Abstract
Background: The link between nocturia and aging male symptoms (AMS) has not been scientifically established. This study aimed to measure the degree of severity of AMS that impacts health-related quality of life (HRQoL) in adult males living with nocturia and to determine the predictive values of nocturnal factors on AMS. Methods: This is an extended analysis of new data collected by using the Hong Kong Traditional AMS (HK-AMS) scale and the Cantonese version of the Pittsburgh Sleep Quality Index (PSQI) in a recently published cross-sectional population-based survey. Results: Of the 781 respondents that completed the set of questionnaires, 68% and 61% of men living with nocturia reported clinically significant (at moderate-to-severe levels) somato-vegetative and sexual AMS; the prevalence and severity were increased with advancing nighttime voiding frequency. Age, the Global PSQI score, certain metabolic diseases, the nocturia-specific QoL (NQoL) score and bedtime voiding frequency were found to be significant predictive factors for composite somato-vegetative and sexual AMS. Conclusions: The current findings suggested the inclusion of nocturia when measuring male-specific HRQoL related to aging.
1. Introduction
Nocturia causes many public health concerns because of its impact on quality of life (QoL), in addition to its associations with numerous illnesses and conditions [1]. Nocturia is often co-morbid with sleep disturbance, significantly impacting life activities and functional levels [2]. Moreover, accumulating evidence links nocturia with certain health risks. Nocturia is a known risk factor for falls, particularly in older people [3]. However, recent research conducted in Korea extended the risk associations of nocturia with slipping and falling to all ages of adult males [4]. In this relation, the Nocturia Quality of life module of the International Consultation on Incontinence Modular Questionnaire (ICIQ-NQoL) has shown to be a useful tool for predicting the risk of falling [5]. Owing to its implications on sleep, nocturia was identified as a stronger predictive symptom than snoring for obstructive sleep apnea. Bedtime voiding frequency was even found to be reflective in the severity of broad sleep-disordered breathing [6,7]. Sleep disturbance caused by nocturia negatively impacts one’s overall well-being, general health, vitality, and essential biological rhythms [8,9].
Our recent research reported that 63% and 80% of Hong Kong adult males, respectively, were living with nocturia [10] and aging male symptoms (AMS) [11] and that both were correlated with age; however, both also shared strong associations with several metabolic and urological conditions which harmed the health-related quality of life (HRQoL). All these conditions were linked with a decline in testosterone levels in men [12]. Testosterone levels decline with age, resulting in the deterioration of men’s health [13,14]. Accumulating evidence supports the benefits of testosterone replacement therapy for treating symptoms of late-onset hypogonadism and improving lower urinary tract symptoms (LUTS) [15] and numerous metabolic conditions, such as insulin resistance, adiposity, and dyslipidemia [16]. The total serum testosterone level was found to be negatively correlated with prostate volume, suggesting a relationship with benign prostate hyperplasia (BPH), insulin level, and an array of obesity-related factors that further linked testosterone to metabolic syndrome [16,17,18]. On the other hand, testosterone also interplayed between nocturia and sleep. The production of testosterone was interfered with when sleep was fragmented to a level failing to show rapid eye movement (REM), as caused by nocturia [19]. Interestingly, recent research indicated the dual roles of testosterone on sleep quality that could be affected by both deprived and excessive supplies in the circulation, whereas obesity was shown to exhibit mediating roles in the inter-correlation between serum testosterone and sleep efficiency [20]. Well-designed sleep restriction experiments clearly show the effects of sleep deprivation on testosterone reduction [21], which may also lead to other health impacts associated with nocturia and AMS. The ICIQ-NQoL questionnaire consisted of two subscales, with one particular measure of the sleep/energy factor [22]. There were also two items in the ‘Aging male’ symptoms (AMS) scale specifically asking about the sleep problem and its consequence [23]. Within the context of men’s HRQoL, considering testosterone may play a central role in nocturia to form part of the AMS. The objective of this study was to determine the predictive values of nocturia and its related factors on AMS.
2. Materials and Methods
2.1. Study Design
This is an extended study of the community-based survey on nocturia that our research team conducted recently [10]; it involves the analysis and interpretation of unpublished data set on AMS and sleep quality that was collected among adult males who were reported as suffering from nocturia. In accordance with the street-intercept and random walk design, respondents with nocturia were also invited to complete the AMS scale and the Pittsburgh Sleep Quality Index (PSQI) on-site after administering the NQoL questionnaire and collecting the demographic information.
2.2. The Instruments and Measurements
The Cantonese version of ICIQ-NQoL questionnaire was used to measure frequency of nocturia and NQoL overall and factor (factor 1: sleep/energy; factor 2: bother/concern) scores [10]. Following the original ICIQ-NQoL construct, frequency of nocturia was counted as 0, 1, 2, 3, or ≥4 episodes of urination per bedtime, which is considered a categorical variable during the data analysis.
The Hong Kong Traditional AMS (HK-AMS) scale [11] and the Cantonese version of the PSQI (CPSQI) [24] were adopted in this study.
The HK-AMS scale consisted of 17 items using the 5-point Likert scale (from 1 to 5) of ‘severity’ to measure the personal perception of respondents on male symptoms or complaints associated with aging. The scores of relevant items were summed to generate the composite score (range of 17–85) and 3 domain scores in 3 dimensions: somato-vegetative (range of 7–35), psychological (range of 5–25), and sexual (range of 5–25). Higher score represented a higher severity of the aging symptoms, whereas the composite score can be further categorized into 4 severity levels as ‘no significant symptoms’ (<26), ‘mild’ (27–36), ‘moderate’ (37–49), and ‘severe’ (>50). Furthermore, the domain scores were categorized into different severity levels according to Heinemann et al. [23]. The psychometric prosperities of similar male population were reported by Yuen et al. [11].
The 19-item CPSQI is a well-validated questionnaire that has been used for measuring the subjective sleep quality of different Chinese populations [24]. The items were categorized into 7 component scores (each ranging 0–3), which are summed to produce a 0–21 global score range, with any scores >5 indicating poor sleeper.
2.3. Data Processing and Statistical Analysis
Data collected from the survey were entered and analyzed using SPSS version 25.0 (IBM, Armonk, NY, USA) and Prism version 9.0 (GraphPad, San Diego, CA, USA). Descriptive statistics were used for reporting the categorical variables (frequency and percentage) and continuous variables (mean and standard deviation (SD)) of the demographics, bedtime voiding frequency, NQoL scores, AMS, and sleep conditions. Significant differences among nominal and continuous variables between groups were evaluated by using Chi-squared (χ2), Student’s t-test, and one-way ANOVA accordingly. Pearson’s correlation analysis was used to assess the linear correlations among continuous variables. Furthermore, stepwise multiple regression analysis was performed to determine the multicollinearity among different variables to identify the significant predictive factors for AMS in the studied population.
3. Results
Those among the studied population that reported prevalence rates of 1, 2, 3, and ≥4 voiding episodes per night were 50.4%, 32.5%, 12.3%, and 4.7%, respectively [10]. Among the 1239 adult men who participated in the survey, all had completed the AMS scale, while the CPSQI was completed by 65.5% and 75% of those with and without nocturia, respectively. Table 1 summarizes and compares the demographics, health conditions, AMS, and sleep quality between non-nocturia and nocturia respondents. The results demonstrated the strong association between nocturia and a spectrum of urological disorders; benign prostatic hyperplasia (BPH) was the most frequently reported by men with nocturia (Table 1). When compared with the non-nocturia respondents, nocturia was also shown to be statistically significantly (p < 0.001) associated with scores and severity levels of all AMS and sleep quality measures (Table 1; Supplementary Tables S1 and S2).

Table 1.
A summary of demographic, health-related characteristics, AMS, and sleep quality measures of the studied adult male population living with and without nocturia.
3.1. High Prevalence of Somato-Vegetative and Sexual AMS among Adult Males Living with Nocturia
The studied male population was not only living with nocturia but also had a mild level of total AMS, with a mean composite score of 31.6 ± 8.4; moderate level of somato-vegetative symptoms, with a mean score of 14.9 ± 4.3; and moderate-to-severe level of sexual symptoms, with a mean score of 9.2 ± 3.6 (Table 1). In addition, almost half of the nocturia population was rated as poor sleepers, with a mean PSQI score of 6.7 ± 3.3 (Table 1).
In comparison, AMS was found to be more commonly (p < 0.001) reported by the respondents with nocturia than those without, particularly with 25% at the moderate level (versus 7% of non-nocturia) and 3% at the severe level (versus 0.4% of non-nocturia) (Supplementary Table S1). The prevalence rates of moderate-to-severe levels of somato-vegetative, sexual, and psychological symptoms were measured as 68%, 61%, and 29%, respectively. As shown in Figure 1, the composite AMS, somato-vegetative, sexual, and Global PSQI scores shared similar significant upward trends (p < 0.001) with increasing bedtime voiding frequency, while all of them except the psychological domain were significantly (<0.001) varied among the episodes of bedtime urination. The linear increase in composite AMS scores plateaued at three voiding episodes per night (Figure 1a). Over 80% of men having 3 voids per night were rated with mild-to-moderate levels of AMS (about half and half in ratio), while the highest percentage of severe AMS was dominant among those having ≥4 voids (Table 2). Although a clear increasing trend was observed with the advancing bedtime voiding frequency for the somato-vegetative symptom scores (Figure 1b), the percentage of the clinically significant (i.e., moderate-to-severe) symptom level was increased from 60% to 76–80% discretely between those with a 1 and ≥2 voiding frequency (Table 2). Psychological symptoms were the least affected by the bedtime voiding frequency. A slight, gradually increasing trend was observed for both the domain score (Figure 1c) and severity classification (Table 2). The prevalence of moderate-to-severe level sexual AMS peaked at 84.3% (51% were at severe level) among those with a voiding frequency of 3 (Table 2). The sexual symptom score followed the trend observed in the Global PSQI score that was linearly increased at voiding frequency ≤3 and significantly dropped (p < 0.001) at frequency ≥4 (Figure 1d,e). The same trend was followed by the prevalence of poor sleepers who had a Global PSQI score >5 (Figure 1f). Particularly, almost 10% of men with nocturia used medications to help their sleep, which was significantly (p < 0.001) more common than those without nocturia at 2.6% (Table 1). Furthermore, the severity levels of the 7 PSQI components in accordance with the bedtime voiding frequency are provided in Supplementary Table S3.
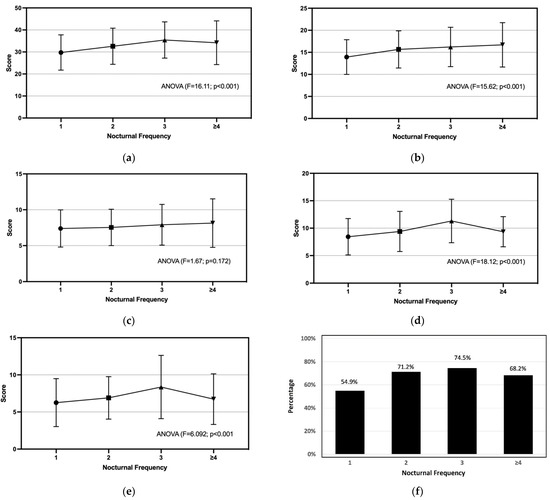
Figure 1.
Severity of AMS and quality of sleep rated by the respondents with nocturia are presented as the scores of: (a) composite AMS, (b) somato-vegetative AMS, (c) psychological AMS, (d) sexual AMS, (e) Global PSQI, and (f) prevalence of poor sleepers against the increasing bedtime voiding frequency.

Table 2.
Prevalence of different severity levels of composite AMS and its domains associated with bedtime voiding frequency.
3.2. NQoL Score and Voiding Frequency Are Predictors for Composite, Somato-Vegetative, and Sexual AMS in Addition to Age and PSQI Score
A correlational analysis indicated that the composite AMS and domain scores were moderately inter-correlated with the bedtime voiding frequency (r = 0.168–0.390; p < 0.001), the NQoL overall score (r = 0.228–0.424; p < 0.001) as well as its factor scores (r = 0.190–0.411; p < 0.001), and the Global PSQI score (r = 0.248–0.408; p < 0.001) (Table 3). Stepwise multiple regression was performed separately with the composite AMS and three domains as dependent variables. The independent variables were evaluated in the order of demographics and illnesses as ‘block 1’, bedtime voiding frequency and NQoL score as ‘block 2’, and Global PSQI score as ‘block 3’. Multicollinearity was observed between the overall NQoL score and its two factor scores (with r = 0.928–0.953; p < 0.001); therefore, only the NQoL score was evaluated by the stepwise multiple regression.

Table 3.
Correlation matrix for the bedtime voiding frequency, PSQI, ICIQ-NqoL, and AMS variables amongst respondents with nocturia.
As summarized in Table 4, based on the standardized coefficient (β) values, from strongest to weakness, the significant independent predictive factors identified for: (1) composite AMS were the NqoL score, Global PSQI score, bedtime voiding frequency, HTN, smoking habit, DM, and age; (2) somato-vegetative AMS were the Global PSQI score, NQoL score, bedtime voiding frequency, comorbidity of DM and HTN, smoking habit, HTN, age, and BPH; (3) psychological AMS were the NQoL score, Global PSQI score, DM, and age; and (4) sexual AMS were the bedtime voiding frequency, Global PSQI score, HTN, NQoL score, and age. Two demographic factors (age and smoking habits) and two metabolic illnesses (diabetes mellitus and high blood pressure) were identified as significant predictive factors for various AMS domains when examined in block 1. However, in the final regression models, independent variables entered in block 2 and block 3 were found to have stronger predictive values than those tested in block 1, where the predictive values of demographics and illnesses became weaker and less significant at p > 0.05 (Table 4). HTN was only predictive for the somato-vegetative and became the least significant predictor of the presence of nocturia and poor sleep quality (Table 4). The current models identified the NQoL score and Global PSQI score as the two strongest predictors for the composite, somato-vegetative, and psychological AMS (Table 4). In relation to the NQoL score, bedtime voiding frequency was also shown to be a significant predictor important for composite, somato-vegetative, and sexual AMS (Table 4).

Table 4.
Predictors of composite AMS and its domains identified by stepwise multiple regression.
4. Discussion
This study found that many of the Hong Kong adult males living with nocturia were also co-morbid with moderate-to-severe levels of somato-vegetative and sexual AMS, which negatively impacted their HRQoL. Both NQoL and AMS questionnaires measured sleep quality, which was believed to be interplayed between nocturia and AMS, among other factors such as age and certain age-related illnesses. The NQoL factors, AMS severity, and sleep quality were shown to be strongly inter-correlated with each other. The current study identified, for the first time, that nocturnal factors (including NQoL scores, voiding frequency, and sleep quality) are strong predictors of AMS. Other well-known predictive risk factors for AMS were demonstrated to become weaker and less significant when those nocturnal factors were introduced into the regression models.
Clinically significant (moderate-to-severe) levels of somato-vegetative (68%) and sexual (61%) AMS were more prevalent in the nocturia population, as compared to more than 80% of the general adult male population living in the city with AMS at little-to-mild levels [11]. Nocturia commonly coexisted with age-related illnesses; in particular, diabetes and hypertension were both characterized by increased urinary frequency and sleep fragmentation [25,26]. Predictably, aging and age-related illnesses linked nocturia to AMS, which are both known to be associated with a decline in androgen [12,13,14]. The consequences of testosterone deficiency in causing nocturia and sleep disturbance were extensively reviewed by Shigehara et al. [27]. Nocturia was shown to be the only significant item of the International Prostate Symptom Score (IPSS) questionnaire that negatively correlated with the serum total testosterone in middle-aged men [28]. Pathologically, the increase in night voiding frequency in aging men could also be due to the interference of diuresis and the anatomical alteration of the lower urinary tract resulting from reduced androgen production, which in turn increases urine production during the night [29]. In this relation, the ‘aging males’ symptoms (AMS) scale was established to measure the symptoms of aging men and their impact on health-related Quality of Life (HRQoL), based on the assumption that men would develop specific complaints during the aging process due to androgen deficiency [23]. Around 10% of men with nocturia reported using medications to help their sleep (Table 1). Considering its influence on circadian rhythms, which is a known factor for lower urinary symptoms (LUS), a recent randomized, controlled trial suggested the therapeutic effects of melatonin on nocturia [30]. This effectiveness could be due to the increase in bladder capacity and decrease in urine volume, as demonstrated in rats treated with melatonin [31].
The high prevalence of nocturia and its correlation with age [10] explained, at least partly, the high prevalence of AMS; however, the strong inter-correlational relationships identified between the variables measured by the three questionnaires in this study further supported the cause-and-effect relationship that nocturia disturbs sleep to cause long-term consequences which harm physical and psychological health, hence affecting the overall HRQoL [32,33,34]. Particularly, results herein indicated that the AMS sexual and PSQI scores were both lower in people with nocturia ≥4, which could be explained by an older subpopulation (average age of 73 [10]) who sleep for shorter durations at night and are commonly less sexually active. The strong correlations of PSQI with both ICIQ-NQoL and AMS scales, as well as its independent predictive value on AMS, suggested sleep quality was important for the HRQoL of men. They also supported the assumption that the overall AMS-associated HRQoL could be modulated by sleep quality affected by nocturia. The ICIQ-NQoL alone measures the nocturia-specific QoL with a focus on two domains, namely, bothersomeness and sleep disturbance [22]. However, the AMS further assesses the general somato-vegetative, psychological, and sexual well-being of men [23]. The AMS scale consists of two items related to sleep disturbance, but none of the items asked about nocturia or related conditions. In the current study, the strong predictive values of NQoL, bedtime voiding frequency, and sleep quality on AMS suggested the combined use of the ICIQ-NQoL questionnaire and AMS scale for measuring the long-term impact on the HRQoL of adult males. Otherwise, items could be added to the AMS scale to cover the bedtime voiding frequency and its bothersome level. The ICIQ-NQoL has been proposed to be a useful tool for predicting the risk of falls [5]; therefore, the current findings support that the same can be used for AMS.
The current study reports, for the first time, the association between nocturia and AMS, which also identifies bedtime voiding frequency as a significant predictor for AMS. However, biological markers should also be evaluated to establish the interrelationship between nocturia and AMS. For instance, nocturia should be precisely measured by using the frequency volume chart (FVC) over a 2–3-day period [35]. Moreover, serum testosterone [36] and melatonin [37] levels should also be included in the predictive model to represent the modulating effects of androgen deficiency and sleep disruption, respectively. More in-depth investigations using cohort studies and interventional designs for further understanding of the underlying pathology on how nocturia and AMS are related to aging are warranted.
5. Conclusions
Nocturia was demonstrated to be associated with clinically significant somato-vegetative and sexual AMS, which might be explained by the androgen deficiency that is implicated with aging and sleep quality. In particular, the NQoL score and nighttime voiding frequency impacted the HRQoL of adult males, which were shown to be strong predictive factors for AMS. The current findings suggested that the occurrence of nocturia can be a reference indicator for poor male-specific HRQoL related to aging, especially when testosterone screening is unavailable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph191811632/s1, Table S1: The comparison between non-nocturia and nocturia on the severity levels of composite AMS and its subscales; Table S2: The comparison between non-nocturia and nocturia on the severity levels of 7 PSQI components; Table S3: Severity levels of the 7 PSQI components associated with bedtime voiding frequency.
Author Contributions
Conceptualization, resources, and data curation, J.W.-M.Y. and C.-F.N.; methodology and software, J.W.-M.Y.; validation, J.W.-M.Y., I.Y.-P.W. and C.-F.N.; formal analysis and supervision, J.W.-M.Y. and I.Y.-P.W.; investigation, J.W.-M.Y., I.Y.-P.W. and C.-F.N.; writing—original draft preparation, J.W.-M.Y.; writing—review and editing, J.W.-M.Y., I.Y.-P.W., P.K.-F.C., J.Y.-C.T., C.-K.C., C.-H.Y. and C.-F.N.; project administration and funding acquisition, J.W.-M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Departmental Research Fund of the Hong Kong Polytechnic University, grant number P0008265.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Human Ethics Sub-committee of the Hong Kong Polytechnic University (Reference number: HSEARS20150805001-01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The raw data presented in this study are available on request from the corresponding author. The data are not publicly available due to the research data governance policy of the institution that gave ethical approval to this study.
Acknowledgments
The authors are grateful to Sara Fung and Chris Lam for their translation service contribution. The authors would also like to thank Jacky Ka-kit Tam, Hilary Hiu-yin Chung, Shermaine Cheuk-ying Ng, Toby Chung-wai Chau, Ka-wai Chan, Yuen-yi Cheung, Chui-yu Lau, Ying-kwan Lau, Wing-yee Lee, Iris Hing-yi Ma, Sau-wan Tang, Pui-yee Tam, Po-yan Lee, Cheuk-yan Leung, Chui-yan Lau, Cho-chak Lee, and Nathania Shing-yan Suen for their efforts in data collection and data entry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Kerrebroeck, P.; Abrams, P.; Chaikin, D.; Donovan, J.; Fonda, D.; Jackson, S.; Jennum, P.; Johnson, T.; Lose, G.; Mattiasson, A.; et al. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology in nocturia: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 179–183. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Bliwise, D.L.; Nørgaard, J.P. The effect of nocturia on sleep. Sleep Med. Rev. 2011, 15, 91–97. [Google Scholar] [CrossRef]
- Stewart, R.B.; Moore, M.T.; May, F.E.; Marks, R.G.; Hale, W.E. Nocturia: A risk factor for falls in the elderly. J. Am. Geriatr. Soc. 1992, 40, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Bang, W.; Kim, M.S.; Park, B.; Kim, J.H.; Choi, H.G. Nocturia Is Associated with Slipping and Falling. PLoS ONE 2017, 12, e0169690. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, T.; Fuse, M.; Yamaguchi, C.; Uchiyama, T.; Kamai, T.; Kurokawa, S.; Morita, T. Nocturia Quality-of-Life questionnaire is a useful tool to predict nocturia and a risk of falling in Japanese outpatients: A cross-sectional survey. Int. J. Urol. 2014, 21, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Krakow, B.; Haynes, P.; Ulibarri, V. Nocturia and snoring: Predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010, 14, 337–343. [Google Scholar] [CrossRef]
- Kaynak, H.; Kaynak, D.; Oztura, I. Does frequency of nocturnal urination reflect the severity of sleep-disordered breathing? J. Sleep Res. 2004, 13, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. To sleep, perchance to live. Sleeping is vital for health, cognitive function, memory and long life. EMBO Rep. 2008, 9, 1070–1073. [Google Scholar] [CrossRef][Green Version]
- Kobelt, G.; Borgström, F.; Mattiasson, A. Productivity, vitality and utility in a group of healthy professionally active individuals with nocturia. BJU Int. 2003, 91, 190–195. [Google Scholar] [CrossRef]
- Yuen, J.W.; Wong, I.Y.; Chiu, P.K.; Teoh, J.Y.; Chan, C.K.; Yee, C.H.; Ng, C.F. A Comprehensive Community-Based Prevalence Study on Nocturia in Hong Kong Male Adults. Int. J. Environ. Res. Public Health 2021, 18, 9112. [Google Scholar] [CrossRef]
- Yuen, J.W.; Ng, C.F.; Chiu, P.K.; Teoh, J.Y.; Yee, C.H. “Aging males” symptoms and general health of adult males: A cross-sectional study. Aging Male 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Traish, A.M. Benefits and Health Implications of Testosterone Therapy in Men With Testosterone Deficiency. Sex. Med. Rev. 2018, 6, 86–105. [Google Scholar] [CrossRef]
- Booth, A.; Johnson, D.R.; Granger, D.A. Testosterone and men’s health. J. Behav. Med. 1999, 22, 1–19. [Google Scholar] [CrossRef]
- Tsujimura, A. The Relationship between Testosterone Deficiency and Men’s Health. World J. Men’s Health 2013, 31, 126–135. [Google Scholar] [CrossRef]
- Rastrelli, G.; Vignozzi, L.; Corona, G.; Maggi, M. Testosterone and Benign Prostatic Hyperplasia. Sex. Med. Rev. 2019, 7, 259–271. [Google Scholar] [CrossRef]
- Xia, B.W.; Zhao, S.C.; Chen, Z.P.; Chen, C.; Liu, T.S.; Yang, F.; Yan, Y. Relationship between serum total testosterone and prostate volume in aging men. Sci. Rep. 2021, 11, 14122. [Google Scholar] [CrossRef]
- Muraleedharan, V.; Jones, T.H. Testosterone and the metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2010, 1, 207–223. [Google Scholar] [CrossRef]
- Gianatti, E.J.; Grossmann, M. Testosterone deficiency in men with Type 2 diabetes: Pathophysiology and treatment. Diabet. Med. 2020, 37, 174–186. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Zabari, Z.; Shen-Orr, Z.; Herer, P.; Lavie, P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J. Clin. Endocrinol. Metab. 2001, 86, 1134–1139. [Google Scholar] [CrossRef]
- Wittert, G. The relationship between sleep disorders and testosterone in men. Asian J. Androl. 2014, 16, 262–265. [Google Scholar] [CrossRef]
- Leproult, R.; Van Cauter, E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA 2011, 305, 2173–2174. [Google Scholar] [CrossRef]
- Abraham, L.; Hareendran, A.; Mills, I.W.; Martin, M.L.; Abrams, P.; Drake, M.J.; MacDonagh, R.P.; Noble, J.G. Development and validation of a quality-of-life measure for men with nocturia. Urology 2004, 63, 481–486. [Google Scholar] [CrossRef]
- Heinemann, L.A.; Saad, F.; Zimmermann, T.; Novak, A.; Myon, E.; Badia, X.; Potthoff, P.; T’Sjoen, G.; Pöllänen, P.; Goncharow, N.P.; et al. The Aging Males’ Symptoms (AMS) scale: Update and compilation of international versions. Health Qual. Life Outcomes 2003, 1, 15. [Google Scholar] [CrossRef]
- Chong, A.M.L.; Cheung, C.k. Factor structure of a Cantonese-version Pittsburgh Sleep Quality Index. Sleep Biol. Rhythms 2012, 10, 118–125. [Google Scholar] [CrossRef]
- Bliwise, D.L.; Wagg, A.; Sand, P.K. Nocturia: A Highly Prevalent Disorder With Multifaceted Consequences. Urology 2019, 133S, 3–13. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Fitzgerald, M.; Goodwin, J.L.; Unruh, M.; Guerra, S.; Quan, S.F. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS ONE 2012, 7, e30969. [Google Scholar] [CrossRef]
- Shigehara, K.; Izumi, K.; Mizokami, A.; Namiki, M. Testosterone Deficiency and Nocturia: A Review. World J. Men’s Health 2017, 35, 14–21. [Google Scholar] [CrossRef]
- Shigehara, K.; Konaka, H.; Koh, E.; Izumi, K.; Kitagawa, Y.; Mizokami, A.; Nakashima, T.; Shimamura, M.; Iwamoto, T.; Namiki, M. Effects of testosterone replacement therapy on nocturia and quality of life in men with hypogonadism: A subanalysis of a previous prospective randomized controlled study in Japan. Aging Male 2015, 18, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Oh, M.M.; Yoon, C.Y.; Bae, J.H.; Kim, J.J.; Moon, D.G. Nocturnal polyuria and decreased serum testosterone: Is there an association in men with lower urinary tract symptoms? Int. J. Urol. 2014, 21, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Leerasiri, P.; Pariyaeksut, P.; Hengrasmee, P.; Asumpinwong, C. Effectiveness of melatonin for the treatment of nocturia: A randomized controlled trial. Int. Urogynecol. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Matsuta, Y.; Yusup, A.; Tanase, K.; Ishida, H.; Akino, H.; Yokoyama, O. Melatonin increases bladder capacity via GABAergic system and decreases urine volume in rats. J. Urol. 2010, 184, 386–391. [Google Scholar] [CrossRef]
- Gulur, D.M.; Mevcha, A.M.; Drake, M.J. Nocturia as a manifestation of systemic disease. BJU Int. 2011, 107, 702–713. [Google Scholar] [CrossRef]
- Li, M.K.; Garcia, L.A.; Rosen, R. Lower urinary tract symptoms and male sexual dysfunction in Asia: A survey of ageing men from five Asian countries. BJU Int. 2005, 96, 1339–1354. [Google Scholar] [CrossRef]
- Fonda, D. Nocturia: A disease or normal ageing? BJU Int. 1999, 84, 13–15. [Google Scholar] [CrossRef]
- Chartier-Kastler, E.; Tubaro, A. The Measurement of Nocturia and Its Impact on Quality of Sleep and Quality of Life in LUTS/BPH. Eur. Urol. Suppl. 2006, 5, 3–11. [Google Scholar] [CrossRef]
- Rivas, A.M.; Mulkey, Z.; Lado-Abeal, J.; Yarbrough, S. Diagnosing and managing low serum testosterone. In Baylor University Medical Center Proceedings; Taylor & Francis: London, UK, 2014; Volume 27, pp. 321–324. [Google Scholar] [CrossRef]
- Fatemeh, G.; Sajjad, M.; Niloufar, R.; Neda, S.; Leila, S.; Khadijeh, M. Effect of melatonin supplementation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2022, 269, 205–216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).