Impact of Socioeconomic Deprivation on the Local Spread of COVID-19 Cases Mediated by the Effect of Seasons and Restrictive Public Health Measures: A Retrospective Observational Study in Apulia Region, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
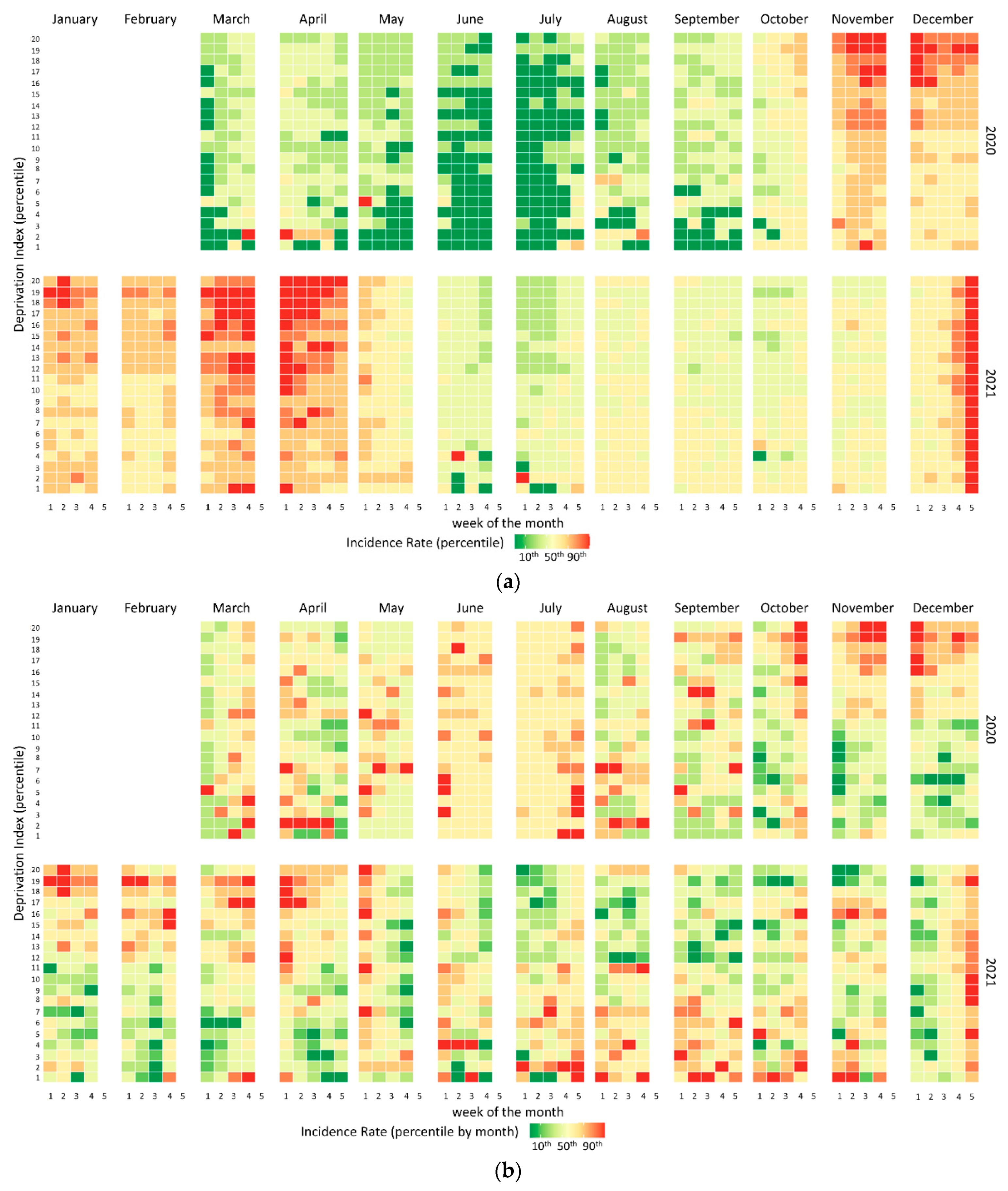
2.2.1. Graphic Method
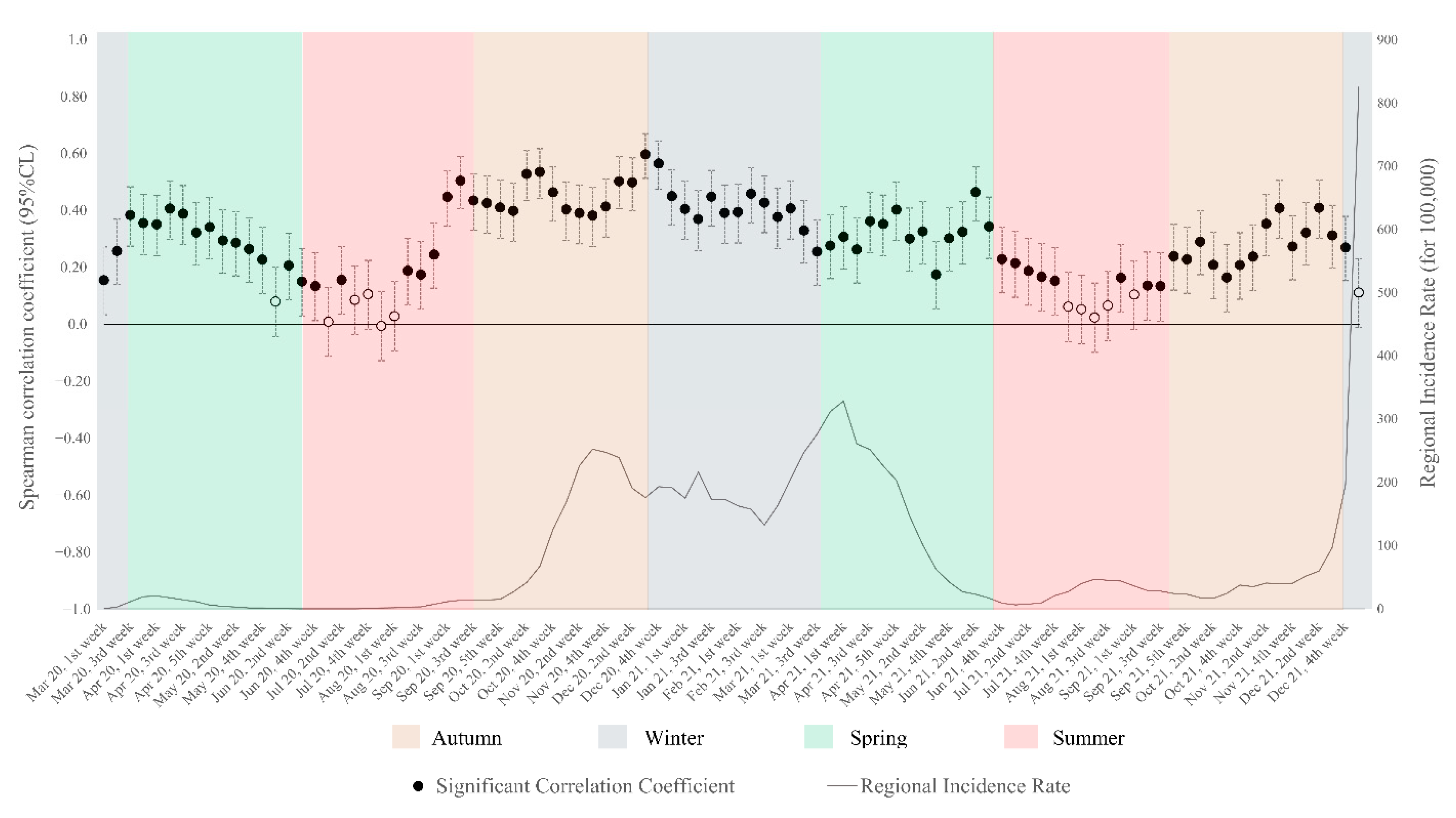
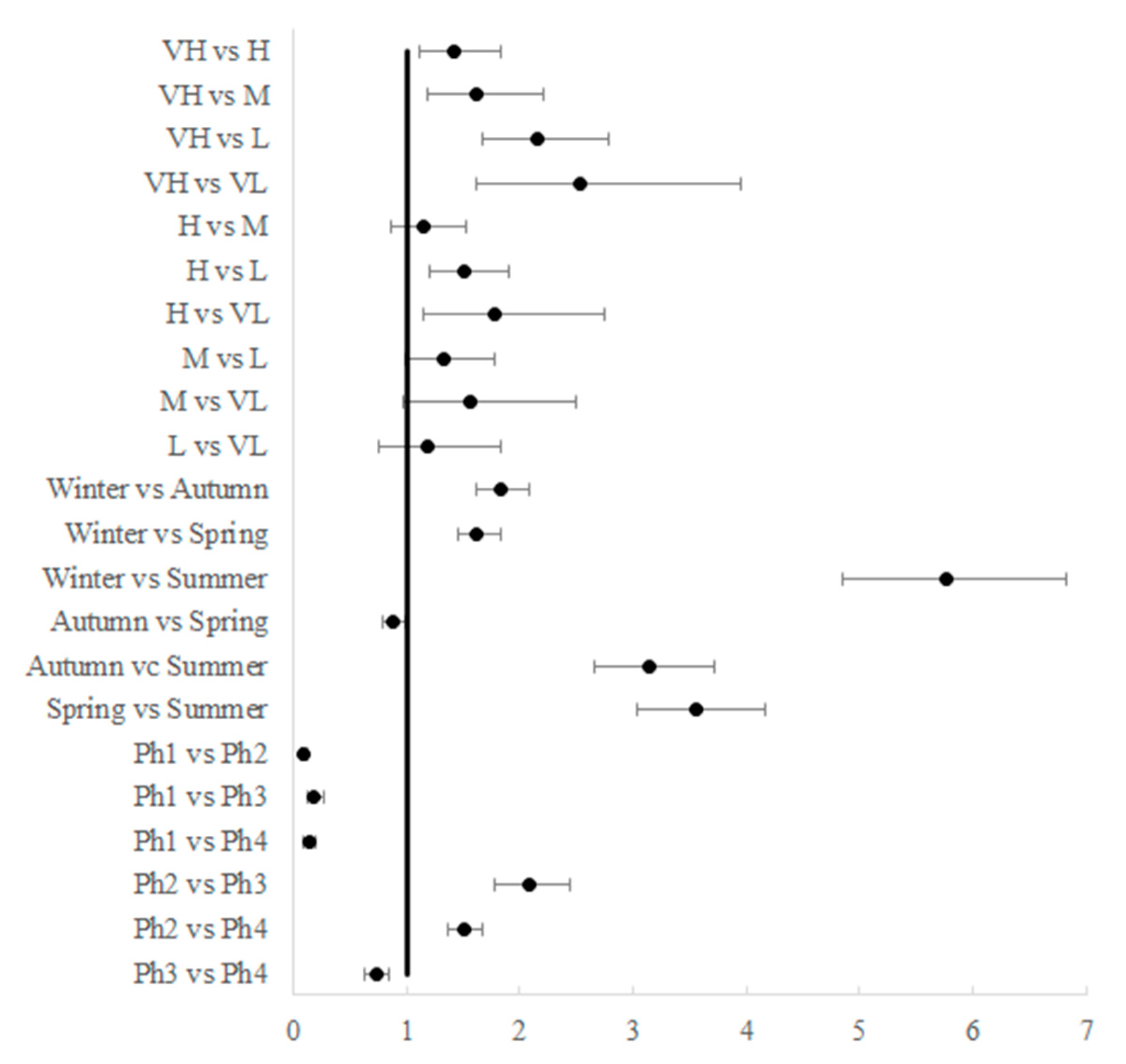
2.2.2. Statistical Method
- Phase 1, from 1 March 2020 to 30 April 2020, “total lockdown”, with a high level of restrictions: ban on leaving the house except out of necessity, suspension of educational services, closure of all commercial activities and public offices [21];
- Phase 2, from 1 May 2020 to 15 June 2020, from 1 October 2020 to 31 December 2020, and from 15 March 2021 to 25 April 2021, “soft lockdown,” with a moderate level of restriction: ban on leaving one’s hometown except for work, suspension of educational services, closure of some commercial activities [22,23];
- Phase 3, from 16 June 2020 to 30 September 2020 and from 26 April 2021 to 18 May 2021, a moderate level of restriction: suspension of indoor educational services, reduction in the number of people accessing commercial activities [24];
- Phase 4, from 8 February 2021 to 14 March 2021 and from 19 May 2021 to 31 December 2021, low level of restrictions.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 ITALIA-Desktop. Available online: https://opendatadpc.maps.arcgis.com/apps/dashboards/b0c68bce2cce478eaac82fe38d4138b1 (accessed on 15 January 2022).
- Odone, A.; Delmonte, D.; Scognamiglio, T.; Signorelli, C. COVID-19 Deaths in Lombardy. Italy: Data in Context. Lancet Public Health 2020, 5, e310. [Google Scholar] [CrossRef]
- Livingston, E.; Bucher, K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [Google Scholar] [CrossRef] [PubMed]
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.M.; Friedrichs, M.; Wagstaff, S.; Sage, K.; LaCross, N.; Bui, D.; McCaffrey, K.; Barbeau, B.; George, A.; Rose, C.; et al. Disparities in COVID-19 Incidence. Hospitalizations. and Testing. by Area-Level Deprivation—Utah, March 3–July 9, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1369–1373. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Lara, N.; Anwar, A.; Garcia, D.C.; Hemaktiathar, S.; Ifebirinachi, C.B.; Anwar, M.; Lin, F.C.; Samuel, R. The Effects of Race/Ethnicity, Age, and Area Deprivation Index (ADI) on COVID-19 Disease Early Dynamics: Washington, D.C. Case Study. J. Racial Ethn. Health Disparities 2022, 1–10. [Google Scholar] [CrossRef]
- Di Girolamo, C.; Bartolini, L.; Caranci, N.; Moro, M.L. Socioeconomic inequalities in overall and COVID-19 mortality during the first outbreak peak in Emilia-Romagna Region (Northern Italy). Epidemiol. Prev. 2020, 44 (Suppl. 2), 288–296. [Google Scholar]
- Yoshikawa, Y.; Kawachi, I. Association of Socioeconomic Characteristics with Disparities in COVID-19 Outcomes in Japan. JAMA Netw. Open 2021, 4, e2117060. [Google Scholar] [CrossRef]
- KC, M.; Oral, E.; Straif-Bourgeois, S.; Rung, A.L.; Peters, E.S. The effect of area deprivation on COVID-19 risk in Louisiana. PLoS ONE 2020, 15, e0243028. [Google Scholar]
- Kitchen, C.; Hatef, E.; Chang, H.Y.; Weiner, J.P.; Kharrazi, H. Assessing the association between area deprivation index on COVID-19 prevalence: A contrast between rural and urban U.S. jurisdictions. AIMS Public Health 2021, 8, 519–530. [Google Scholar] [CrossRef]
- Ossimetha, A.; Ossimetha, A.; Kosar, C.M.; Rahman, M. Socioeconomic Disparities in Community Mobility Reduction and COVID-19 Growth. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; Volume 96, pp. 78–85. [Google Scholar]
- Caranci, N.; Biggeri, A.; Grisotto, L.; Pacelli, B.; Spadea, T.; Costa, G. The Italian deprivation index at census block level: Definition, description and association with general mortality. Epidemiol. Prev. 2010, 34, 167–176. (In Italian) [Google Scholar]
- Liu, X.; Huang, J.; Li, C.; Zhao, Y.; Wang, D.; Huang, Z.; Yang, K. The role of seasonality in the spread of COVID-19 pandemic. Env. Res. 2021, 195, 110874. [Google Scholar] [CrossRef] [PubMed]
- Jamison, J.C.; Bundy, D.; Jamison, D.T.; Spitz, J.; Verguet, S. Comparing the impact on COVID-19 mortality of self-imposed behavior change and of government regulations across 13 countries. Health Serv. Res. 2021, 56, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Tan, M.M.; Turk, E.; Sridhar, D.; Leung, G.M.; Shibuya, K.; Asgari, N.; Oh, J.; García-Basteiro, A.L.; Hanefeld, J.; et al. Lessons learnt from easing COVID-19 restrictions: An analysis of countries and regions in Asia Pacific and Europe. Lancet 2020, 396, 1525–1534. [Google Scholar] [CrossRef]
- Bartolomeo, N.; Giotta, M.; Trerotoli, P. In-Hospital Mortality in Non-COVID-19-Related Diseases before and during the Pandemic: A Regional Retrospective Study. Int. J. Env. Res. Public Health 2021, 18, 10886. [Google Scholar] [CrossRef] [PubMed]
- WHO. Go.Data: Managing Complex Data in Outbreaks. Available online: https://www.who.int/godata (accessed on 16 January 2022).
- Demo Istat. Available online: http://demo.istat.it/pop2019/index.html (accessed on 20 January 2022).
- Rosano, A.; Pacelli, B.; Zengarini, N.; Costa, G.; Cislaghi, C.; Caranci, N. Update and review of the 2011 Italian deprivation index calculated at the census section level. Epidemiol. Prev. 2020, 44, 162–170. (In Italian) [Google Scholar]
- Bonett, D.G.; Wright, T.A. Sample size requirements for estimating pearson, kendall and spearman correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Urgent Measures in Containement and Management of the Epidemiological Emergency from COVID-19. 2020. Available online: https://www.gazzettaufficiale.it/eli/id/2020/02/23/20G00020/sg (accessed on 15 January 2022).
- Further Implementing Provisions of the D.L. 23 February, No 6, Containing Urgent Measures on Containment and Management of the Epidemiological Emergency from COVID-19, Applicable on the Entire National Territory. 2020. Available online: https://www.gazzettaufficiale.it/eli/id/2020/04/27/20A02352/sg (accessed on 15 January 2022).
- Urgent Measures Connected with the Extension of the Declaration of the State of Epidemiological Emergency from COVID-19, for the Deferral of Electoral Consultations for the Year 2020 and for the Continuity of Operation of the COVID Alert System, as Well as for the Implementation of the Directive EU 2020/739 of 3 June 2020, and Urgent Provions on Tax Collection. 2020. Available online: https://www.gazzettaufficiale.it/eli/id/2020/10/07/20G00144/sg (accessed on 15 January 2022).
- Further Implementing Provisions of the D.L. No 19 of 25 March 2020, Containing Urgent Measures to Cope with the Epidemiological Emergency from COVID-19, and D.L. No 33 of 16 May 2020, Containing Additional Urgent Measures to Cope with the Epidemiological Emergency form COVID-19. 2020. Available online: https://www.gazzettaufficiale.it/eli/id/2020/06/11/20A03194/sg (accessed on 15 January 2022).
- Ballinger, G.A. Using Generalized Estimating Equations for Longitudinal Data Analysis. Organ Res. Methods 2004, 7, 127–150. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 January 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation; R package version 1.0.7. 2021. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 30 January 2022).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2021. Available online: http://qgis.osgeo.org (accessed on 15 April 2022).
- Whittle, R.S.; Diaz-Artiles, A. An ecological study of socioeconomic predictors in detection of COVID-19 cases across neighborhoods in New York City. BMC Med. 2020, 18, 271. [Google Scholar] [CrossRef]
- Ho, F.K.; Celis-Morales, C.A.; Gray, S.R.; Katikireddi, S.V.; Niedzwiedz, C.L.; Hastie, C.; Ferguson, L.D.; Berry, C.; Mackay, D.F.; Gill, J.M.; et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: Results from a UK Biobank prospective cohort study. BMJ Open 2020, 10, e040402. [Google Scholar] [CrossRef]
- Baena-Díez, J.M.; Barroso, M.; Cordeiro-Coelho, S.I.; Díaz, J.L.; Grau, M. Impact of COVID-19 outbreak by income: Hitting hardest the most deprived. J. Public Health 2020, 42, 698–703. [Google Scholar] [CrossRef]
- Magesh, S.; John, D.; Li, W.T.; Li, Y.; Mattingly-App, A.; Jain, S.; Chang, E.Y.; Ongkeko, W.M. Disparities in COVID-19 Outcomes by Race, Ethnicity, and Socioeconomic Status: A Systematic-Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2134147. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Urdiales, A.; Fabiani, M.; Rosano, A.; Vescio, M.F.; Del Manso, M.; Bella, A.; Riccardo, F.; Pezzotti, P.; Regidor, E.; Andrianou, X. Socioeconomic patterns and COVID-19 outcomes before, during and after the lockdown in Italy (2020). Health Place 2021, 71, 102642. [Google Scholar] [CrossRef] [PubMed]
- Orenes-Piñero, E.; Baño, F.; Navas-Carrillo, D.; Moreno-Docón, A.; Marín, J.M.; Misiego, R.; Ramírez, P. Evidences of SARS-CoV-2 virus air transmission indoors using several untouched surfaces: A pilot study. Sci. Total Env. 2021, 751, 142317. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Miao, T.; Liu, L.; Zheng, X.; Luo, D.; Li, Y. Indoor transmission of SARS-CoV-2. Indoor Air 2021, 31, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Walk, R.A.; Bourne, L.S. Ghettos in Canada’s cities? Racial segregation, ethnic enclaves and poverty concentration in Canadian urban areas. Can. Geogr. 2006, 50, 273–297. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 2020, 20, 911–919. [Google Scholar] [CrossRef]
- Leso, V.; Fontana, L.; Iavicoli, I. Susceptibility to Coronavirus (COVID-19) in Occupational Settings: The Complex Interplay between Individual and Workplace Factors. Int. J. Env. Res. Public Health 2021, 18, 1030. [Google Scholar] [CrossRef]
- Morrissey, K.; Spooner, F.; Salter, J.; Shaddick, G. Area level deprivation and monthly COVID-19 cases: The impact of government policy in England. Soc. Sci. Med. 2021, 289, 114413. [Google Scholar] [CrossRef]
- Riou, J.; Panczak, R.; Althaus, C.L.; Junker, C.; Perisa, D.; Schneider, K.; Criscuolo, N.G.; Low, N.; Egger, M. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: A population-based analysis. Lancet Public Health 2021, 6, e683–e691. [Google Scholar] [CrossRef]
- Escobar, G.J.; Adams, A.S.; Liu, V.X.; Soltesz, L.; Chen, Y.F.; Parodi, S.M.; Ray, G.T.; Myers, L.C.; Ramaprasad, C.M.; Dlott, R.; et al. Racial disparities in COVID-19 testing and outcomes: Retrospective cohort study in an integrated health system. Ann. Intern. Med. 2021, 174, 786–793. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S.; Das, K.; Basu, T.; Das, M.; Dutta, I. Modeling the effect of area deprivation on COVID-19 incidences: A study of Chennai megacity, India. Public Health 2020, 185, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, T.L.; Brown, C.; Smith, R.; Perri, M.; Ziegler, C.; Pinto, A.D. Social determinants of COVID-19 incidence and outcomes: A rapid review. PLoS ONE 2021, 16, e0248336. [Google Scholar] [CrossRef] [PubMed]
- Vandentorren, S.; Smaïli, S.; Chatignoux, E.; Maurel, M.; Alleaume, C.; Neufcourt, L.; Kelly-Irving, M.; Delpierre, C. The effect of social deprivation on the dynamic of SARS-CoV-2 infection in France: A population-based analysis. Lancet Public Health 2022, 7, e240–e249. [Google Scholar] [CrossRef]

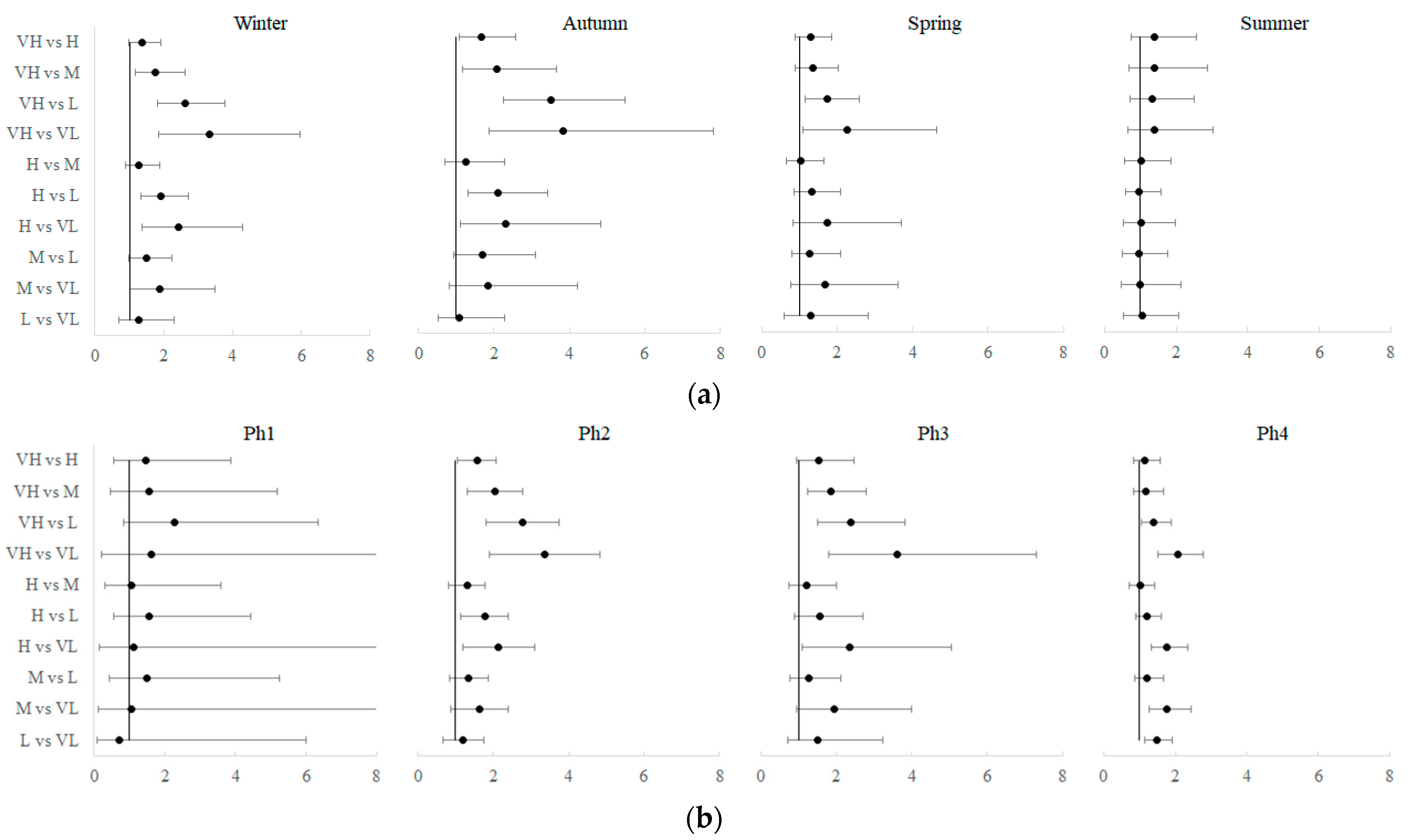
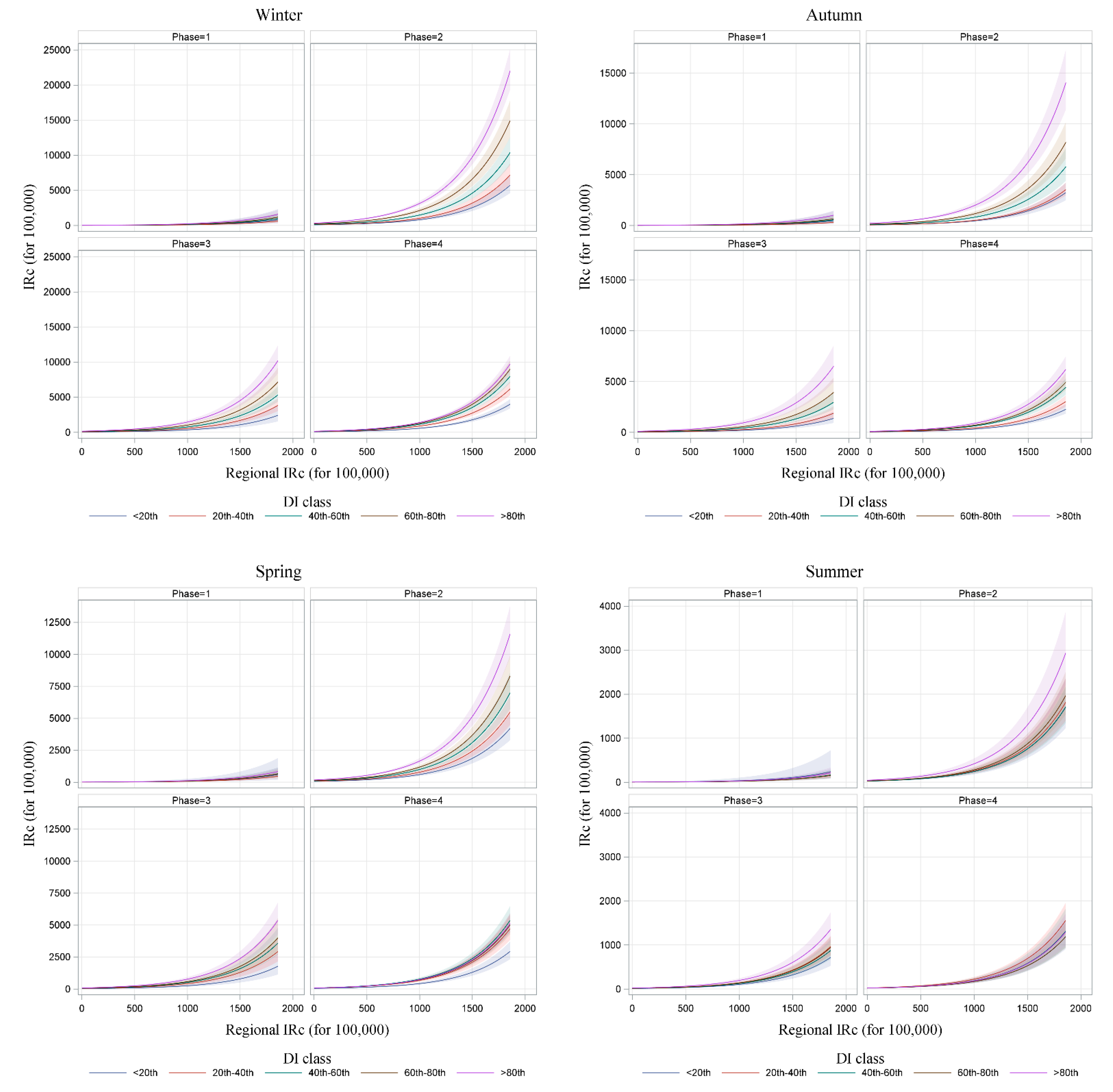
| Month | 2020 | 2021 | ||
|---|---|---|---|---|
| n | IRc | n | IRc | |
| January | - | - | 28,929 | 736.7 |
| February | - | - | 24,154 | 615.1 |
| March | 1334 | 34.0 | 40,870 | 1040.8 |
| April | 2757 | 70.2 | 49,841 | 1269.2 |
| May | 399 | 10.2 | 13,824 | 352.0 |
| June | 48 | 1.2 | 2997 | 76.3 |
| July | 92 | 2.3 | 2818 | 71.8 |
| August | 605 | 15.4 | 6897 | 175.6 |
| September | 2683 | 68.3 | 5504 | 140.2 |
| October | 10,273 | 261.6 | 3766 | 95.9 |
| November | 35,103 | 893.9 | 6101 | 155.4 |
| December | 38,877 | 990.0 | 48,286 | 1229.6 |
| Effect | Deprivation Index | |||||
|---|---|---|---|---|---|---|
| VH | H | M | L | VL | ||
| Season | Autumn | 68.5 ± 5.3 | 41.2 ± 4 | 33 ± 4.7 | 19.5 ± 1.9 | 17.9 ± 3.3 |
| Winter | 107.2 ± 7.5 | 78 ± 4.9 | 60.8 ± 5.3 | 41 ± 3.2 | 32.1 ± 4.8 | |
| Spring | 52.8 ± 2.9 | 40.8 ± 3.4 | 39.2 ± 3.9 | 30.4 ± 3 | 23.3 ± 4.5 | |
| Summer | 13.1 ± 1.8 | 9.4 ± 0.9 | 9.3 ± 1.3 | 9.8 ± 1 | 9.3 ± 1.5 | |
| Level of restrictions | Ph1 | 9.4 ± 1.7 | 6.4 ± 1.3 | 6.1 ± 1.7 | 4.1 ± 0.9 | 5.8 ± 3.3 |
| Ph2 | 138.4 ± 6.9 | 88.2 ± 5.3 | 67.5 ± 4.5 | 49.8 ± 3.2 | 41.2 ± 3.5 | |
| Ph3 | 64.1 ± 4.7 | 41.7 ± 4.7 | 34.3 ± 3.1 | 26.8 ± 3 | 17.7 ± 3.3 | |
| Ph4 | 61 ± 3.7 | 52.7 ± 3.3 | 52.2 ± 4 | 43.7 ± 2.3 | 29.7 ± 1.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomeo, N.; Giotta, M.; Tafuri, S.; Trerotoli, P. Impact of Socioeconomic Deprivation on the Local Spread of COVID-19 Cases Mediated by the Effect of Seasons and Restrictive Public Health Measures: A Retrospective Observational Study in Apulia Region, Italy. Int. J. Environ. Res. Public Health 2022, 19, 11410. https://doi.org/10.3390/ijerph191811410
Bartolomeo N, Giotta M, Tafuri S, Trerotoli P. Impact of Socioeconomic Deprivation on the Local Spread of COVID-19 Cases Mediated by the Effect of Seasons and Restrictive Public Health Measures: A Retrospective Observational Study in Apulia Region, Italy. International Journal of Environmental Research and Public Health. 2022; 19(18):11410. https://doi.org/10.3390/ijerph191811410
Chicago/Turabian StyleBartolomeo, Nicola, Massimo Giotta, Silvio Tafuri, and Paolo Trerotoli. 2022. "Impact of Socioeconomic Deprivation on the Local Spread of COVID-19 Cases Mediated by the Effect of Seasons and Restrictive Public Health Measures: A Retrospective Observational Study in Apulia Region, Italy" International Journal of Environmental Research and Public Health 19, no. 18: 11410. https://doi.org/10.3390/ijerph191811410
APA StyleBartolomeo, N., Giotta, M., Tafuri, S., & Trerotoli, P. (2022). Impact of Socioeconomic Deprivation on the Local Spread of COVID-19 Cases Mediated by the Effect of Seasons and Restrictive Public Health Measures: A Retrospective Observational Study in Apulia Region, Italy. International Journal of Environmental Research and Public Health, 19(18), 11410. https://doi.org/10.3390/ijerph191811410







