The Association between Osteoporosis and Peripheral Artery Disease: A Population-Based Longitudinal Follow-Up Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
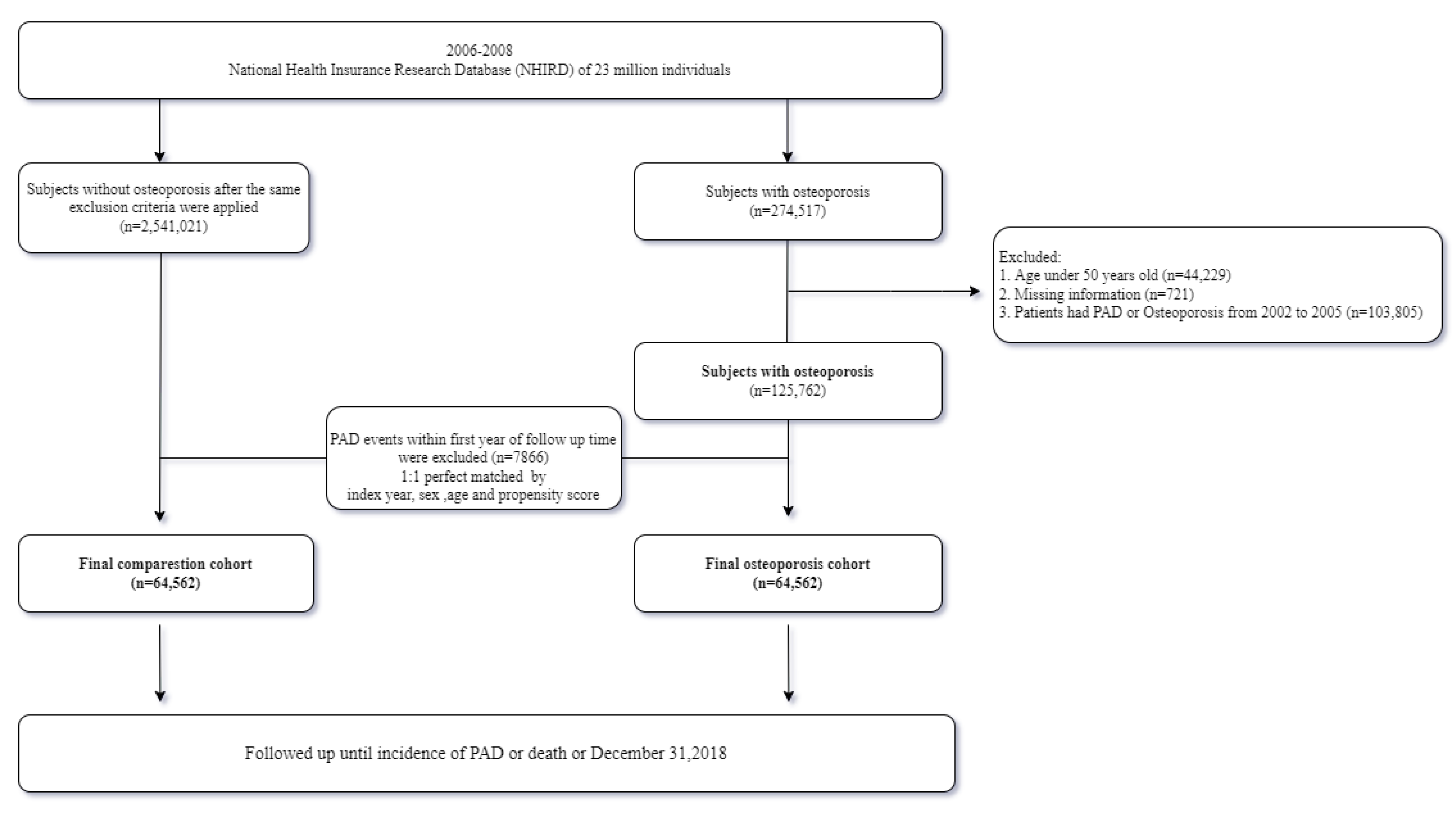
2.2. Study Population
2.3. Outcome Measure and Confounding Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauersachs, R.; Zeymer, U.; Briere, J.-B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, E.M.; Wang, K.; Keo, H.H.; Duval, S.; Smolderen, K.G.; Cohen, D.J.; Steg, G.; Bhatt, D.L.; Hirsch, A.T. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Cardiovasc. Qual. Outcomes 2010, 3, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Newman, C.; Crossman, D.C. Cardiovascular disease and bone. Arch. Biochem. Biophys 2010, 503, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Cassar, A.; Poldermans, D.; Rihal, C.S.; Gersh, B.J. The management of combined coronary artery disease and peripheral vascular disease. Eur. Heart J. 2010, 31, 1565–1572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities Between Peripheral Artery Disease and Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1982–1989. [Google Scholar] [CrossRef]
- Anagnostis, P.; Karagiannis, A.; Kakafika, A.I.; Tziomalos, K.; Athyros, V.G.; Mikhailidis, D.P. Atherosclerosis and osteoporosis: Age-dependent degenerative processes or related entities? Osteoporos Int. 2009, 20, 197–207. [Google Scholar] [CrossRef]
- Hu, X.; Ma, S.; Yang, C.; Wang, W.; Chen, L. Relationship between senile osteoporosis and cardiovascular and cerebrovascular diseases. Exp. Ther. Med. 2019, 17, 4417–4420. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, J.; Zhang, K.; Tang, Z. A community-based study of the relationship between coronary artery disease and osteoporosis in Chinese postmenopausal women. Coron. Artery Dis. 2016, 27, 59–64. [Google Scholar] [CrossRef]
- von Mühlen, D.; Allison, M.; Jassal, S.K.; Barrett-Connor, E. Peripheral arterial disease and osteoporosis in older adults: The Rancho Bernardo Study. Osteoporos. Int. 2009, 20, 2071–2078. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Policha, A.; Maldonado, T.; Hiramoto, J.S.; Honig, S.; Conte, M.S.; Berger, J.; Rockman, C.B. Novel association between bone mineral density scores and the prevalence of peripheral artery disease in both sexes. Vasc. Med. 2017, 22, 13–20. [Google Scholar] [CrossRef]
- van der Klift, M.; Pols, H.A.P.; Hak, A.E.; Witteman, J.C.M.; Hofman, A.; de Laet, C.E.D.H. Bone mineral density and the risk of peripheral arterial disease: The Rotterdam Study. Calcif. Tissue Int. 2002, 70, 443–449. [Google Scholar] [CrossRef]
- Lin, A.C.-C.; Chao, E.; Yang, C.-M.; Wen, H.-C.; Ma, H.-L.; Lu, T.-C. Costs of staged versus simultaneous bilateral total knee arthroplasty: A population-based study of the Taiwanese National Health Insurance Database. J. Orthop. Surg. Res. 2014, 9, 59. [Google Scholar] [CrossRef]
- Hsiao, F.-Y.; Huang, Y.-T.; Yang, C.-L.; Huang, W.-F. Using Taiwan’s National Health Insurance Research Databases for Pharmacoepidemiology Research. J. Food Drug Anal. 2007, 15, 99–108. [Google Scholar] [CrossRef]
- Laroche, M.; Pécourneau, V.; Blain, H.; Breuil, V.; Chapurlat, R.; Cortet, B.; Sutter, B.; Degboé, Y. Osteoporosis and ischemic cardiovascular disease. Jt. Bone Spine 2017, 84, 427–432. [Google Scholar] [CrossRef]
- Zhang, Y.; He, B.; Wang, H.; Shi, J.; Liang, H. Associations between bone mineral density and coronary artery disease: A meta-analysis of cross-sectional studies. Arch. Osteoporos. 2020, 15, 24. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, W.-C.; Kuo, C.-N.; Yu, H.-C.; Yang, C.-C.; Lin, Y.-W.; Hung, K.-S. A population-based five-year study on the risk of stroke in patients with osteoporosis in Taiwan. Bone 2015, 72, 9–13. [Google Scholar] [CrossRef]
- Wong, S.Y.-S.; Kwok, T.; Woo, J.; Lynn, H.; Griffith, J.F.; Leung, J.; Tang, Y.Y.N.; Leung, P.C. Bone mineral density and the risk of peripheral arterial disease in men and women: Results from Mr. and Ms Os, Hong Kong. Osteoporos. Int. 2005, 16, 1933–1938. [Google Scholar] [CrossRef]
- Liang, Y.S.; Yeh, K.C.; Pan, S.L. Osteoporosis and the long-term risk of peripheral artery disease: A population-based longitudinal follow-up study in Taiwan. Osteoporos. Int. 2022, 33, 1117–1123. [Google Scholar] [CrossRef]
- Gaudio, A.; Xourafa, A.; Rapisarda, R.; Castellino, P.; Signorelli, S.S. Peripheral artery disease and osteoporosis: Not only age-related (Review). Mol. Med. Rep. 2018, 18, 4787–4792. [Google Scholar] [CrossRef]
- Demková, K.; Kozárová, M.; Malachovská, Z.; Javorský, M.; Tkáč, I. Osteoprotegerin concentration is associated with the presence and severity of peripheral arterial disease in type 2 diabetes mellitus. Vasa 2018, 47, 131–135. [Google Scholar] [CrossRef]
- Kapetanios, D.; Karkos, C.; Giagtzidis, I.; Papazoglou, K.; Kiroplastis, K.; Spyridis, C. Vascular calcification biomarkers and peripheral arterial disease. Int. Angiol. 2016, 35, 455–459. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Narula, R.; Tauseef, M.; Ahmad, I.A.; Agarwal, K.; Ashok, A.; Anjana, A. Vitamin d deficiency among postmenopausal women with osteoporosis. J. Clin. Diagn. Res. 2003, 7, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Weng, S.; Felton, S.K.; Bhandare, S.; Riek, A.; Butler, B.; Proctor, B.M.; Petty, M.; Chen, Z.; Schechtman, K.B.; et al. 1,25(OH) 2 Vitamin D Inhibits Foam Cell Formation and Suppresses Macrophage Cholesterol Uptake in Patients With Type 2 Diabetes Mellitus. Circulation 2009, 120, 687–698. [Google Scholar] [CrossRef]
- Sharma, J.; Raggi, P.; Melamed, K.L.; Muntner, P.; Michos, E.D.; Uribarri, J.; Weber, C. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: Results from NHANES 2001 to 2004. Arterioscler Thromb. Vasc. Biol. 2008, 28, 1179–1185. [Google Scholar]
- Tzoulaki, I.; Murray, G.D.; Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G.R. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005, 112, 976–983. [Google Scholar] [CrossRef]
- Pande, R.L.; Brown, J.; Buck, S.; Redline, W.; Doyle, J.; Plutzky, J.; Creager, M.A. Association of monocyte tumor necrosis factorαexpression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J. Vasc. Surg. 2015, 61, 155–161. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Fortin, M.; Guthrie, B.; Hemmelgarn, B.R.; James, M.T.; Klarenbach, S.W.; Lewanczuk, R.; Manns, B.J.; Ronksley, P.; et al. Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med. Inform. Decis. Mak. 2015, 15, 31. [Google Scholar]
| Case (N = 64,562) | Comparison (N = 64,562) | p | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Year | |||||
| 2006 | 23,955 | 37.1 | 23,898 | 37.02 | 0.6959 |
| 2007 | 21,325 | 33.03 | 21,243 | 32.9 | |
| 2008 | 19,282 | 29.87 | 19,421 | 30.08 | |
| Sex | |||||
| Female | 50,454 | 78.15 | 50,555 | 78.3 | 0.4958 |
| Male | 14,108 | 21.85 | 14,007 | 21.7 | |
| Age | |||||
| 49–55 | 11,059 | 17.13 | 11,059 | 17.13 | 1 |
| ≥55 | 53,503 | 82.87 | 53,503 | 82.87 | |
| Mean (SD) | 66.25 (10.34) | 66.25 (10.34) | 1 | ||
| Baseline Comorbidity | |||||
| Hypertension | 6202 | 9.61 | 4971 | 7.7 | <0.001 |
| Hyperlipidemia | 22,044 | 34.14 | 17,545 | 27.18 | <0.001 |
| COPD | 18,190 | 28.17 | 11,772 | 18.23 | <0.001 |
| Diabetes mellitus | 16,025 | 24.82 | 12,700 | 19.67 | <0.001 |
| Liver disease | 1580 | 2.45 | 795 | 1.23 | <0.001 |
| CHF | 6926 | 10.73 | 5130 | 7.95 | <0.001 |
| RA | 126 | 0.2 | 87 | 0.13 | 0.0075 |
| CKD | 3969 | 6.15 | 2185 | 3.38 | <0.001 |
| Gout | 11,227 | 17.39 | 7980 | 12.36 | <0.001 |
| Ischemic stroke | 2809 | 4.35 | 1261 | 1.95 | <0.001 |
| Raloxifene | 806 | 1.25 | 1024 | 1.59 | <0.001 |
| Denosumab | 2393 | 3.71 | 1547 | 2.4 | <0.001 |
| Oral bisphosphate | 1203 | 1.86 | 2260 | 3.5 | <0.001 |
| Zoledronic acid | 508 | 0.79 | 623 | 0.96 | 0.0006 |
| Ibandronate | 342 | 0.53 | 391 | 0.61 | 0.0695 |
| Teriparatide | 244 | 0.38 | 338 | 0.52 | 0.0209 |
| Case (N = 64,562) | Comparison (N = 64,562) | |||||
| N | % | Incidence Rate | N | % | Incidence Rate | |
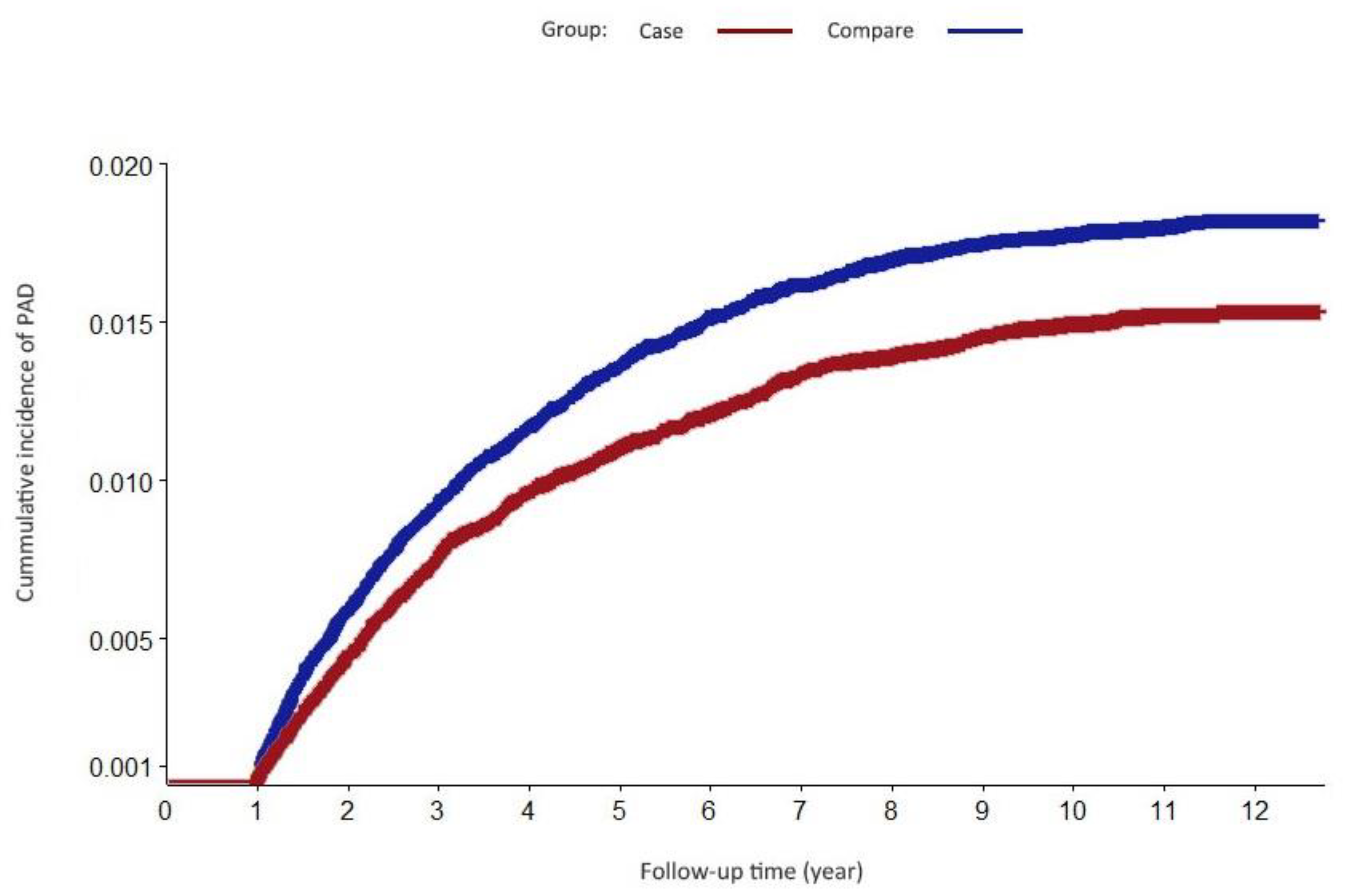
| PAD | 1105 | 1.71 | 172.9 | 898 | 1.39 | 145.5 |
| Case vs. Comparison | ||||||
| Crude Hazard Ratio (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |||
| PAD | 1.21 (1.10–1.32) | <0.001 | 1.18(1.08–1.29) | <0.001 | ||
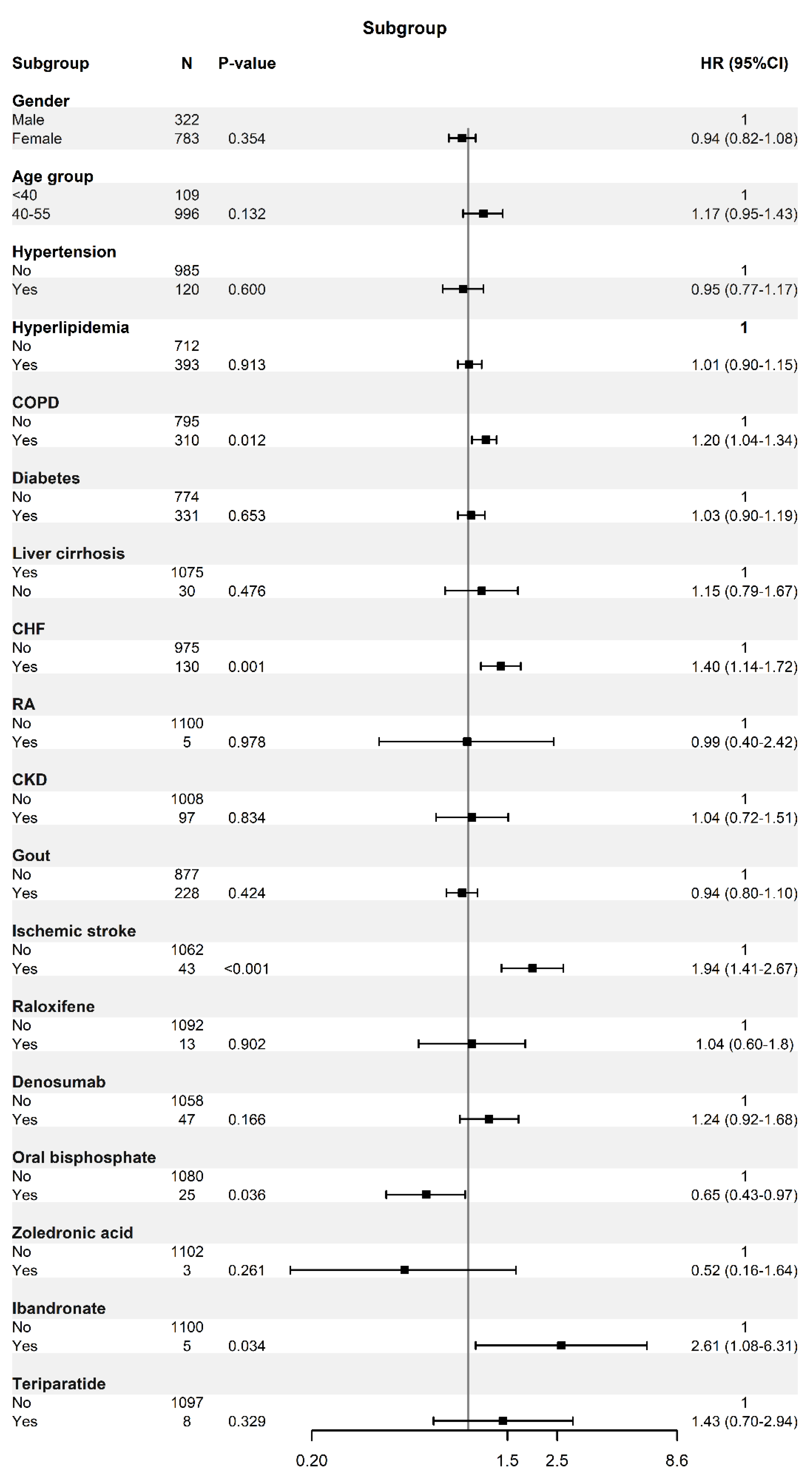
| PAD | ||
|---|---|---|
| aHR (95% CI) | p-Value | |
| Gender | <0.001 | |
| Male | 1 | |
| Female | 0.658 (0.595–0.727) | |
| Age | <0.001 | |
| 49–55 | 1 | |
| ≥55 | 1.729 (1.497–1.995) | |
| Hypertension | 0.5842 | |
| No | 1 | |
| Yes | 1.045 (0.893–1.223) | |
| Hyperlipidemia | 0.3151 | |
| No | 1 | |
| Yes | 0.949 (0.857–1.051) | |
| COPD | 0.2621 | |
| No | 1 | |
| Yes | 0.942 (0.848–1.046) | |
| Diabetes | <0.001 | |
| No | 1 | |
| Yes | 1.291 (1.162–1.434) | |
| Liver cirrhosis | 0.7197 | |
| No | 1 | |
| Yes | 0.94 (0.669–1.32) | |
| CHF | 0.1491 | |
| No | 1 | |
| Yes | 1.12 (0.96–1.307) | |
| RA | 0.0034 | |
| No | 1 | |
| Yes | 2.664 (1.382–5.135) | |
| CKD | 0.0029 | |
| No | 1 | |
| Yes | 1.558 (1.163–2.086) | |
| Gout | 0.1728 | |
| No | 1 | |
| Yes | 1.086 (0.964–1.224) | |
| Ischemic stroke or SE | 0.3575 | |
| No | 1 | |
| Yes | 0.887 (0.687–1.145) | |
| Raloxifene | 0.3124 | |
| No | 1 | |
| Yes | 0.815 (0.548–1.212) | |
| Denosumab | 0.2987 | |
| No | 1 | |
| Yes | 1.132 (0.896–1.432) | |
| Oral bisphosphate | 0.2789 | |
| No | 1 | |
| Yes | 1.151 (0.892–1.485) | |
| Zoledronic acid | 0.8917 | |
| No | 1 | |
| Yes | 0.969 (0.612–1.533) | |
| Ibandronate | 0.8755 | |
| No | 1 | |
| Yes | 0.955 (0.538–1.697) | |
| Teriparatide | 0.1937 | |
| No | 1 | |
| Yes | 1.433 (0.833–2.465) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syu, D.-K.; Hsu, S.-H.; Yeh, P.-C.; Lee, T.-L.; Kuo, Y.-F.; Huang, Y.-C.; Jiang, C.-C.; Chen, M. The Association between Osteoporosis and Peripheral Artery Disease: A Population-Based Longitudinal Follow-Up Study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 11327. https://doi.org/10.3390/ijerph191811327
Syu D-K, Hsu S-H, Yeh P-C, Lee T-L, Kuo Y-F, Huang Y-C, Jiang C-C, Chen M. The Association between Osteoporosis and Peripheral Artery Disease: A Population-Based Longitudinal Follow-Up Study in Taiwan. International Journal of Environmental Research and Public Health. 2022; 19(18):11327. https://doi.org/10.3390/ijerph191811327
Chicago/Turabian StyleSyu, De-Kai, Shu-Hua Hsu, Ping-Chun Yeh, Tsung-Lin Lee, Yu-Feng Kuo, Yen-Chun Huang, Ching-Chuan Jiang, and Mingchih Chen. 2022. "The Association between Osteoporosis and Peripheral Artery Disease: A Population-Based Longitudinal Follow-Up Study in Taiwan" International Journal of Environmental Research and Public Health 19, no. 18: 11327. https://doi.org/10.3390/ijerph191811327
APA StyleSyu, D.-K., Hsu, S.-H., Yeh, P.-C., Lee, T.-L., Kuo, Y.-F., Huang, Y.-C., Jiang, C.-C., & Chen, M. (2022). The Association between Osteoporosis and Peripheral Artery Disease: A Population-Based Longitudinal Follow-Up Study in Taiwan. International Journal of Environmental Research and Public Health, 19(18), 11327. https://doi.org/10.3390/ijerph191811327






