Socioeconomic Inequalities and Vaccine Uptake: An Umbrella Review Protocol
Abstract
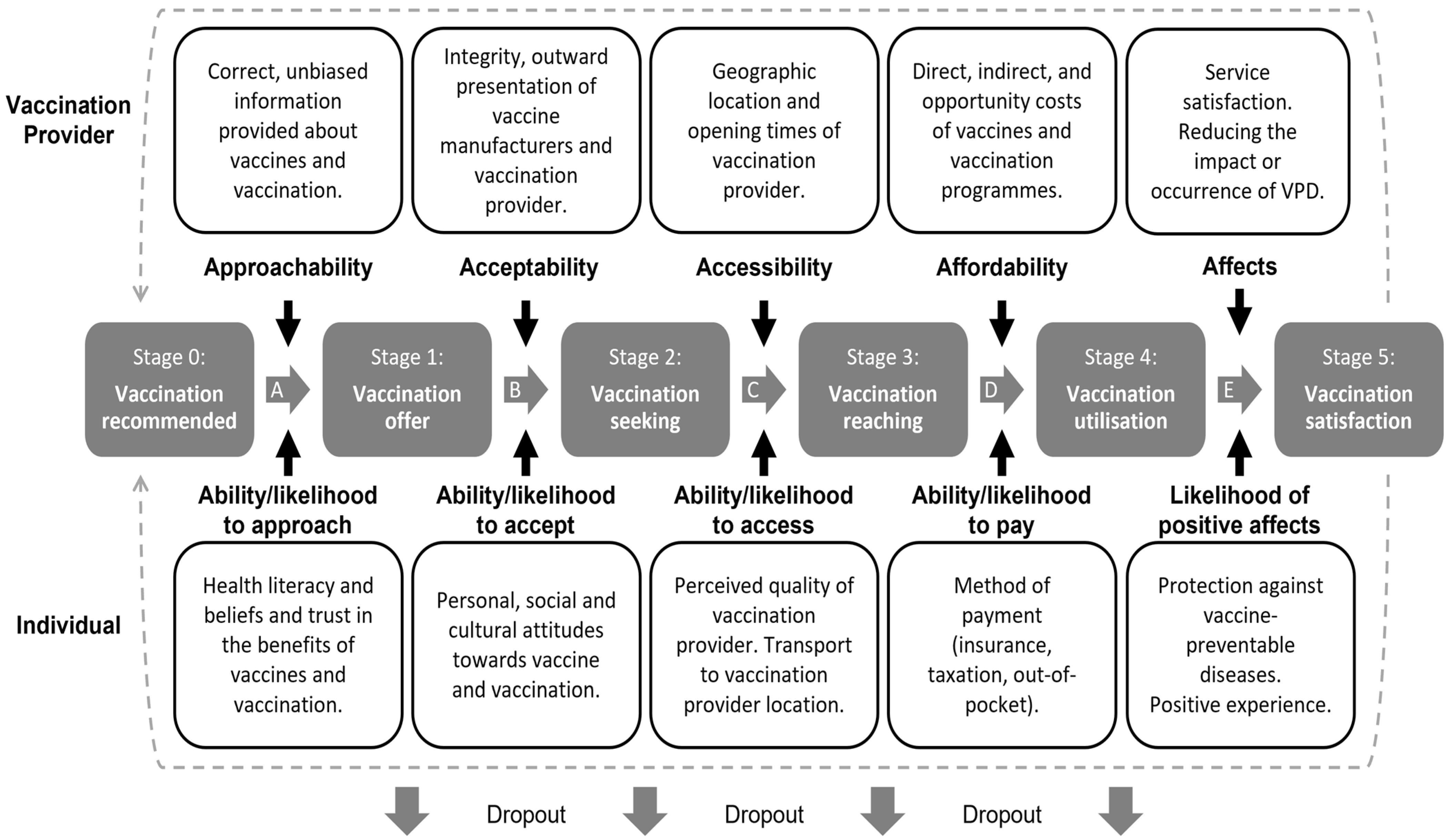
:1. Introduction
2. Methods
2.1. Research Questions
- Primary: Are there socioeconomic inequalities in vaccine uptake?If so, which vaccines, countries, and measures of socioeconomic status are affected?
- Secondary: Are any potentially impactful mechanisms or pathways of socioeconomic inequalities in vaccine uptake identified?If so, what mechanisms or pathways are identified?
2.2. Inclusion Criteria
- Population: All countries, and demographical and social groups, will be eligible for inclusion.
- Interventions: The intervention, or phenomena of interest, are WHO universally recommended routine vaccinations. All WHO recommended routine vaccines will be considered (see Table 2 for more information on what constitutes a routine vaccination), including influenza and COVID-19, to account for reviews published in response to the Coronavirus pandemic.
- Comparison: Systematic reviews will be included irrespective of whether their primary studies had controls or not. Control groups may include randomised or matched designs.
- Outcomes: Variation in the rate or proportion of a target population which have been vaccinated, according to socioeconomic determinants. The SE determinants will be: the level of education, occupational classification, measures of area-level deprivation (e.g., the English Indices of Multiple Deprivation [54]), and income.
- Study Design: Only systematic reviews or studies which attempt to synthesise quantitative or qualitative primary studies will be included. The quantitative reviews do not have to include a meta-analysis.
- Were inclusion/exclusion criteria reported?
- Was the search adequate?
- Were the included studies synthesised?
- Was the quality of the included studies assessed?
- Are sufficient details about the individual included studies presented?
2.3. Information Sources and Search Strategy
2.4. Screening and Selection
2.5. Data Extraction
2.6. Quality Appraisal
2.7. Dealing with Overlap
3. Results
3.1. Synthesis
3.2. Pilot Search
- Pilot search 1 consisted of the study design, population, and intervention elements of the search strategy. The search returned 2087 results, of which all nine key papers were included (see supplementary material Annex S8).
- Pilot search 2 consisted of the study design, population, intervention, and outcome elements of the search strategy. The searched returned 1090 results, of which all nine key papers were included (see supplementary material Annex S9).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Environmental Programme. Escaping the ‘Era of Pandemics’: Experts Warn Worse Crises to Come Options Offered to Reduce Risk; UN Environment Programme: Bonn, Germany, 2020; Available online: https://www.unep.org/news-and-stories/press-release/escaping-era-pandemics-experts-warn-worse-crises-come-options (accessed on 15 June 2022).
- SAGE Working Group. Report of the SAGE Working Group of Vaccine Hesitancy. 2014. Available online: https://www.asset-scienceinsociety.eu/sites/default/files/sage_working_group_revised_report_vaccine_hesitancy.pdf (accessed on 15 June 2022).
- Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 15 June 2022).
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Graham, H. Unequal Lives: Health and Socioeconomic Inequalities; Open University Press: Maidenhead, UK, 2007. [Google Scholar]
- Welch, V.; Petticrew, M.; Tugwell, P.; White, H.; PRISMA-Equity Bellagio Group. PRISMA-Equity 2012 extension: Reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012, 9, e1001333. [Google Scholar] [CrossRef] [PubMed]
- Levesque, J.F.; Harris, M.F.; Russell, G. Patient-centred access to health care: Conceptualising access at the interface of health systems and populations. Int. J. Equity Health 2013, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Moscelli, G.; Siciliani, L.; Gutacker, N.; Cookson, R. Socioeconomic inequality of access to healthcare: Does choice explain the gradient? J. Health Econ. 2018, 57, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Booysen, F.; Mbonigaba, J. Socio-economic inequalities in the multiple dimensions of access to healthcare: The case of South Africa. BMC Public Health 2020, 20. [Google Scholar] [CrossRef]
- Walters, S.; Suhrcke, M. Socioeconomic Inequalities in Health and Health Care Access in Central and Eastern Europe and the CIS: A Review of the Recent Literature; WHO European Office for Investment for Health and Development: Geneva, Switzerland, 2005; p. 1. Available online: https://www.euro.who.int/__data/assets/pdf_file/0006/125457/e94412.pdf (accessed on 17 June 2022).
- O’Neill, J.; Tabish, H.; Welch, V.; Petticrew, M.; Pottie, K.; Clarke, M.; Evans, T.; Pardo Pardo, J.; Waters, E.; White, H.; et al. Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J. Clin. Epidemiol. 2014, 67, 56–64. [Google Scholar] [CrossRef]
- Norman, C.; Wildman, J.M.; Sowden, S. COVID-19 at the Deep End: A Qualitative Interview Study of Primary Care Staff Working in the Most Deprived Areas of England during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 8689. [Google Scholar] [CrossRef]
- PROGRESS-Plus. Available online: https://methods.cochrane.org/equity/projects/evidence-equity/progress-plus (accessed on 15 June 2022).
- Coronavirus and Vaccination Rates in People Aged 18 Years or Over by Socio-Demographic Characteristic and Region, England; Office for National Statistics: London, UK, 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthinequalities/datasets/coronavirusandvaccinationratesinpeopleaged18yearsandoverbysociodemographiccharacteristicandregionengland (accessed on 28 July 2022).
- Wu, A.C.; Wisler-Sher, D.J.; Griswold, K.; Colson, E.; Shapiro, E.D.; Holmboe, E.S. Postpartum mothers’ attitudes, knowledge, and trust regarding vaccination. Matern. Child Health J. 2008, 12, 766–773. [Google Scholar] [CrossRef]
- Antai, D. Faith and child survival: The role of religion in childhood immunization in Nigeria. J. Biosoc. Sci. 2009, 41, 57–76. [Google Scholar] [CrossRef]
- Antai, D. Gender inequities, relationship power, and childhood immunization uptake in Nigeria: A population-based cross-sectional study. Int. J. Infect. Dis. 2012, 16, e136–e145. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Obaida-Nasrin, S. Factors affecting acceptance of complete immunization coverage of children under five years in rural Bangladesh. Salud Publica Mex. 2010, 52, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Mullooly, J.P.; Goodman, M.; McCarty, M.C.; Hanson, A.M.; Crane, B.; Nordin, J.D. Identification and characteristics of vaccine refusers. BMC Pediatr. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.; Simboro, S.; Kouyaté, B.; Dugas, M.; Graham, J.; Bibeau, G. Assessment of factors associated with complete immunization coverage in children aged 12-23 months: A cross-sectional study in Nouna district, Burkina Faso. BMC Int. Health Hum. Rights 2009, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Fournier, P.; Kobiane, J.F.; Sondo, B.K. Rates of coverage and determinants of complete vaccination of children in rural areas of Burkina Faso (1998–2003). BMC Public Health 2009, 9. [Google Scholar] [CrossRef]
- Patra, N. A probe into the ways to stimulate immunisation in India: Findings from National Family Health Survey-III. IJCP 2012, 5, 65–84. [Google Scholar]
- Babalola, S. Maternal reasons for non-immunisation and partial immunisation in northern Nigeria. J. Paediatr. Child Health 2011, 47, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, R.; Adedokun, B.; Lawoyin, T. Children not receiving adequate immunization in Ibadan, Nigeria: What reasons and beliefs do their mothers have? Niger. J. Clin. Pract. 2010, 13, 173–178. [Google Scholar]
- Kumar, D.; Aggarwal, A.; Gomber, S. Immunization status of children admitted to a tertiary-care hospital of north India: Reasons for partial immunization or non-immunization. J. Health Popul. Nutr. 2010, 28, 300–304. [Google Scholar] [CrossRef]
- Patel, T.; Pandit, N. Why infants miss vaccination during routine immunization sessions? Study in a rural area of Anand district, Gujarat. Indian J. Public Health 2011, 55, 321–323. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, Y.; Zhang, J.X.; Kang, C.Y.; Duan, P. Status of mother’s KAP on child immunization in minority areas, Guizhou Province. Beijing Da Xue Xue Bao Yi Xue Ban 2007, 39, 136–139. [Google Scholar]
- Akmatov, M.K.; Mikolajczyk, R.T.; Kretzschmar, M.; Krämer, A. Attitudes and beliefs of parents about childhood vaccinations in post-soviet countries: The example of Kyrgyzstan. Pediatr. Infect Dis. J. 2009, 28, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, M.S.; Irigoyen, M.; Martinez, R.A.; Findley, S. How Parents’ Negative Experiences at Immunization Visits Affect Child Immunization Status in a Community in New York City. Public Health Rep. 2011, 126, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mapatano, M.; Kayembe, K.; Piripiri, L.; Nyandwe, K. Immunisation-related knowledge, attitudes and practices of mothers in Kinshasa, Democratic Republic of the Congo. SA Fam. Pract. 2008, 50, 61. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Frimpong, J.A.; Rivers, P.A.; Kronenfeld, J.J. Effects of Maternal and Provider Characteristics on Up-to-Date Immunization Status of Children Aged 19 to 35 Months. Am. J. Public Health 2007, 97, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yin, Z.; Suraratdecha, C.; Liu, X.; Li, Y.; Hills, S.; Zhang, K.; Chen, Y.; Liang, X. Knowledge, attitudes and practices of caregivers regarding Japanese encephalitis in Shaanxi Province, China. Public Health 2011, 125, 79–83. [Google Scholar] [CrossRef]
- Sinno, D.D.; Shoaib, H.A.; Musharrafieh, U.M.; Hamadeh, G.N. Prevalence and predictors of immunization in a health insurance plan in a developing country. Pediatr. Int. 2009, 51, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, K.; El-Hai, R.A.; Amit-Aharon, A.; Nehama, H.; Gondia, M.; Davidovitch, N.; Goren, S.; Cohen, D. Risk factors of underutilization of childhood immunizations in ultraorthodox Jewish communities in Israel despite high access to health care services. Vaccine 2012, 30, 2109–2115. [Google Scholar] [CrossRef]
- Phukan, R.K.; Barman, M.P.; Mahanta, J. Factors Associated with immunization coverage of children in Assam, India: Over the first year of life. J. Trop Pediatr. 2009, 55, 249–252. [Google Scholar] [CrossRef]
- Chhabra, P.; Nair, P.; Gupta, A.; Sandhir, M.; Kannan, A.T. Immunization in urbanized villages of Delhi. Indian J. Pediatr. 2007, 74, 131–134. [Google Scholar] [CrossRef]
- Rammohan, A.; Awofeso, N.; Fernandez, R.C. Paternal education status significantly influences infants’ measles vaccination uptake, independent of maternal education status. BMC Public Health 2012, 12, 336. [Google Scholar] [CrossRef]
- Vikram, K.; Vanneman, R.; Desai, S. Linkages between maternal education and childhood immunization in India. Soc. Sci Med. 2012, 75, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Danis, K.; Georgakopoulou, T.; Stavrou, T.; Laggas, D.; Panagiotopoulos, T. Socioeconomic factors play a more important role in childhood vaccination coverage than parental perceptions: A cross-sectional study in Greece. Vaccine 2012, 28, 1861–1869. [Google Scholar] [CrossRef]
- Uwemedimo, O.T.; Findley, S.E.; Andres, R.; Irigoyen, M.; Stockwell, M.S. Determinants of Influenza Vaccination Among Young Children in an Inner-City Community. J. Community Health 2012, 37, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, R.E.; Lawoyin, T.O.; Adedokun, B.O. Immunization status and its determinants among children of female traders in Ibadan, South-Western Nigeria. Afr. J. Med. Med. Sci. 2009, 38, 9–15. [Google Scholar] [PubMed]
- Mitchell, S.; Andersson, N.; Ansari, N.M.; Omer, K.; Soberanis, J.L.; Cockcroft, A. Equity and vaccine uptake: A cross-sectional study of measles vaccination in Lasbela District, Pakistan. BMC Int. Health Hum. Rights 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, N.; Siddiqi, A.E.A.; Nisar, N.; Khan, A. Mothers’ knowledge about EPI and its relation with age-appropriate vaccination of infants in peri-urban Karachi. J. Pak. Med. Assoc. 2010, 60, 940–944. [Google Scholar] [PubMed]
- Arat, A.; Burström, B.; Östberg, V.; Hjern, A. Social inequities in vaccination coverage among infants and pre-school children in Europe and Australia—A systematic review. BMC Public Health 2019, 19. [Google Scholar] [CrossRef]
- Jeudin, P.; Liveright, E.; del Carmen, M.G.; Perkins, R.B. Race, ethnicity and income as factors for HPV vaccine acceptance and use. Hum. Vaccin Immunother. 2013, 9, 1413–1420. [Google Scholar] [CrossRef]
- Fernández de Casadevante, V.; Gil Cuesta, J.; Cantarero-Arévalo, L. Determinants in the Uptake of the Human Papillomavirus Vaccine: A Systematic Review Based on European Studies. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef]
- Glymour, M.M.; Avendano, M.; Kawachi, I. Socioeconomic Status and Health. In Social Epidemiology, 2nd ed.; Berkman, L., Kawachi, I., Glymour, M., Eds.; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Sun, X. World Health Systems; John Wiley & Sons Incorporated: Hoboken, NJ, USA, 2019. [Google Scholar]
- Fisher, H.; Trotter, C.L.; Audrey, S.; MacDonald-Wallis, K.; Hickman, M. Inequalities in the uptake of human papillomavirus vaccination: A systematic review and meta-analysis. Int. J. Epidemiol. 2013, 42, 896–908. [Google Scholar] [CrossRef]
- Glossary: Out-of-of-Pocket Expenditure on Healthcare. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Out-of-pocket_expenditure_on_healthcare#:~:text=Household%20out%2Dof%2Dpocket%20payment,the%20use%20of%20the%20services (accessed on 16 June 2022).
- Martinic, K.M.; Pieper, D.; Glatt, A.; Puljak, L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med. Res. Methodol. 2019, 19, 203. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Tsagris, M.; Fragkos, K.C. Umbrella Reviews, Overviews of Reviews, and Meta-epidemiologic Studies: Similarities and Differences. In Umbrella Reviews: Evidence Synthesis with Overviews of Reviews and Meta-Epidemiologic Studies; Biondi-Zoccai, G., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- English Indices of Deprivation. Available online: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed on 7 June 2022).
- Database of Abstracts of Reviews of Effects (DARE): Quality Assessed Reviews. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285222/ (accessed on 16 June 2022).
- World Health Organization. Global Vaccine Action Plan. 2011–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/global-vaccine-action-plan-2011-2020 (accessed on 16 June 2022).
- World Health Organization. Table 1: Summary of WHO Position Papers—Recommendations for Routine Immunization; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/who-recommendations-for-routine-immunization---summary-tables (accessed on 16 June 2022).
- Rayyan; Rayyan Systems, Inc.: Cambridge, UK, 2016; Available online: https://www.rayyan.ai/ (accessed on 16 June 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, 4008. [Google Scholar] [CrossRef] [PubMed]
- Lunny, C.; Pieper, D.; Thabet, P.; Kanji, S. Managing overlap of primary study results across systematic reviews: Practical considerations for authors of overviews of reviews. BMC Med. Res. Methodol. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.; Fernandes, R.; Becker, L.; Pieper, D.; Hartling, L. Chapter V: Overviews of Reviews. In Cochrane Handbook for Systematic Reviews of Interventions, 6.3; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Available online: https://training.cochrane.org/handbook/current/chapter-v (accessed on 16 June 2022).
- Campbell, M.; McKenzie, J.; Sowden, A.; Katikireddi, S.; Brennan, S.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting. BMJ 2020, 368, I6890. [Google Scholar] [CrossRef] [PubMed]
- Crocker-Buque, T.; Mindra, G.; Duncan, R.; Mounier-Jack, S. Immunization, urbanization and slums—A systematic review of factors and interventions. BMC Public Health 2017, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.E.; Kadokura, E.; Eckert, L.O.; Miyake, S.; Mounier-Jack, S.; Aldea, M.; Ross, D.A.; Watson-Jones, D. Factors influencing completion of multi-dose vaccine schedules in adolescents: A systematic review. BMC Public Health 2016, 16, 1–17. [Google Scholar] [CrossRef]
- Tabacchi, G.; Costantino, C.; Napoli, G.; Marchese, V.; Cracchiolo, M.; Casuccio, A.; Vitale, F.; The Esculapio Working Group. Determinants of European parents’ decision on the vaccination of their children against measles, mumps and rubella: A systematic review and meta-analysis. Hum. Vaccin Immunother. 2016, 12, 1909–1923. [Google Scholar] [CrossRef]
- Forshaw, J.; Gerver, S.M.; Gill, M.; Cooper, E.; Manikam, L.; Ward, H. The global effect of maternal education on complete childhood vaccination: A systematic review and meta-analysis. BMC Infect Dis. 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Bocquier, A.; Wardm, J.; Raude, J.; Peretti-Watel, P.; Verger, P. Socioeconomic differences in childhood vaccination in developed countries: A systematic review of quantitative studies. Expert Rev. Vaccine 2017, 16, 1107–1118. [Google Scholar] [CrossRef]
- Study Design Search Filters. Available online: https://bestpractice.bmj.com/info/toolkit/learn-ebm/study-design-search-filters/ (accessed on 16 June 2022).
- Todd, A.; Bambra, C. Learning from past mistakes? The COVID-19 vaccine and the inverse equity hypothesis. Eur. J. Public Health 2021, 31, 2. [Google Scholar] [CrossRef]
| Barrier | Promoter | |
|---|---|---|
| Low income/SES | USA [15] Nigeria [16] | Nigeria [17] Bangladesh [18] |
| High income/SES | USA [19] | Burkina Faso [20,21] India [22] Bangladesh [18] |
| Low education | Nigeria [16,17,23,24] India [25,26] China [27] Kyrgyzstan [28] USA [29] DR Congo [30] | USA [31] |
| High education | China [32] Lebanon [33] Israel [34] Bangladesh [18] USA [19] DR Congo [30] | India [22,25,35,36,37,38] Greece [39] The Netherlands [40] Nigeria [41] Pakistan [42,43] |
| Inclusion | Exclusion |
|---|---|
| Access to the full text. | |
| Reviews published after 2011–present day. Any language (interpreters will be sourced if required). | |
Inclusion: Population
| Exclusion: Population
|
Inclusion: Intervention
| Exclusion: Intervention
|
Inclusion: Comparison
| Exclusions: Comparison
|
Inclusion: Outcome
| Exclusion: Outcome
|
Inclusion: Study Design
| Exclusion: Study Design
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacre, A.; Bambra, C.; Wildman, J.M.; Thomson, K.; Sowden, S.; Todd, A. Socioeconomic Inequalities and Vaccine Uptake: An Umbrella Review Protocol. Int. J. Environ. Res. Public Health 2022, 19, 11172. https://doi.org/10.3390/ijerph191811172
Sacre A, Bambra C, Wildman JM, Thomson K, Sowden S, Todd A. Socioeconomic Inequalities and Vaccine Uptake: An Umbrella Review Protocol. International Journal of Environmental Research and Public Health. 2022; 19(18):11172. https://doi.org/10.3390/ijerph191811172
Chicago/Turabian StyleSacre, Amber, Clare Bambra, Josephine M. Wildman, Katie Thomson, Sarah Sowden, and Adam Todd. 2022. "Socioeconomic Inequalities and Vaccine Uptake: An Umbrella Review Protocol" International Journal of Environmental Research and Public Health 19, no. 18: 11172. https://doi.org/10.3390/ijerph191811172
APA StyleSacre, A., Bambra, C., Wildman, J. M., Thomson, K., Sowden, S., & Todd, A. (2022). Socioeconomic Inequalities and Vaccine Uptake: An Umbrella Review Protocol. International Journal of Environmental Research and Public Health, 19(18), 11172. https://doi.org/10.3390/ijerph191811172






