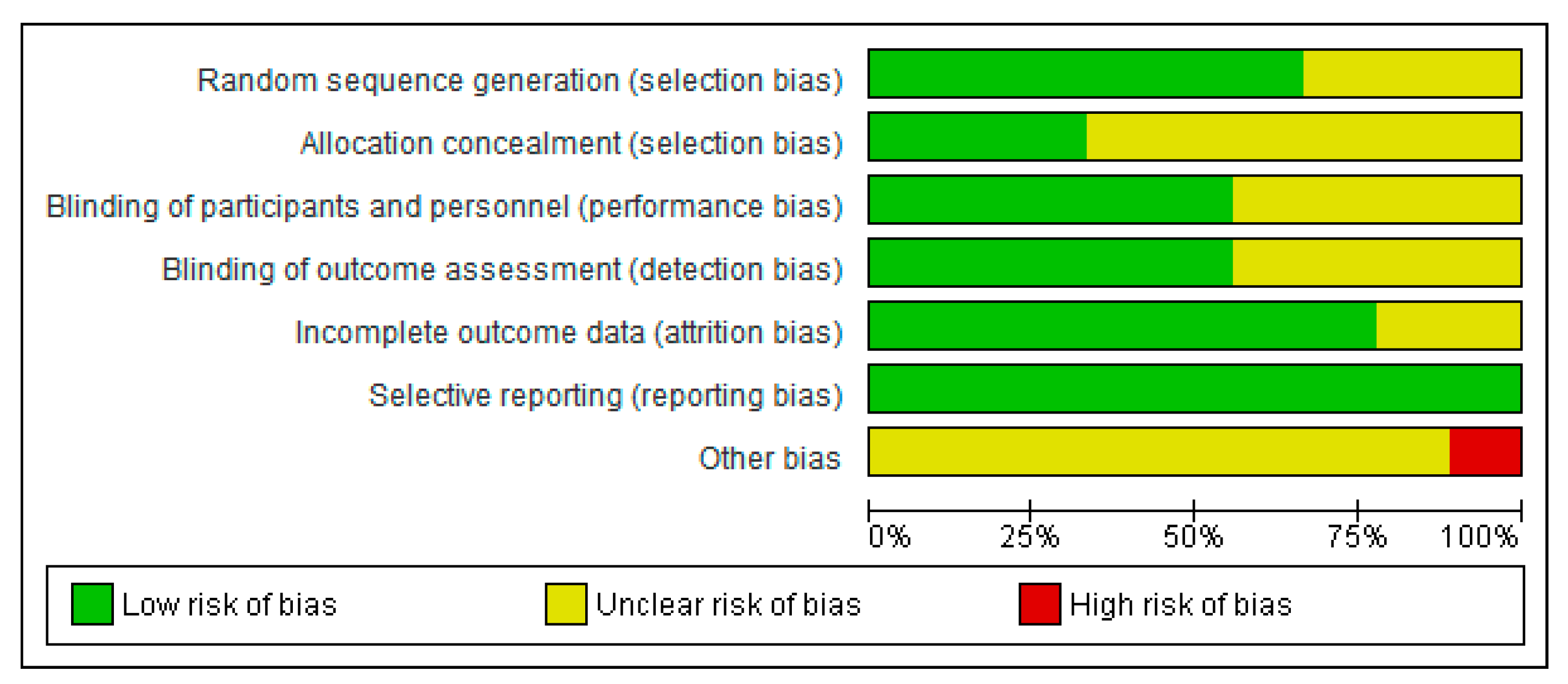
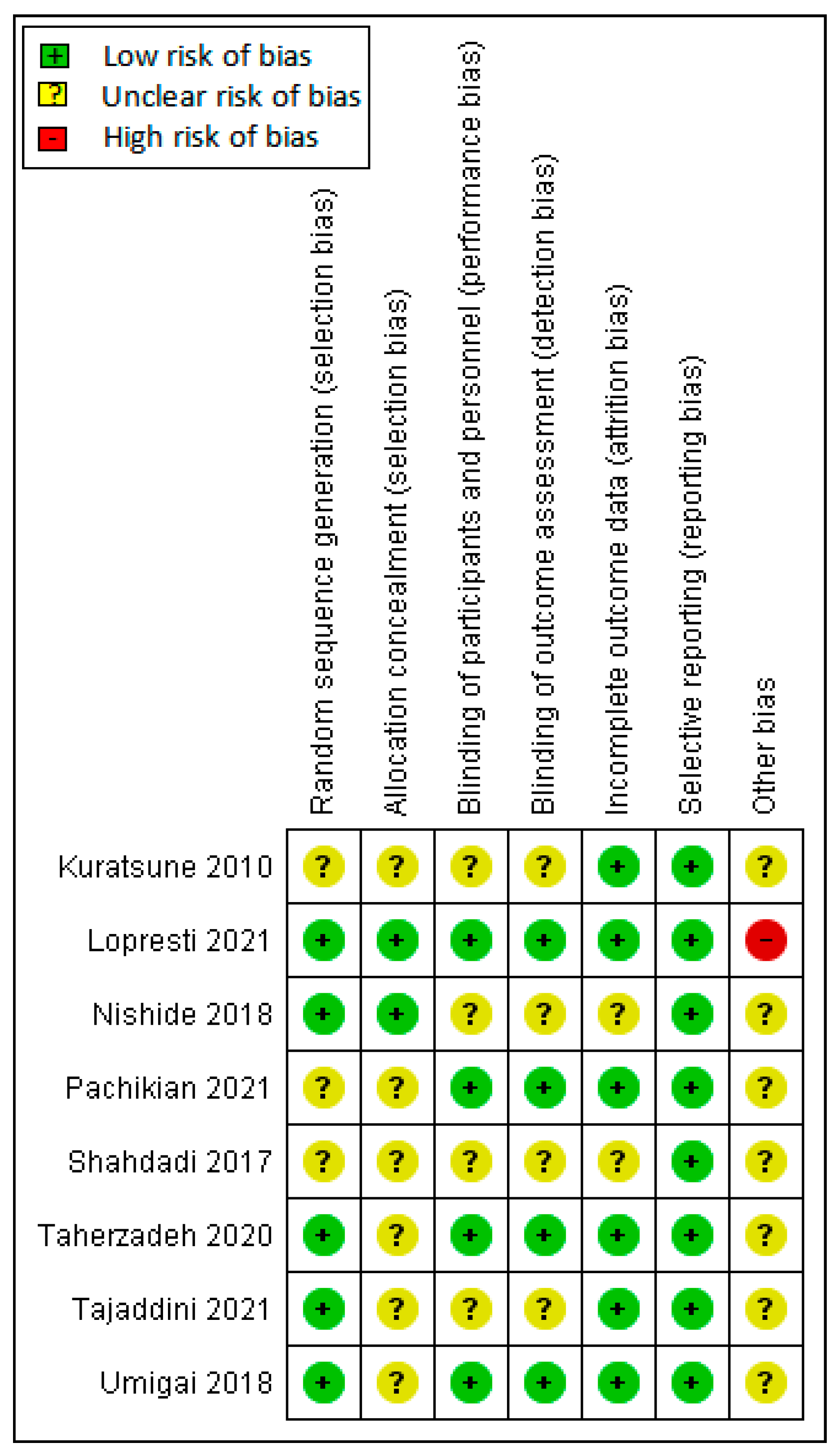
2.3. Quality Assessment
2.4. Statistical Analyses
3. Results
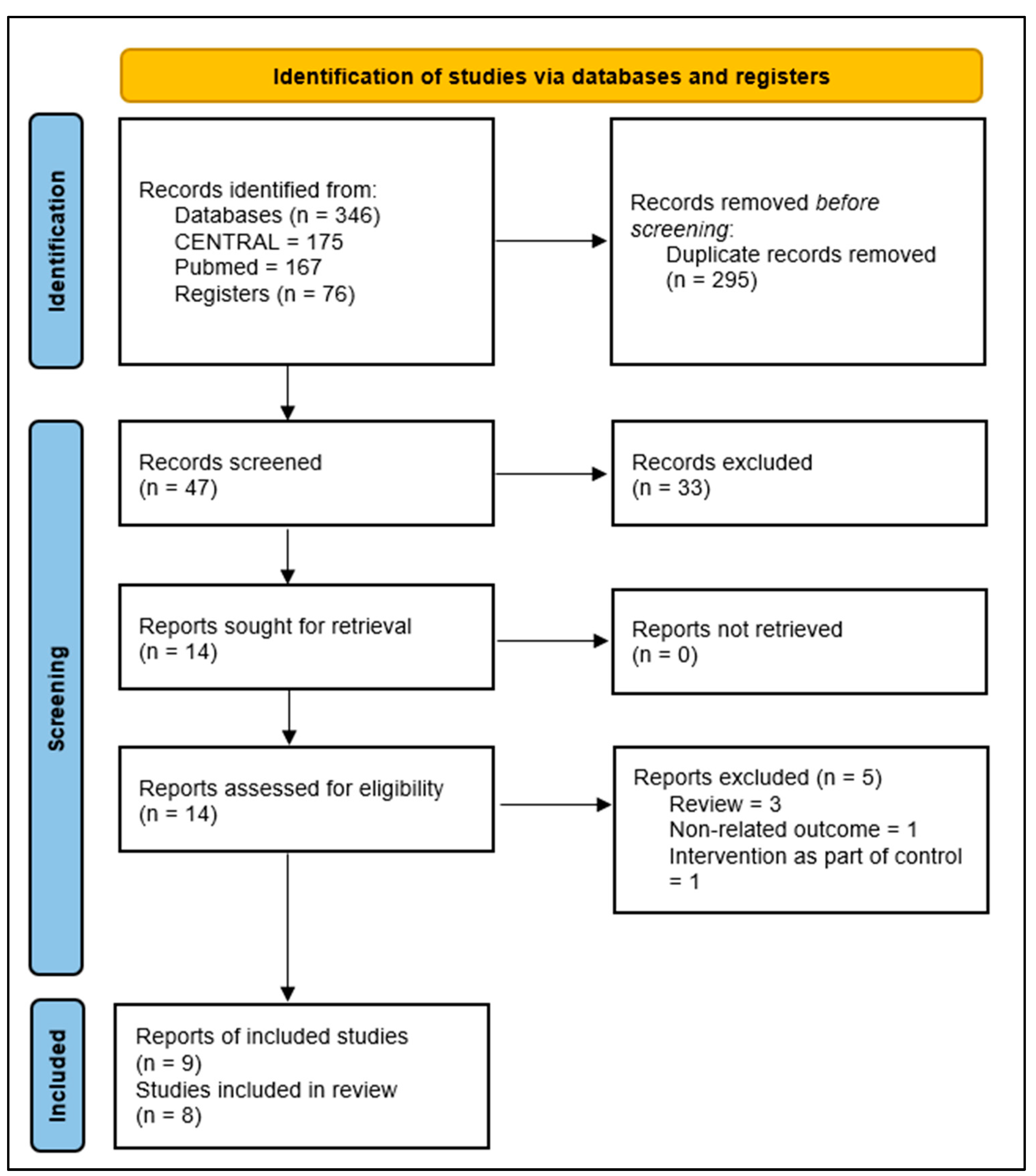
3.1. Results of the Search
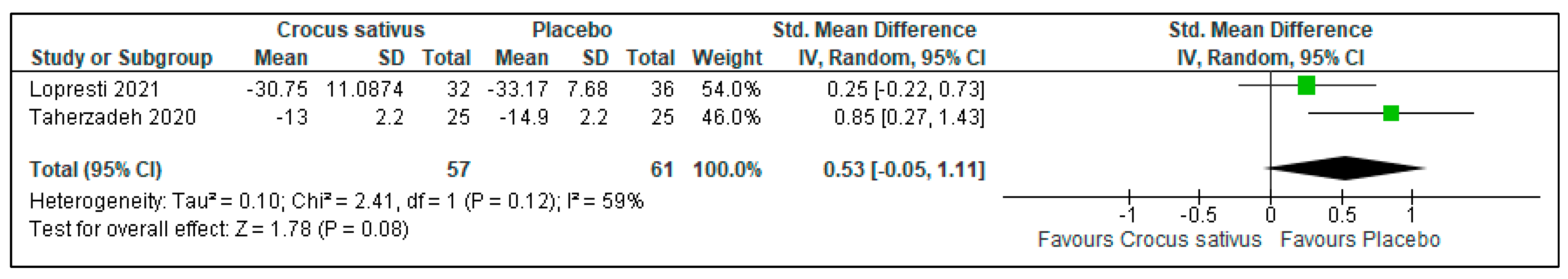
3.2. Insomnia Severity
3.3. Sleep Quality
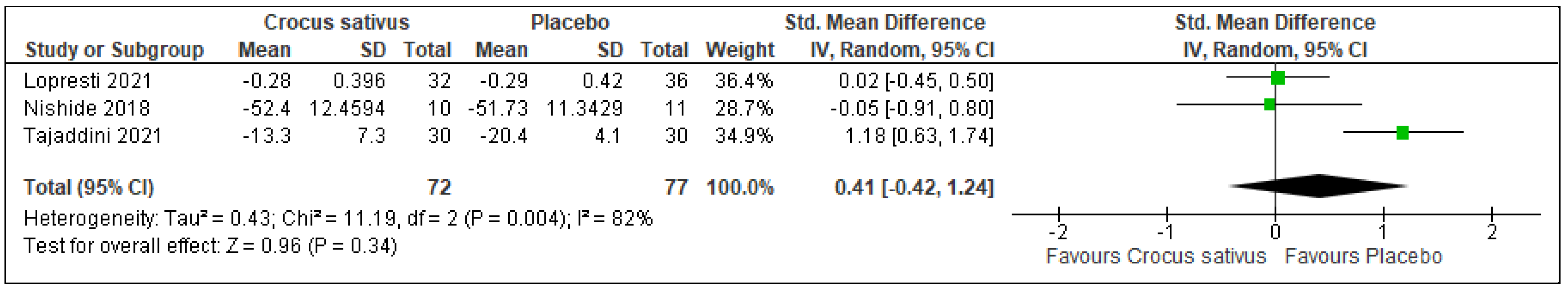
3.4. Sleep Latency
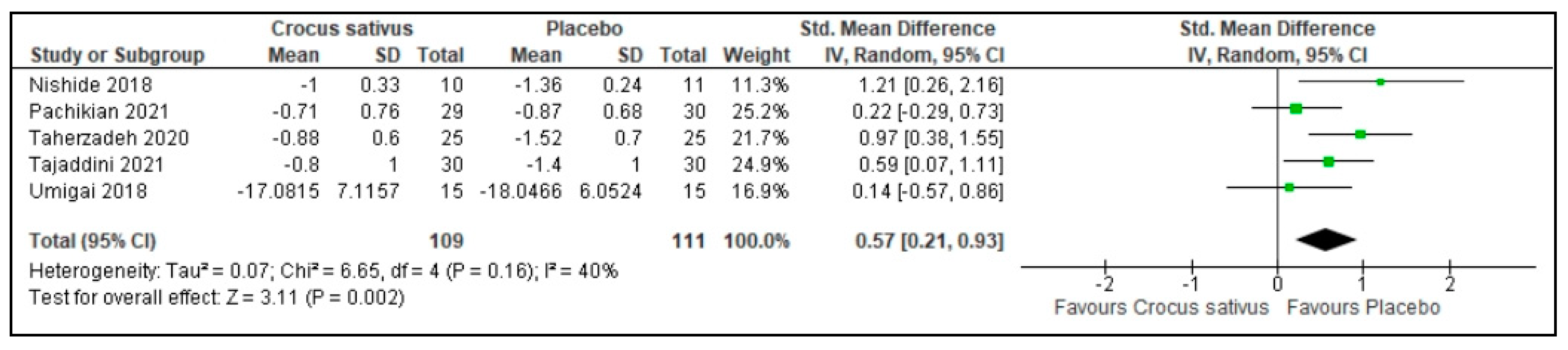
3.5. Sleep Duration
3.6. Sleep Efficiency
3.7. Sleep Disturbances
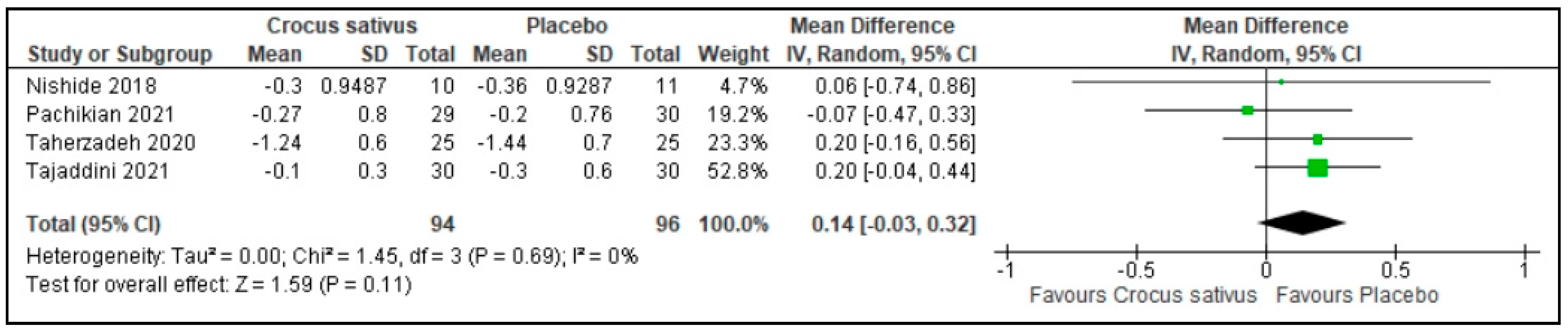
3.8. Use of Sleep Medications
3.9. Daytime Dysfunction
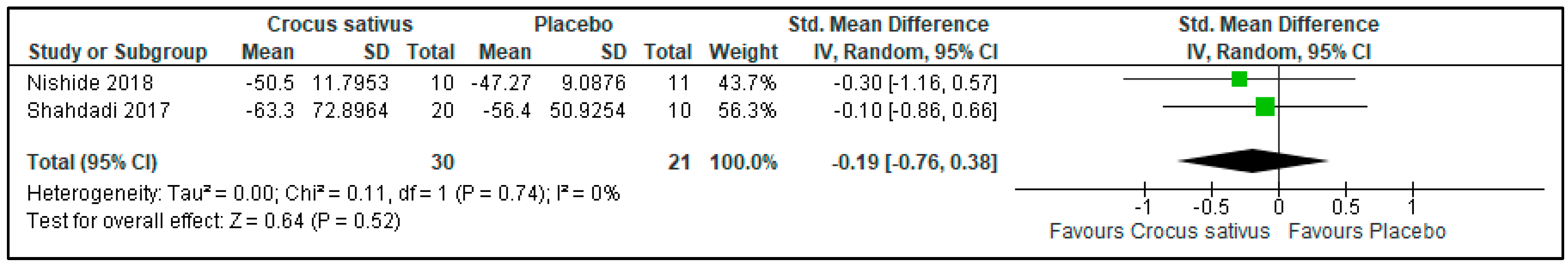
3.10. Anxiety Level
3.11. Depression Level
3.12. Restorative Sleep
3.13. Quality of Life
4. Discussion
4.1. Implications for Clinical Practice
4.2. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
| #1 | Saffron |
| #2 | Crocus sativus |
| #3 | Crocetin |
| #4 | MeSH descriptor: [Saffron] explode all trees |
| #5 | MeSH descriptor: [Crocus sativus] explode all trees |
| #6 | MeSH descriptor: [Crocetin] explode all trees |
| #7 | Insomnia |
| #8 | Difficulty in sleeping |
| #9 | Disorder of sleep initiation and maintenance |
| #10 | Sleep initiation and maintenance disorder |
| #11 | Sleep-wake disorder (SWD) |
| #12 | MeSH descriptor: [Insomnia] explode all trees |
| #13 | MeSH descriptor: [Difficulty in sleeping] explode all trees |
| #14 | MeSH descriptor: [Disorder of sleep initiation and maintenance] explode all trees |
| #15 | MeSH descriptor: [Sleep initiation and maintenance disorder] explode all trees |
| #16 | MeSH descriptor: [Sleep-wake disorder] explode all trees |
| #17 | MeSH descriptor: [SWD] explode all trees |
| #18 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 |
| #18 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| #19 | #18 AND #19 |
References
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Möller, H.J. Effectiveness and safety of benzodiazepines. J. Clin. Psychopharmacol. 1999, 19, 2s–11s. [Google Scholar] [CrossRef][Green Version]
- Del Barrio, V. Diagnostic and statistical manual of mental disorders. In Encyclopedia of Applied Psychology; Spielberger, C.D., Ed.; Elsevier: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Buysse, D.J. Insomnia. JAMA 2013, 309, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Terzano, M.G.; Parrino, L.; Cirignotta, F.; Ferini-Strambi, L.; Gigli, G.; Rudelli, G.; Sommacal, S. Studio Morfeo: Insomnia in primary care, a survey conducted on the Italian population. Sleep Med. 2004, 5, 67–75. [Google Scholar] [CrossRef]
- Bhaskar, S.; Hemavathy, D.; Prasad, S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J. Fam. Med. Prim. Care 2016, 5, 780–784. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Rocks, T.; Ruusunen, A.; Loughman, A.; Lopresti, A.; Marshall, S.; Berk, M.; Jacka, F.; Dean, O.M. Effect of saffron supplementation on symptoms of depression and anxiety: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 557–571. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z.; et al. The efficacy of saffron in the treatment of mild to moderate depression: A meta-analysis. Planta Med. 2019, 85, 24–31. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Emami, S.A.; Hosseinzadeh, H. Razi’s Al-Hawi and saffron (Crocus sativus): A review. Iran J. Basic Med. Sci. 2015, 18, 1153–1166. [Google Scholar]
- Nemati, H.; Boskabady, M.H.; Ahmadzadef Vostakolaei, H. Stimulatory effect of Crocus sativus (saffron) on beta2-adrenoceptors of guinea pig tracheal chains. Phytomedicine 2008, 15, 1038–1045. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.H.; Liu, T.Y.; Hong, Z.Y.; Urade, Y.; Huang, Z.L.; Qu, W.M. Safranal enhances non-rapid eye movement sleep in pentobarbital-treated mice. CNS Neurosci. Ther. 2012, 18, 623–630. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.B.; Mehri, S.; Hosseinzadeh, H. Toxicology effects of saffron and its constituents: A review. Iran J. Basic Med. Sci. 2017, 20, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Ke, L.; Li, J.; Zhao, H.; Lu, T.; Mentis, A.F.A.; Wang, Y.; Wang, Z.; Polissiou, M.G.; Tang, L.; et al. Saffron (Crocus sativus L.) and health outcomes: A meta-research review of meta-analyses and an evidence mapping study. Phytomedicine 2021, 91, 153699. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Updated July 2019; The Cochrane Collaboration: London, UK, 2019. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. What is “quality of evidence” and why is it important to clinicians? BMJ 2008, 336, 995–998. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Salobrar-García, E.; Pinazo-Durán, M.D.; Ramírez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408–1416. [Google Scholar] [CrossRef]
- Siddiqui, M.J.; Saleh, M.S.M.; Basharuddin, S.; Zamri, S.H.B.; Mohd Najib, M.H.B.; Che Ibrahim, M.Z.B.; Binti Mohd Noor, N.A.; Binti Mazha, H.N.; Mohd Hassan, N.; Khatib, A. Saffron (Crocus sativus L.): As an antidepressant. J. Pharm. Bioallied. Sci. 2018, 10, 173–180. [Google Scholar] [CrossRef]
- Zare, M.; Bazrafshan, A.; Malekpour Afshar, R.; Mazloomi, S.M. Saffron (adjunct) for people with schizophrenia who have antipsychotic-induced metabolic syndrome. Cochrane Database Syst. Rev. 2018, 2018, CD012950. [Google Scholar] [CrossRef]
- Ahmadpanah, M.; Ramezanshams, F.; Ghaleiha, A.; Akhondzadeh, S.; Sadeghi Bahmani, D.; Brand, S. Crocus sativus L. (saffron) versus sertraline on symptoms of depression among older people with major depressive disorders-a double-blind, randomized intervention study. Psychiatry Res. 2019, 282, 112613. [Google Scholar] [CrossRef]
- Milajerdi, A.; Jazayeri, S.; Shirzadi, E.; Hashemzadeh, N.; Azizgol, A.; Djazayery, A.; Esmaillzadeh, A.; Akhondzadeh, S. The effects of alcoholic extract of saffron (Crocus satious L.) on mild to moderate comorbid depression-anxiety, sleep quality, and life satisfaction in type 2 diabetes mellitus: A double-blind, randomized and placebo-controlled clinical trial. Complementary Ther. Med. 2018, 41, 196–202. [Google Scholar] [CrossRef]
- Kuratsune, H.; Umigai, N.; Takeno, R.; Kajimoto, Y.; Nakano, T. Effect of crocetin from Gardenia jasminoides Ellis on sleep: A pilot study. Phytomedicine 2010, 17, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. An investigation into an evening intake of a saffron extract (affron(R)) on sleep quality, cortisol, and melatonin concentrations in adults with poor sleep: A randomised, double-blind, placebo-controlled, multi-dose study. Sleep Med. 2021, 86, 7–18. [Google Scholar] [CrossRef]
- Nishide, A.; Fujita, T.; Nagaregawa, Y.; Shoyama, Y.; Ohnuki, K.; Shimizu, K.; Yamamoto, T.; Watanabe, T.; Ohnuki, K. Sleep enhancement by saffron extract in randomized control trial. Jpn. Pharmacol. Ther. 2018, 46, 1407–1415. [Google Scholar]
- Pachikian, B.D.; Copine, S.; Suchareau, M.; Deldicque, L. Effects of saffron extract on sleep quality: A randomized double-blind controlled clinical trial. Nutrients 2021, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Shahdadi, H.; Balouchi, A.; Dehghanmehr, S. Effect of saffron oral capsule on anxiety and quality of sleep of diabetic patients in a tertiary healthcare facility in southeastern Iran: A quasi-experimental study. Trop. J. Pharm. Res. 2017, 16, 2749–2753. [Google Scholar] [CrossRef]
- Taherzadeh, Z.; Khaluyan, H.; Iranshahy, M.; Rezaeitalab, F.; Eshaghi Ghalibaf, M.H.; Javadi, B. Evaluation of sedative effects of an intranasal dosage form containing saffron, lettuce seeds and sweet violet in primary chronic insomnia: A randomized, double-dummy, double-blind placebo controlled clinical trial. J. Ethnopharmacol. 2020, 262, 113116. [Google Scholar] [CrossRef]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Hadi, A.; Ostadrahimi, A. Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: A double-blind, placebo-controlled, randomised clinical trial. Int. J. Clin. Pract. 2021, 75, e14334. [Google Scholar] [CrossRef]
- Umigai, N.; Takeda, R.; Mori, A. Effect of crocetin on quality of sleep: A randomized, double-blind, placebo-controlled, crossover study. Complementary Ther. Med. 2018, 41, 47–51. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Kravitz, H.M.; Sowers, M.F.; Moul, D.E.; Buysse, D.J.; Hall, M. Psychometric evaluation of the Insomnia Symptom Questionnaire: A self-report measure to identify chronic insomnia. J. Clin. Sleep Med. 2009, 5, 41–51. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Raniti, M.B.; Waloszek, J.M.; Schwartz, O.; Allen, N.B.; Trinder, J. Factor structure and psychometric properties of the Pittsburgh Sleep Quality Index in community-based adolescents. Sleep 2018, 41, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, H.; Zhao, M.; Li, Z.; Cook, C.E.; Buysse, D.J.; Zhao, Y.; Yao, Y. Reliability, validity, and factor structure of Pittsburgh Sleep Quality Index in community-based centenarians. Front. Psychiatry 2020, 11, 573530. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Wegener, S.T. Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Rheum. 2003, 49, S184–S196. [Google Scholar] [CrossRef]
- Morfeld, M.; Petersen, C.; Krüger-Bödeker, A.; von Mackensen, S.; Bullinger, M. The assessment of mood at workplace—Psychometric analyses of the revised Profile of Mood States (POMS) questionnaire. Psychosoc. Med. 2007, 4, Doc06. [Google Scholar]
- Brown, T.A.; Chorpita, B.F.; Korotitsch, W.; Barlow, D.H. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 1997, 35, 79–89. [Google Scholar] [CrossRef]
- Ali, A.M.; Alkhamees, A.A.; Hori, H.; Kim, Y.; Kunugi, H. The Depression Anxiety Stress Scale 21: Development and validation of the Depression Anxiety Stress Scale 8-Item in psychiatric patients and the general public for easier mental health measurement in a post COVID-19 world. Int. J. Environ. Res. Public Health 2021, 18, 10142. [Google Scholar] [CrossRef]
- Drake, C.L.; Hays, R.D.; Morlock, R.; Wang, F.; Shikiar, R.; Frank, L.; Downey, R.; Roth, T. Development and evaluation of a measure to assess restorative sleep. J. Clin. Sleep Med. 2014, 10, 733E–741E. [Google Scholar] [CrossRef]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef] [PubMed]
- Farabi, S.S.; Quinn, L.; Carley, D.W. Validity of actigraphy in measurement of sleep in young adults with type 1 diabetes. J. Clin. Sleep Med. 2017, 13, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Mahmoudi, M. Review on the effects of saffron extract and its constituents on factors related to nervous system, cardiovascular and gastrointestinal diseases. J. Clin. Excell. 2014, 3, 108–127. [Google Scholar]
- Taavoni, S.; Ekbatani, N.N.; Haghani, H. Effect of Tribulus terrestris, ginger, saffron, and Cinnamomum on menopausal symptoms: A randomised, placebo-controlled clinical trial. Prz. Menopauzalny 2017, 16, 19–22. [Google Scholar] [CrossRef]
- Taavoni, S.; Ekbatani, N.; Kashaniyan, M.; Haghani, H. Effect of valerian on sleep quality in postmenopausal women: A randomized placebo-controlled clinical trial. Menopause 2011, 18, 951–955. [Google Scholar] [CrossRef]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Van de Water, A.T.; Holmes, A.; Hurley, D.A. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—A systematic review. J. Sleep Res. 2011, 20, 183–200. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2018, 14, 1231–1237. [Google Scholar] [CrossRef]
- Topalidis, P.; Florea, C.; Eigl, E.S.; Kurapov, A.; Leon, C.A.B.; Schabus, M. Evaluation of a low-cost commercial actigraph and its potential use in detecting cultural variations in physical activity and sleep. Sensors 2021, 21, 3774. [Google Scholar] [CrossRef]




| Author (Year) | Sample Size | Patient Characteristics | CS Form/Route/Daily Dose | Control | Follow-Up |
|---|---|---|---|---|---|
| Kuratsune et al. (2010) [24] | I: 17 C: 17 | Healthy adults with insomnia (PSQIG ≧ 6) | I: Extract in capsule/Oral/7.5 mg | Placebo capsule (dextrin) | 6 weeks |
| Lopresti et al. (2021) [25] | I₁: 40 I₂: 40 C: 40 | Healthy adults with insomnia | I: Extract in capsule/Oral/14 mg and 28 mg | Placebo capsule (cellulose and calcium) | 4 weeks |
| I: 33 C: 30 | |||||
| Nishide et al. (2018) [26] | I: 10 C: 11 | Healthy adults | I: Extract in capsule/Oral/0.6 mg | Placebo capsule (cellulose, starch, calcium) | 4 weeks |
| Pachikian et al. (2021) [27] | I: 34 C: 32 | Healthy adults with insomnia (ISI 7-21) | I: Extract in capsule/Oral/15.5 mg | Placebo capsule (dextrin) | 6 weeks |
| Shahdadi et al. (2017) [28] | I: 25 C: 25 | Diabetes Mellitus with insomnia | I: Extract in capsule/Oral/300 mg | Placebo capsule | 1 week |
| Taherzadeh et al. (2020) [29] | I: 25 C: 25 | Healthy adults with insomnia | I: Extract in liquid/Intranasal/0.02 mg/mL | Placebo (sesame oil) | 8 weeks |
| Tajaddini et al. (2021) [30] | I: 35 C: 35 | Diabetes Mellitus with insomnia | I: Extract in capsule/Oral/100 mg | Placebo capsule (dextrin) | 8 weeks |
| Umigai et al. (2018) [31] | I: 15 C: 15 | Healthy adults with insomnia | I: Extract in capsule/Oral/30 mg | Placebo capsule (dextrin) | 14 days |
| Outcomes | No. of Participants (Studies) | No. of Participants | Anticipated Absolute Effects | Certainty of Evidence (GRADE) | Comments | |||
|---|---|---|---|---|---|---|---|---|
| Placebo | CS | MD | 95%CI | p-Value | ||||
| Insomnia severity | 118 (2 RCT) | 61 | 57 | 0.53 higher | −0.05 to 1.11 | 0.08 | ⨁⨁⨁◯ Moderate | Risk of bias: not serious Inconsistency: not serious Indirectness: not serious Imprecision: serious |
| Sleep quality | 308 (6 RCT) | 157 | 151 | 0.89 higher | 0.10 to 1.68 | 0.03 | ⨁◯◯◯ Very low | Risk of bias: not serious Inconsistency: serious Indirectness: serious Imprecision: serious |
| Sleep latency | 190 (4 RCT) | 96 | 94 | 0.10 higher | −0.19 to 0.38 | 0.51 | ⨁⨁◯◯ Low | Risk of bias: not serious Inconsistency: not serious Indirectness: serious Imprecision: serious |
| Sleep efficiency | 233 (5 RCT) | 117 | 116 | 0.08 higher | −0.18 to 0.34 | 0.54 | ⨁⨁◯◯ Low | Risk of bias: not serious Inconsistency: not serious Indirectness: serious Imprecision: serious |
| Sleep duration | 220 (5 RCT) | 111 | 109 | 0.57 higher | 0.21 to 0.93 | 0.002 | ⨁⨁⨁◯ Moderate | Risk of bias: not serious Inconsistency: not serious Indirectness: not serious Imprecision: serious |
| Use of sleep medications | 190 (4 RCT) | 96 | 94 | 0.14 higher | −0.03 to 0.32 | 0.11 | ⨁⨁⨁◯ Moderate | Risk of bias: not serious Inconsistency: not serious Indirectness: not serious Imprecision: serious |
| Anxiety Level | 51 (2 RCT) | 21 | 30 | 0.19 lower | −0.76 to 0.38 | 0.52 | ⨁⨁◯◯ Low | Risk of bias: not serious Inconsistency: not serious Indirectness: serious Imprecision: serious |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munirah, M.P.; Norhayati, M.N.; Noraini, M. Crocus Sativus for Insomnia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11658. https://doi.org/10.3390/ijerph191811658
Munirah MP, Norhayati MN, Noraini M. Crocus Sativus for Insomnia: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(18):11658. https://doi.org/10.3390/ijerph191811658
Chicago/Turabian StyleMunirah, Mohd Puad, Mohd Noor Norhayati, and Mohamad Noraini. 2022. "Crocus Sativus for Insomnia: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 18: 11658. https://doi.org/10.3390/ijerph191811658
APA StyleMunirah, M. P., Norhayati, M. N., & Noraini, M. (2022). Crocus Sativus for Insomnia: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(18), 11658. https://doi.org/10.3390/ijerph191811658






