Association between 3-Year Repetitive Isolated Hematuria and eGFR Deterioration in an Apparently Healthy Population: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
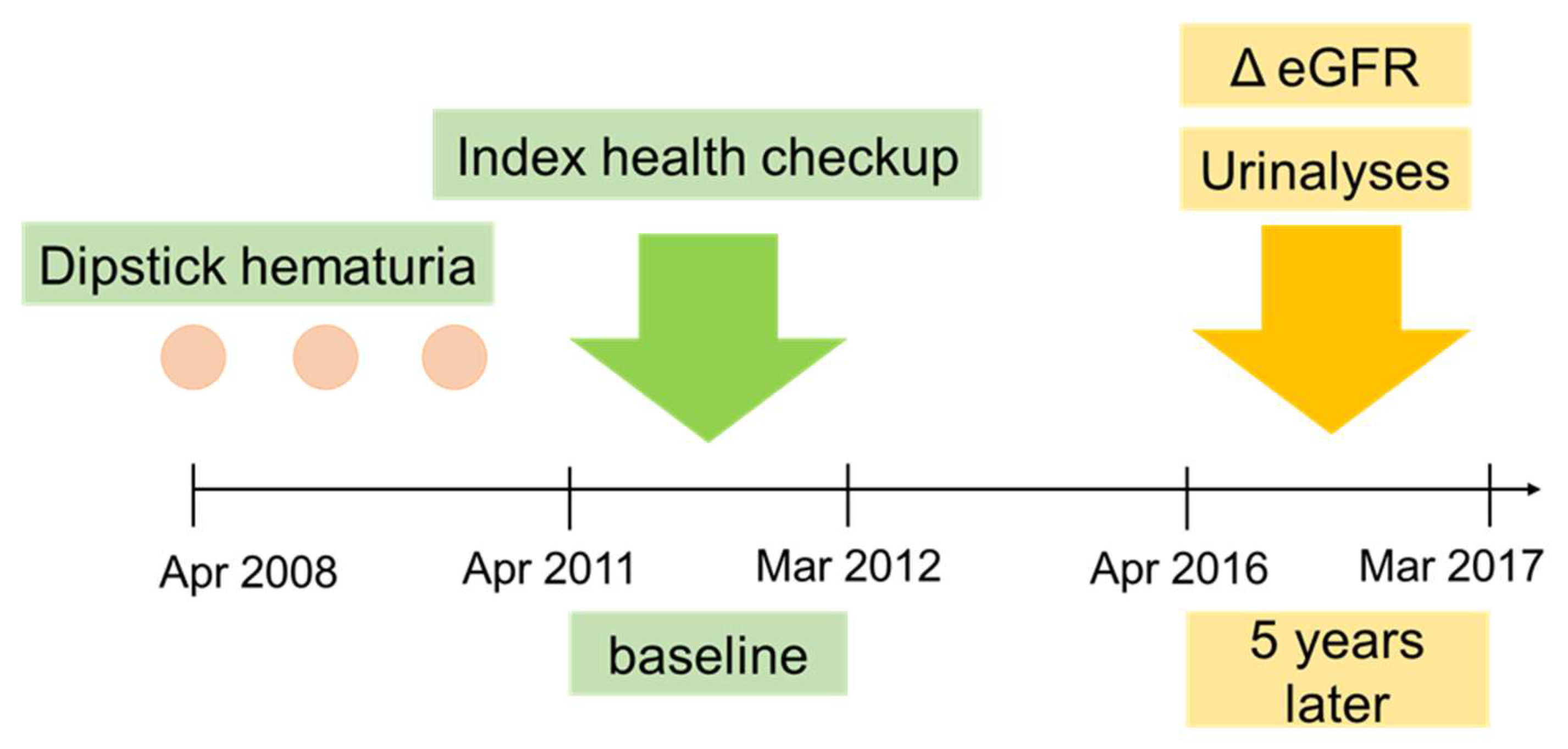
2.1. Study Design and Population
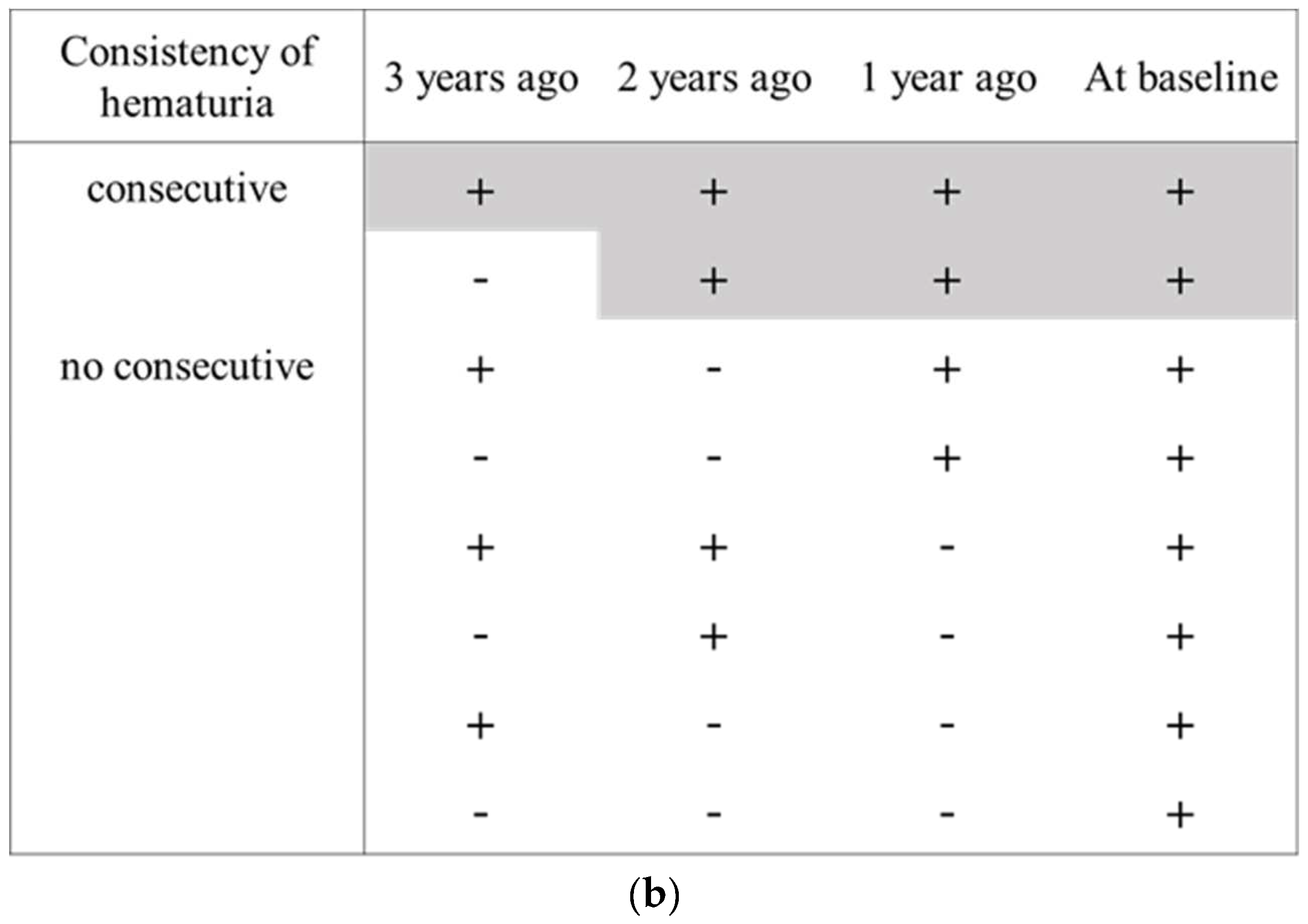
2.2. Persistent Hematuria
2.3. Outcomes
2.4. Confounding Factors
2.5. Statistical Analyses
2.6. Ethical Consideration
3. Results
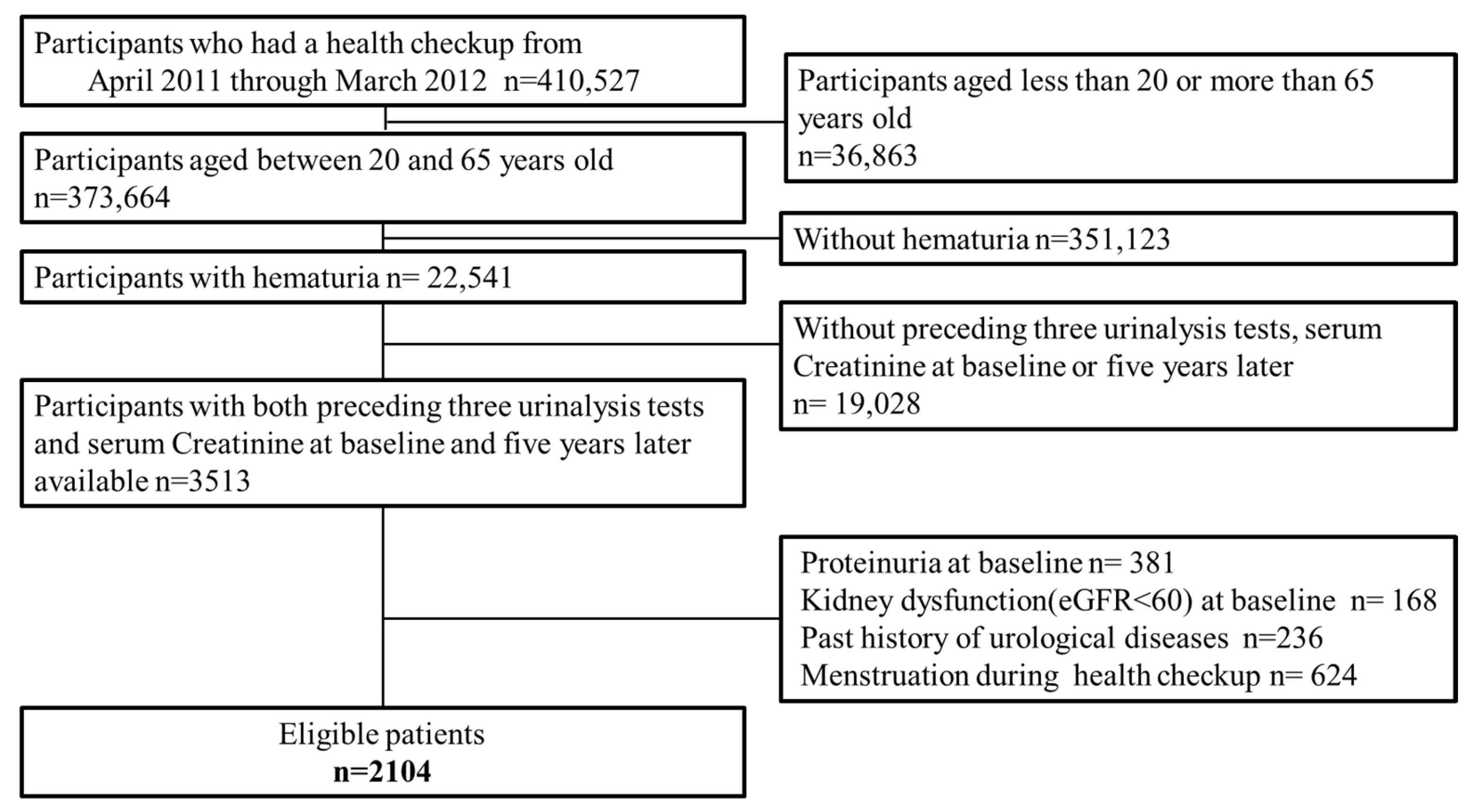
3.1. Study Participant
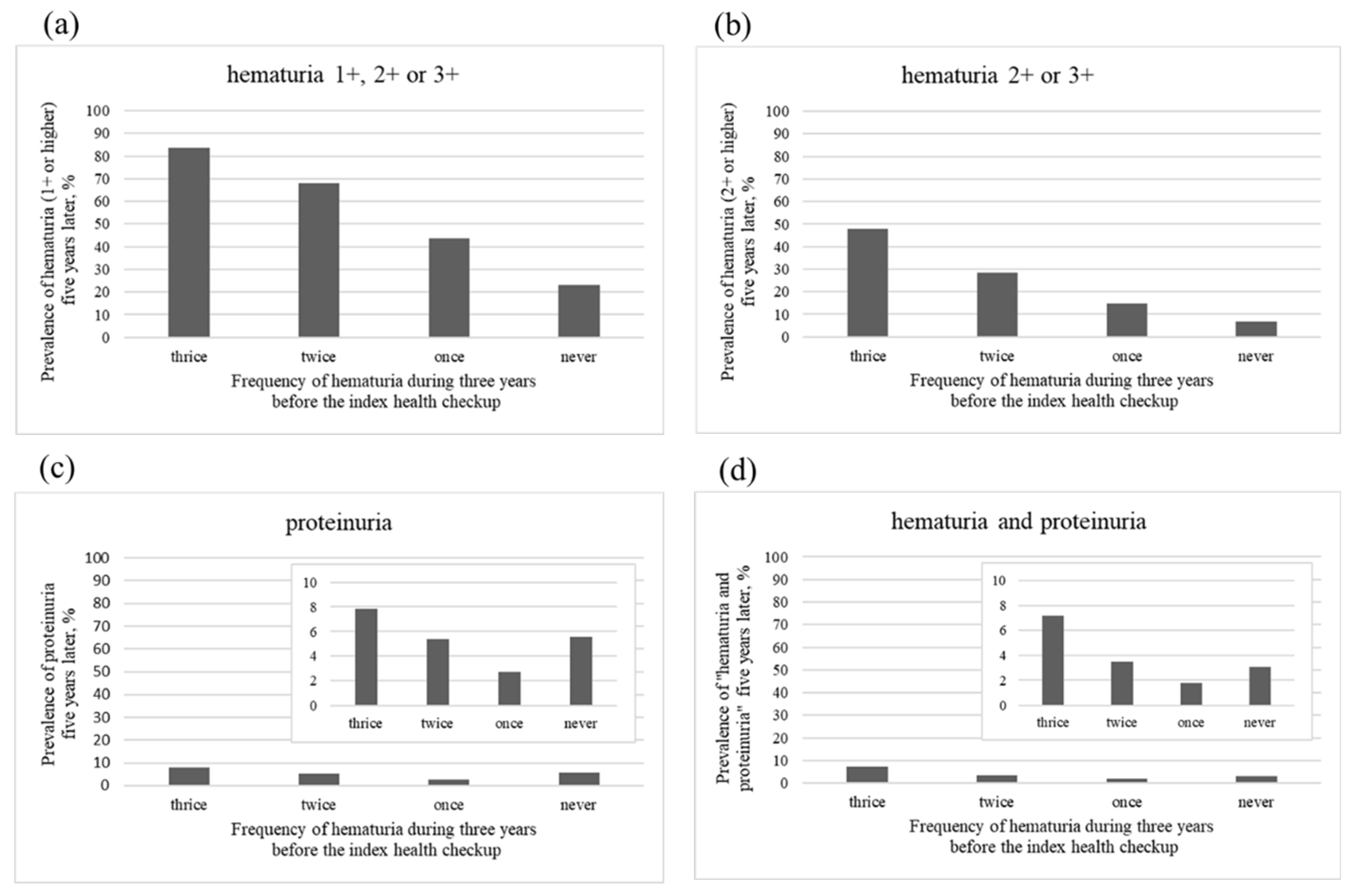
3.2. Main Analysis
3.3. Additional Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Xu, Y.; Zhang, W.; Bundy, J.D.; Chen, C.-S.; Kelly, T.N.; Chen, J.; He, J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015, 88, 950–957. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Watanabe, T.; Iseki, K.; Yamagata, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Moriyama, T.; Ando, Y.; et al. Prevalence of chronic kidney disease in the Japanese general population. Clin. Exp. Nephrol. 2009, 13, 621–630. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Boulware, E.; Coresh, J.; Stevens, L.A.; Miller, E.R.; Saran, R.; Messer, K.L.; Levey, A.S.; Powe, N.R. Patient Awareness of Chronic Kidney Disease. Arch. Intern. Med. 2008, 168, 2268–2275. [Google Scholar] [CrossRef]
- Chu, C.D.; McCulloch, C.E.; Banerjee, T.; Pavkov, M.E.; Burrows, N.R.; Gillespie, B.W.; Saran, R.; Shlipak, M.G.; Powe, N.R.; Tuot, D.S.; et al. CKD Awareness Among US Adults by Future Risk of Kidney Failure. Am. J. Kidney Dis. 2020, 76, 174–183. [Google Scholar] [CrossRef]
- Yamagata, K.; Iseki, K.; Nitta, K.; Imai, H.; Iino, Y.; Matsuo, S.; Makino, H.; Hishida, A. Chronic kidney disease perspectives in Japan and the importance of urinalysis screening. Clin. Exp. Nephrol. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Japanese Clinical Practice Guidelines for Hematuria Diagnosis 2013 Published (Japanese). Available online: https://cdn.jsn.or.jp/guideline/pdf/hugl2013.pdf (accessed on 7 July 2022).
- Iseki, K.; Ikemiya, Y.; Iseki, C.; Takishita, S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003, 63, 1468–1474. [Google Scholar] [CrossRef]
- Iseki, K.; Konta, T.; Asahi, K.; Yamagata, K.; Fujimoto, S.; Tsuruya, K.; Narita, I.; Kasahara, M.; Shibagaki, Y.; Moriyama, T.; et al. Association of dipstick hematuria with all-cause mortality in the general population: Results from the specific health check and guidance program in Japan. Nephrol. Dial. Transplant. 2018, 33, 825–832. [Google Scholar] [CrossRef]
- Iseki, K.; Konta, T.; Asahi, K.; Yamagata, K.; Fujimoto, S.; Tsuruya, K.; Narita, I.; Kasahara, M.; Shibagaki, Y.; Moriyama, T.; et al. Higher cardiovascular mortality in men with persistent dipstick hematuria. Clin. Exp. Nephrol. 2021, 25, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, M.; Cha, M.-U.; Nam, K.H.; An, S.Y.; Park, S.; Jhee, J.H.; Yun, H.-R.; Kee, Y.K.; Park, J.T.; et al. Microscopic hematuria is a risk factor of incident chronic kidney disease in the Korean general population: A community-based prospective cohort study. QJM Int. J. Med. 2018, 111, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Takahashi, H.; Tomida, C.; Yamagata, Y.; Koyama, A. Prognosis of Asymptomatic Hematuria and/or Proteinuria in Men. Nephron Exp. Nephrol. 2002, 91, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.; Kwan, B.C.; Li, P.; Szeto, C.C. Asymptomatic isolated microscopic haematuria: Long-term follow-up. QJM Int. J. Med. 2004, 97, 739–745. [Google Scholar] [CrossRef][Green Version]
- Vivante, A. Persistent Asymptomatic Isolated Microscopic Hematuria in Israeli Adolescents and Young Adults and Risk for End-Stage Renal Disease. JAMA 2011, 306, 729–736. [Google Scholar] [CrossRef]
- Hoshino, Y.; Kaga, T.; Abe, Y.; Endo, M.; Wakai, S.; Tsuchiya, K.; Nitta, K. Renal biopsy findings and clinical indicators of patients with hematuria without overt proteinuria. Clin. Exp. Nephrol. 2015, 19, 918–924. [Google Scholar] [CrossRef]
- Lee, H.M.; Hyun, J.I.; Min, J.-W.; Lee, K.; Kim, Y.K.; Choi, E.J.; Song, H.C. The Natural Course of Biopsy-Proven Isolated Microscopic Hematuria: A Single Center Experience of 350 Patients. J. Korean Med. Sci. 2016, 31, 909–914. [Google Scholar] [CrossRef]
- Evidence-Based Clinical Guideline for IgA Nephropathy 2020 Published, (Japanese). Available online: https://jsn.or.jp/academicinfo/report/evidence_IgA_guideline2020.pdf (accessed on 7 July 2022).
- JCCLS (Japanese Committee for Clinical Laboratory Standards). Available online: https://www.jccls.org/ (accessed on 7 July 2022).
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Imai, E.; Yamagata, K.; Iseki, K.; Iso, H.; Horio, M.; Mkino, H.; Hishida, A.; Matsuo, S.; Fine, D.M.; Garg, N.; et al. Kidney Disease Screening Program in Japan: History, Outcome, and Perspectives. Clin. J. Am. Soc. Nephrol. 2007, 2, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Igarashi, M.; Kobayashi, M. Natural history and risk factors for immunoglobulin a nephropathy in Japan. Am. J. Kidney Dis. 1997, 29, 526–532. [Google Scholar] [CrossRef]
- Manno, C.; Strippoli, G.; D’Altri, C.; Torres, D.; Rossini, M.; Schena, F.P. A Novel Simpler Histological Classification for Renal Survival in IgA Nephropathy: A Retrospective Study. Am. J. Kidney Dis. 2007, 49, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P. Immunoglobulin a nephropathy with mild renal lesions: A call in the forest for physicians and nephrologists. Am. J. Med. 2001, 110, 499–500. [Google Scholar] [CrossRef]
- Tiebosch, A.T.; Wolters, J.; Frederik, P.F.; van der Wiel, T.W.; Zeppenfeldt, E.; Vriesman, P.J.V.B.; van Rie, H. Epidemiology of idiopathic glomerular disease: A prospective study. Kidney Int. 1987, 32, 112–116. [Google Scholar] [CrossRef]
- Simon, P.; Ramée, M.-P.; Autuly, V.; Laruelle, E.; Charasse, C.; Cam, G.; Ang, K.S. Epidemiology of primary glomerular diseases in a French region. Variations according to period and age. Kidney Int. 1994, 46, 1192–1198. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Haas, M.; Reich, H.N. IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef]
- Szeto, C.-C.; Lai, F.M.-M.; To, K.-F.; Wong, T.Y.-H.; Chow, K.-M.; Choi, P.C.-L.; Lui, S.-F.; Li, P.K.-T. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am. J. Med. 2001, 110, 434–437. [Google Scholar] [CrossRef]
- Shen, P.; He, L.; Huang, D. Clinical course and prognostic factors of clinical early IgA nephropathy. Neth. J. Med. 2008, 66, 242–247. [Google Scholar]
- Sevillano, A.M.; Gutierrez, J.A.M.; Yuste, C.; Cavero, T.; Mérida, E.; Rodríguez, P.; García, A.; Morales, E.; Fernández, C.; Martínez, M.A.; et al. Remission of Hematuria Improves Renal Survival in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3089–3099. [Google Scholar] [CrossRef]


| Total (n = 2104) | Thrice (n = 680) | Twice (n = 429) | Once (n = 440) | Never (n = 555) | p Value | |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 48.3 (8.22) | 50.3 (7.79) | 48.0 (8.20) | 47.6 (8.12) | 46.7 (8.36) | <0.001 |
| Male, n (%) | 1138 (54.1) | 335 (49.3) | 222 (51.7) | 233 (53.0) | 348 (62.7) | <0.001 |
| BMI, n (%) | 0.03 | |||||
| <18.5 | 238 (11.3) | 82 (12.1) | 50 (11.7) | 61 (13.9) | 45 (8.1) | |
| ≥18.5, <25 | 1511 (71.8) | 498 (73.3) | 308 (71.8) | 303 (68.9) | 402 (72.4) | |
| ≥25, <30 | 306 (14.6) | 90 (13.3) | 61 (14.2) | 68 (15.5) | 87 (15.7) | |
| ≥30 | 48 (2.3) | 9 (1.3) | 10 (2.3) | 8 (1.8) | 21 (3.8) | |
| Dipstick hematuria, n (%) | <0.001 | |||||
| 1+ | 1348 (64.1) | 315 (46.3) | 303 (70.6) | 334 (75.9) | 396 (71.4) | |
| 2+ | 639 (30.4) | 313 (46.0) | 118 (27.5) | 88 (20.0) | 120 (21.6) | |
| 3+ | 117 (5.6) | 52 (7.7) | 8 (1.9) | 18 (4.1) | 39 (7.0) | |
| Serum creatinine, mg/dL, mean (SD) | 0.740 (0.130) | 0.732 (0.129) | 0.735 (0.132) | 0.729 (0.129) | 0.752 (0.140) | 0.02 |
| eGFR, mL/min/1.73 m2, mean (SD) | 80.0 (11.9) | 78.38 (11.7) | 79.99 (12.3) | 80.92 (11.0) | 81.36 (12.5) | <0.001 |
| Hypertension, n (%) | 651 (31.0) | 232 (34.2) | 127 (29.7) | 118 (26.8) | 174 (31.4) | 0.06 |
| Diabetes mellitus, n (%) | 302 (14.4) | 83 (12.2) | 64 (14.9) | 68 (15.5) | 87 (15.7) | 0.3 |
| Dyslipidemia, n (%) | 869 (41.4) | 296 (43.5) | 167 (39.0) | 185 (42.1) | 221 (39.9) | 0.4 |
| Hyperuricemia, n (%) | 289 (13.8) | 95 (14) | 49 (11.4) | 54 (12.3) | 91 (16.4) | 0.1 |
| Risk Ratio | |||
|---|---|---|---|
| Thrice | Twice | Once | |
| Dipstick hematuria | |||
| 1+, 2+ or 3+ | 3.64 (3.11–4.25) | 2.97 (2.52–3.51) | 1.91 (1.58–2.30) |
| 2+ or 3+ | 7.13 (5.17–9.83) | 4.26 (3.02–6.02) | 2.23 (1.52–3.27) |
| Dipstick proteinuria | 1.39 (0.91–2.14) | 0.96 (0.57–1.62) | 0.49 (0.26–0.94) |
| Hematuria and proteinuria | 2.35 (1.37–4.03) | 1.14 (0.58–2.26) | 0.60 (0.26–1.37) |
| CKD, eGFR < 60 | 1.22 (0.79–1.89) | 0.61 (0.33–1.11) | 0.87 (0.51–1.47) |
| GFR 30% reduction | 1.09 (0.25–4.84) | 2.16 (0.52–8.97) | 1.26 (0.26–6.22) |
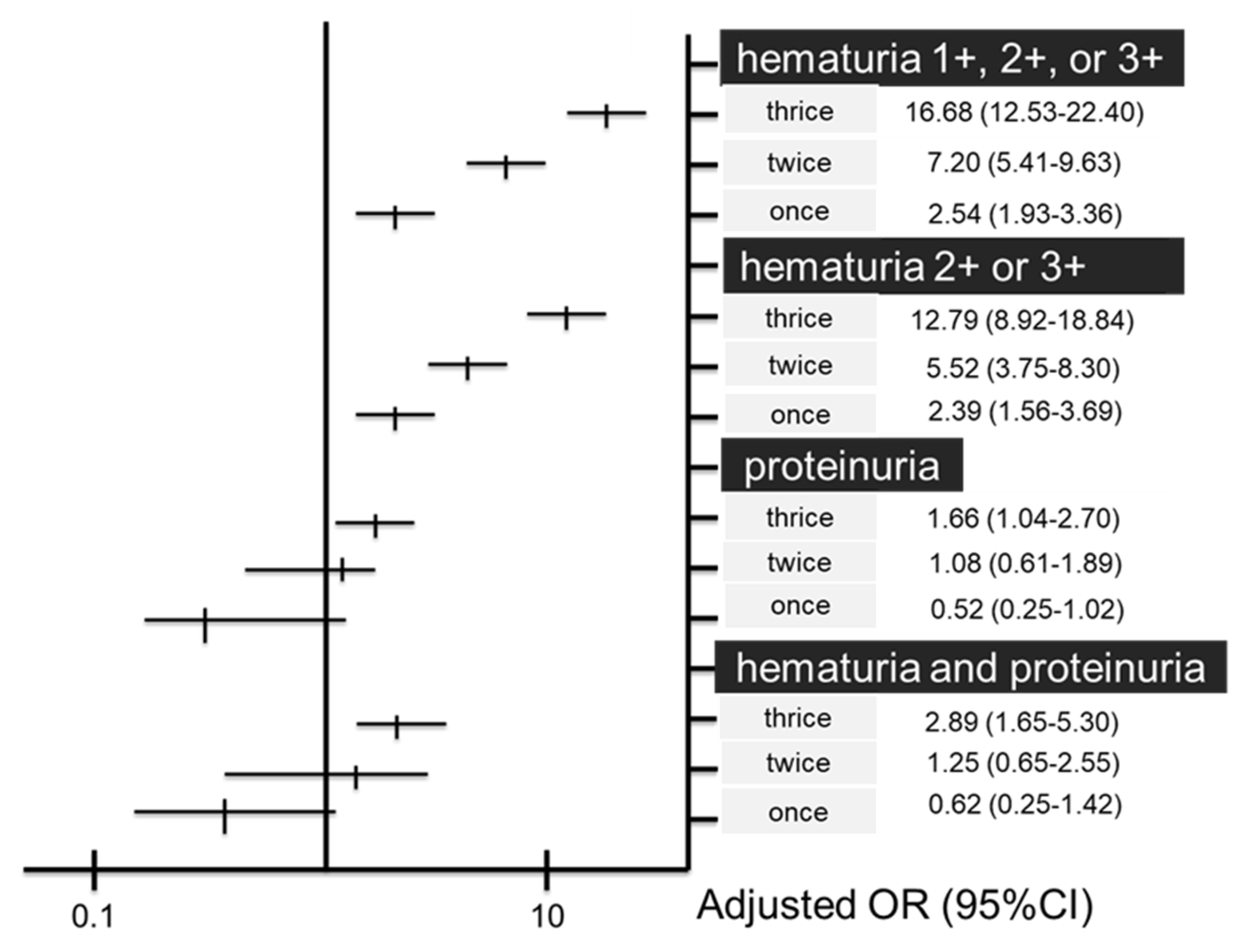
| Multivariate Odds Ratio * | |||
|---|---|---|---|
| Thrice | Twice | Once | |
| Dipstick hematuria | |||
| 1+, 2+ or 3+ | 16.68 (12.53–22.40) | 7.2 (5.41–9.63) | 2.54 (1.93–3.36) |
| 2+ or 3+ | 12.79 (8.92–18.84) | 5.52 (3.75–8.30) | 2.39 (1.56–3.69) |
| Dipstick proteinuria | 1.66 (1.04–2.70) | 1.08 (0.61–1.89) | 0.52 (0.25–1.02) |
| Hematuria and proteinuria | 2.89 (1.65–5.30) | 1.25 (0.61–2.55) | 0.62 (0.25–1.42) |
| CKD, eGFR < 60 | 0.79 (0.47–1.35) | 0.83 (0.45–1.51) | 0.94 (0.50–1.72) |
| GFR 30% reduction | 1.31 (0.27–7.11) | 2.44 (0.56–1.26) | 1.33 (0.24–7.54) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, M.; Matsuzaki, K.; Suzuki, H.; Suzuki, Y.; Kawamura, T.; Marunaka, Y.; Iwami, T. Association between 3-Year Repetitive Isolated Hematuria and eGFR Deterioration in an Apparently Healthy Population: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 11466. https://doi.org/10.3390/ijerph191811466
Ishida M, Matsuzaki K, Suzuki H, Suzuki Y, Kawamura T, Marunaka Y, Iwami T. Association between 3-Year Repetitive Isolated Hematuria and eGFR Deterioration in an Apparently Healthy Population: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(18):11466. https://doi.org/10.3390/ijerph191811466
Chicago/Turabian StyleIshida, Mami, Keiichi Matsuzaki, Hitoshi Suzuki, Yusuke Suzuki, Takashi Kawamura, Yoshinori Marunaka, and Taku Iwami. 2022. "Association between 3-Year Repetitive Isolated Hematuria and eGFR Deterioration in an Apparently Healthy Population: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 18: 11466. https://doi.org/10.3390/ijerph191811466
APA StyleIshida, M., Matsuzaki, K., Suzuki, H., Suzuki, Y., Kawamura, T., Marunaka, Y., & Iwami, T. (2022). Association between 3-Year Repetitive Isolated Hematuria and eGFR Deterioration in an Apparently Healthy Population: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 19(18), 11466. https://doi.org/10.3390/ijerph191811466






