Pain Assessment with the BPS and CCPOT Behavioral Pain Scales in Mechanically Ventilated Patients Requiring Analgesia and Sedation
Abstract
1. Introduction
2. Material and Methods
3. Statistical Analysis Methods
4. Results
5. Discussion
6. Study Limitations
7. Implications to Practice
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, 825–873. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.B.; Marcon, S.S.; de Macedo, C.R.L.; Jorge, A.C.; Duarte, P.A.D. Sedation and memories of patients subjected to mechanical ventilation in an intensive care unit. Rev. Bras. De Ter. Intensiva 2014, 26, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Reade, M.C.; Finfer, S. Sedation and Delirium in the Intensive Care Unit. New Engl. J. Med. 2014, 370, 444–454. [Google Scholar] [CrossRef]
- Ayasrah, S. Care-related pain in critically ill mechanically ventilated patients. Anaesth. Intensive Care 2016, 44, 458–465. [Google Scholar] [CrossRef]
- Arroyo-Novoa, C.M.; Milagros, I.; Figueroa-Ramos, R.N.; Puntillo, K. Occurrence and Practices for Pain, Agitation and Delirium in Intensive Care Unit Patients. Puerto Rico Health Sci. J. 2019, 38, 156–162. [Google Scholar]
- Olsen, B.F.; Valeberg, B.; Jacobsen, M.; Småstuen, M.; Puntillo, K.; Rustøen, T. Pain in intensive care unit patients—A longitudinal study. Nurs. Open 2021, 8, 224–231. [Google Scholar] [CrossRef]
- Chanques, G.; Pohlman, A.; Kress, J.P.; Molinari, N.; de Jong, A.; Jaber, S.; Hall, J.B. Psychometric comparison of three behavioural scales for the assessment of pain in critically ill patients unable to self-report. Crit. Care 2014, 18, R160. [Google Scholar] [CrossRef]
- Puntillo, K.A.; Max, A.; Timsit, J.-F.; Vignoud, L.; Chanques, G.; Robleda, G.; Roche-Campo, F.; Mancebo, J.; Divatia, J.V.; Soares, M.; et al. Determinants of Procedural Pain Intensity in the Intensive Care Unit. The Europain® Study. Am. J. Respir. Crit. Care Med. 2014, 189, 39–47. [Google Scholar] [CrossRef]
- Gélinas, C.; Boitor, M.; Puntillo, K.A.; Arbour, C.; Topolovec-Vranic, J.; Cusimano, M.D.; Choinière, M.; Streiner, D.L. Behaviors Indicative of Pain in Brain-Injured Adult Patients With Different Levels of Consciousness in the Intensive Care Unit. J. Pain Symptom Manag. 2019, 57, 761–773. [Google Scholar] [CrossRef]
- Rijkenberg, S.; van der Voort, P.H. Can the critical-care pain observation tool (CPOT) be used to assess pain in delirious ICU patients? J. Thorac. Dis. 2016, 8, e285. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Ely, E.W.; Pandharipande, P.P.; Patel, M.B. The ABCDEF Bundle in Critical Care. Crit. Care Clin. 2017, 33, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Kirankumar, D.H. Behavioural pain scale to assess pain in sedated and conscious patients. Int. J. Med. Biomed. Stud. 2019, 3, 31–34. [Google Scholar] [CrossRef]
- Damico, V.; Macchi, G.; Murano, L.; Forastieri Molinari, A. Incidence of pain at rest and during nursing procedures in ICU patients: A longitudinal observational study. Ann. Ig. 2020, 32, 407–418. [Google Scholar] [PubMed]
- Payen, J.-F.; Bosson, J.-L.; Chanques, G.; Mantz, J.; Labarere, J.; for the DOLOREA Investigators. Pain Assessment Is Associated with Decreased Duration of Mechanical Ventilation in the Intensive Care Unit: A Post HocAnalysis of the DOLOREA Study. Anesthesiology 2009, 111, 1308–1316. [Google Scholar] [CrossRef]
- Olsen, B.F.; Rustøen, T.; Sandvik, L.; Miaskowski, C.; Jacobsen, M.; Valeberg, B.T. Development of a pain management algorithm for intensive care units. Hear. Lung 2015, 44, 521–527. [Google Scholar] [CrossRef]
- Kotfis, K.; Zegan-Barańska, M.; Żukowski, M.; Kusza, K.; Kaczmarczyk, M.; Ely, E.W. Multicenter assessment of sedation and delirium practices in the intensive care units in Poland—Is this common practice in Eastern Europe? BMC Anesthesiol. 2017, 2, 120. [Google Scholar] [CrossRef]
- Pandharipande, P.; Ely, E.; Arora, R.; Balas, M.; Boustani, M.; La Calle, G.; Cunningham, C.; Devlin, J.; Elefante, J.; Han, J.; et al. The intensive care delirium research agenda: A multinational, interprofessional perspective. Intensive Care Med. 2017, 43, 1329–1339. [Google Scholar] [CrossRef]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef]
- Barnes-Daly, M.A.; Phillips, G.; Ely, E.W. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: Implementing PAD guidelines via the ABCDEF bundle in 6064 patients. Crit. Care Med. 2017, 45, 171–178. [Google Scholar] [CrossRef]
- Vincent, J.L. Optimizing sedation in the ICU: The eCASH concept. Signa Vitae 2017, 13, 10–13. [Google Scholar] [CrossRef][Green Version]
- Baron, R.; Binder, A.; Biniek, R.; Braune, S.; Buerkle, H.; Dall, P.; Demirakca, S.; Eckardt, R.; Eggers, V.; Eichler, I.; et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)–short version. GMS Ger. Med. Sci. 2015, 13, 1–42. [Google Scholar]
- Misiołek, H.; Zajączkowska, R.; Daszkiewicz, A.; Woroń, J.; Dobrogowski, J.; Wordliczek, J.; Owczuk, R. Postępowanie w bólu pooperacyjnym 2018: Stanowisko Sekcji Znieczulenia Regionalnego i Terapii Bólu Polskiego Towarzystwa Anestezjologii i Intensywnej Terapii, Polskiego Towarzystwa Znieczulenia Regionalnego i Leczenia Bólu, Polskiego Towarzystwa Badania Bólu oraz Konsultanta Krajowego w dziedzinie anestezjologii i intensywnej terapii. Anestezjol. Intensywna Ter. 2018, 50, 175–203. [Google Scholar]
- Kotfis, K.; Zegan-Barańska, M.; Strzelbicka, M.; Safranow, K.; Żukowski, M.; Ely, E.W.; the POL-CPOT Study Group. Validation of the Polish version of the Critical Care Pain Observation Tool (CPOT) to assess pain intensity in adult, intubated intensive care unit patients: The POL-CPOT study. Arch. Med. Sci. 2018, 14, 880–889. [Google Scholar] [CrossRef]
- Gutysz-Wojnicka, A.; Ozga, D.; Mayzner-Zawadzka, E.; Dyk, D.; Majewski, M.; Doboszyńska, A. Psychometric Assessment of Physiologic and Behavioral Pain Indicators in Polish Versions of the Pain Assessment Scales. Pain Manag. Nurs. 2019, 20, 292–301. [Google Scholar] [CrossRef]
- Chanques, G.; Tarri, T.; Ride, A.; Prades, A.; De Jong, A.; Carr, J.; Molinari, N.; Jaber, S. Analgesia nociception index for the assessment of pain in critically ill patients: A diagnostic accuracy study. Br. J. Anaesth. 2017, 119, 812–820. [Google Scholar] [CrossRef]
- Gélinas, C.; Fillion, L.; Puntillo, K.A.; Viens, C.; Fortier, M. Validation of the critical-care pain observation tool in adult patients. Am. J. Crit. Care 2006, 15, 420–427. [Google Scholar] [CrossRef]
- Wøien, H. Movements and trends in intensive care pain treatment and sedation: What matters to the patient? J. Clin. Nurs. 2020, 29, 1129–1140. [Google Scholar] [CrossRef]
- Severgnini, P.; Pelosi, P.; Contino, E.; Serafinelli, E.; Novario, R.; Chiaranda, M. Accuracy of critical care pain observation tool and behavioral pain scale to assess pain in critically ill conscious and unconscious patients: Prospective, observational study. J. Intensive Care. 2016, 4, 68. [Google Scholar] [CrossRef]
- Al Darwish, Z.Q.; Hamdi, R.; Fallatah, S. Evaluation of pain assessment tools in patients receiving mechanical ventilation. AACN Adv. Crit. Care 2016, 27, 162–172. [Google Scholar] [CrossRef]
- Joffe, A.M.; McNulty, B.; Boitor, M.; Marsh, R.; Gélinas, C. Validation of the Critical-Care Pain Observation Tool in brain-injured critically ill adults. J. Crit. Care 2016, 36, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Echegaray-Benites, C.; Kapoustina, O.; Gélinas, C. Validation of the use of the Critical-Care Pain Observation Tool (CPOT) with brain surgery patients in the neurosurgical intensive care unit. Intensive Crit. Care Nurs. 2014, 30, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sulla, F.; De Souza Ramos, N.; Terzi, N.; Trenta, T.; Uneddu, M.; Zaldivar Cruces, M.A.; Sarli, L. Validation of the Italian version of the Critical Pain Observation Tool in brain-injured critically ill adults. Acta Biomed. 2017, 30, 48–54. [Google Scholar]
- Kanji, S.; MacPhee, H.; Singh, A.; Johanson, C.; Fairbairn, J.; Lloyd, T.; MacLean, R.; Rosenberg, E. Validation of the Critical Care Pain Observation Tool in Critically Ill Patients With Delirium: A Prospective Cohort Study. Crit. Care Med. 2016, 44, 943–947. [Google Scholar] [CrossRef]
- Puntillo, K.; Gélinas, C.; Chanques, G. Next steps in ICU pain research. Intensive Care Med. 2017, 43, 1386–1388. [Google Scholar] [CrossRef]
- Vadelka, A.; Busnelli, A.; Bonetti, L. Comparison between two behavioural scales for the evaluation of pain in critical patients, as related to the state of sedation: An observational study. Scenario 2017, 34, 4–14. [Google Scholar]
- Pudas-Tähkä, S.-M.; Axelin, A.; Aantaa, R.; Lund, V.; Salanterä, S. Pain assessment tools for unconscious or sedated intensive care patients: A systematic review. J. Adv. Nurs. 2009, 65, 946–956. [Google Scholar] [CrossRef]
- Mehta, S.; McCullagh, I.; Burry, L. Current sedation practices: Lessons learned from international surveys. Anesthesiol. Clin. 2011, 29, 607–624. [Google Scholar] [CrossRef]
- Hughes, C.G.; McGrane, S.; Pandharipande, P.P. Sedation in the intensive care setting. Clin. Pharmacol. Adv. Appl. 2012, 4, 53–63. [Google Scholar]
- Park, S.Y.; Lee, H.B. Prevention and management of delirium in critically ill adult patients in the intensive care unit: A review based on the 2018 PADIS guidelines. Acute Crit. Care 2019, 34, 117–125. [Google Scholar] [CrossRef]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Balzer, F.; Weiß, B.; Kumpf, O.; Treskatsch, S.; Spies, C.; Wernecke, K.D.; Krannich, A.; Kastrup, M. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit. Care 2015, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Stylsvig, M.; Toft, P. Long-term psychological effects of a no-sedation protocol in critically ill patients. Crit. Care 2011, 15, R293. [Google Scholar] [CrossRef] [PubMed]
- Shehabi, Y.; Bellomo, R.; Reade, M.C.; Bailey, M.; Bass, F.; Howe, B.; McArthur, C.; Murray, L.; Seppelt, I.M.; Webb, S.; et al. for the Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators and the Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Early Goal-Directed Sedation Versus Standard Sedation in Mechanically Ventilated Critically Ill Patients: A Pilot Study. Crit. Care Med. 2013, 41, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Boncyk, C.; Nahrwold, D.A.; Hughes, C.G. Targeting light versus deep sedation for patients receiving mechanical ventilation. J. Emerg. Crit. Care Med. 2018, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Z.Y.; Zhou, W.H.; Hu, B.; Rao, X.; Li, J.G. A national multicenter survey on management of pain, agitation and delirium in intensive care units in China. Chin. Med. J. 2017, 130, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef]
- Egerod, I.; Albarran, J.W.; Ring, M.; Blackwood, B. Sedation practice in Nordic and non-Nordic ICUs: A European survey. Nurs. Crit. Care 2013, 18, 166–175. [Google Scholar] [CrossRef]
- Mansouri, P.; Javadpour, S.; Zand, F.; Ghodsbin, F.; Sabetian, G.; Masjedi, M.; Tabatabaee, H.R. Implementation of a protocol for integrated management of pain, agitation, and delirium can improve clinical outcomes in the intensive care unit: A randomized clinical trial. J. Crit. Care 2013, 28, 918–922. [Google Scholar] [CrossRef]
- Hager, D.N.; Dinglas, V.D.; Subhas, S.; Rowden, A.M.; Neufeld, K.J.; Bienvenu, O.J.; Touradji, P.; Colantuoni, E.; Reddy, D.R.; Brower, R.G.; et al. Reducing deep sedation and delirium in acute lung injury patients: A quality improvement project. Crit. Care Med. 2013, 41, 1435–1442. [Google Scholar] [CrossRef]
- Rijkenberg, S.; Stilma, W.; Endeman, H.; Bosman, R.J.; Oudemans-van Straaten, H.M. Pain measurement in mechanically ventilated critically ill patients: Behavioral Pain Scale versus Critical-Care Pain Observation Tool. J. Crit. Care 2015, 30, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Herr, K. Evaluation of Two Observational Pain Assessment Tools in Chinese Critically Ill Patients. Pain Med. 2015, 16, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Birkedal, H.C.; Larsen, M.H.; Steindal, S.A.; Solberg, M.T. Comparison of two behavioural pain scales for the assessment of procedural pain: A systematic review. Nurs. Open 2021, 8, 2050–2060. [Google Scholar] [CrossRef]
- Matthews, E.E. Sleep disturbances and fatigue in critically ill patients. AACN Adv. Crit. Care 2011, 22, 204–224. [Google Scholar] [CrossRef]
- Edvardsen, J.B.; Hetmann, F. Promoting Sleep in the Intensive Care Unit. SAGE Open Nurs. 2020, 6, 2377960820930209. [Google Scholar] [CrossRef]
- Pain Management in Intensive Care EfCCNa Recommendations. 2017. Available online: https://www.efccna.org/images/stories/publication/2017_PM_neu.pdf (accessed on 5 May 2022).
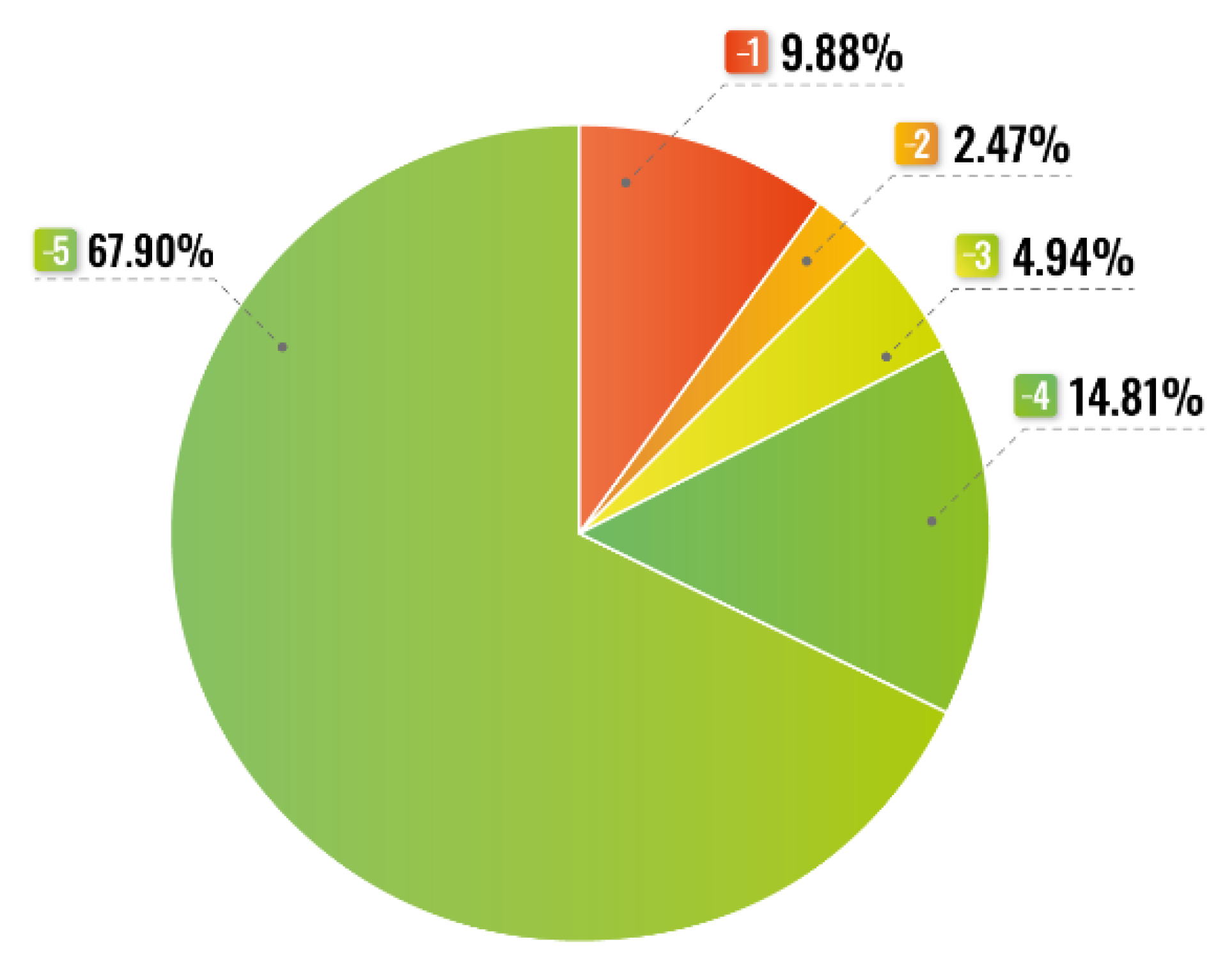
| Type of Drug | Dose i.v. |
|---|---|
| Oxycodone | 0.5–9.5 mg/h |
| Propofol | 10–300 mg/h |
| Midazolam | 1–30 mg/h |
| Dexmedetomidine | 0.008–0.18 mg/h |
| Thiopental | 70–240 mg/h |
| Ketamine | 10–250 mg/h |
| Pain Scale Scores | Before Intervention n (%) | During Intervention n (%) | After Intervention n (%) |
|---|---|---|---|
| BPS | |||
| <5 points | 959 (95.42%) | 678 (67.46%) | 937 (93.23%) |
| ≥5 points | 46 (4.58%) | 327 (32.54%) | 68 (6.77%) |
| CCPOT | |||
| <3 points | 970 (96.52%) | 706 (70.25%) | 958 (95.33%) |
| ≥3 points | 35 (3.48%) | 299 (29.75%) | 47 (4.67%) |
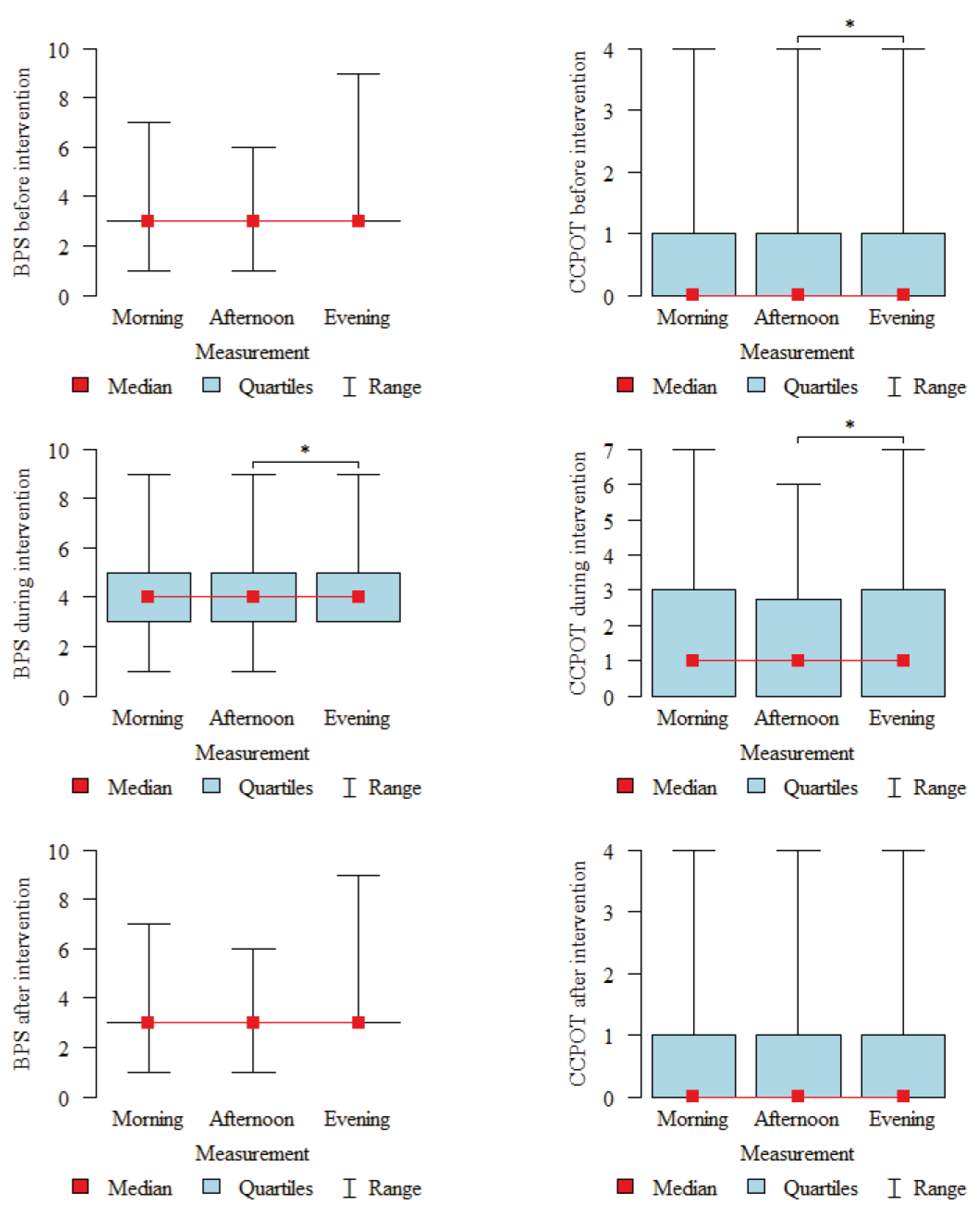
| Before Intervention (BI) | During Intervention (I) | After Intervention (AI) | p | |
|---|---|---|---|---|
| BPS | ||||
| mean ± SD | 3.2 ± 0.58 | 4.12 ± 1.37 | 3.25 ± 0.66 | p < 0.001 |
| median | 3 | 4 | 3 | |
| quartiles | 3–3 | 3–5 | 3–3 | I > BI,AI |
| CCPOT | ||||
| mean ± SD | 0.47 ± 0.78 | 1.66 ± 1.73 | 0.52 ± 0.85 | p < 0.001 |
| median | 0 | 1 | 0 | |
| quartiles | 0–1 | 0–3 | 0–1 | I > BI,AI |
| Parameter | RASS |
|---|---|
| Spearman Correlation Coefficient (R) | |
| BPS before intervention | R = 0.279, p = 0.012 * |
| BPS during intervention | R = 0.444, p < 0.001 * |
| BPS after intervention | R = 0.293, p = 0.008 * |
| CCPOT before intervention | R = 0.438, p < 0.001 * |
| CCPOT during intervention | R = 0.556, p < 0.001 * |
| CCPOT after intervention | R = 0.446, p < 0.001 * |
| Measurement | Spearman Correlation Coefficient (R) between BPS and CCPOT | p |
|---|---|---|
| Before intervention | 0.695 | p < 0.001 * |
| During intervention | 0.907 | p < 0.001 * |
| After intervention | 0.622 | p < 0.001 * |
| Scales Scores | Correlations with Age | |
|---|---|---|
| (R) | p | |
| BPS before interventions | −0.073 | p = 0.027 * |
| BPS during interventions | 0.04 | p = 0.222 |
| BPS after interventions | −0.029 | p = 0.379 |
| CCPOT before interventions | −0.011 | p = 0.731 |
| CCPOT during interventions | 0.061 | p = 0.062 |
| CCPOT after interventions | −0.007 | p = 0.839 |
| Scales Scores | Correlations with Length of Hospitalization | |
|---|---|---|
| R | p | |
| BPS before interventions | 0.055 | p = 0.09 |
| BPS during interventions | 0.092 | p = 0.005 * |
| BPS after interventions | 0.11 | p = 0.001 * |
| CCPOT before interventions | 0.054 | p = 0.095 |
| CCPOT during interventions | 0.093 | p = 0.004 * |
| CCPOT after interventions | 0.083 | p = 0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnar-Gruszka, K.; Sega, A.; Płaszewska-Żywko, L.; Wojtan, S.; Potocka, M.; Kózka, M. Pain Assessment with the BPS and CCPOT Behavioral Pain Scales in Mechanically Ventilated Patients Requiring Analgesia and Sedation. Int. J. Environ. Res. Public Health 2022, 19, 10894. https://doi.org/10.3390/ijerph191710894
Wojnar-Gruszka K, Sega A, Płaszewska-Żywko L, Wojtan S, Potocka M, Kózka M. Pain Assessment with the BPS and CCPOT Behavioral Pain Scales in Mechanically Ventilated Patients Requiring Analgesia and Sedation. International Journal of Environmental Research and Public Health. 2022; 19(17):10894. https://doi.org/10.3390/ijerph191710894
Chicago/Turabian StyleWojnar-Gruszka, Katarzyna, Aurelia Sega, Lucyna Płaszewska-Żywko, Stanisław Wojtan, Marcelina Potocka, and Maria Kózka. 2022. "Pain Assessment with the BPS and CCPOT Behavioral Pain Scales in Mechanically Ventilated Patients Requiring Analgesia and Sedation" International Journal of Environmental Research and Public Health 19, no. 17: 10894. https://doi.org/10.3390/ijerph191710894
APA StyleWojnar-Gruszka, K., Sega, A., Płaszewska-Żywko, L., Wojtan, S., Potocka, M., & Kózka, M. (2022). Pain Assessment with the BPS and CCPOT Behavioral Pain Scales in Mechanically Ventilated Patients Requiring Analgesia and Sedation. International Journal of Environmental Research and Public Health, 19(17), 10894. https://doi.org/10.3390/ijerph191710894






