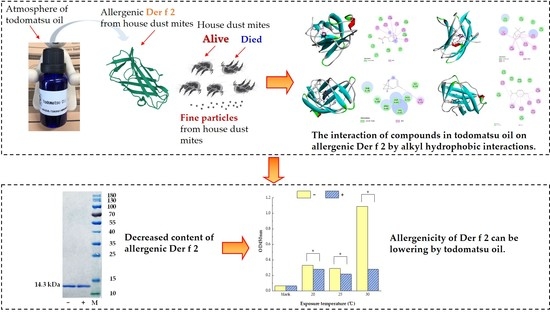
Novel Approaches for Inhibiting the Indoor Allergen Der f 2 Excreted from House Dust Mites by Todomatsu Oil Produced from Woodland Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment of Samples by Todomatsu Oil
2.2. Der f 2 Allergenicity Identification via Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Der f 2 Content Determination Using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
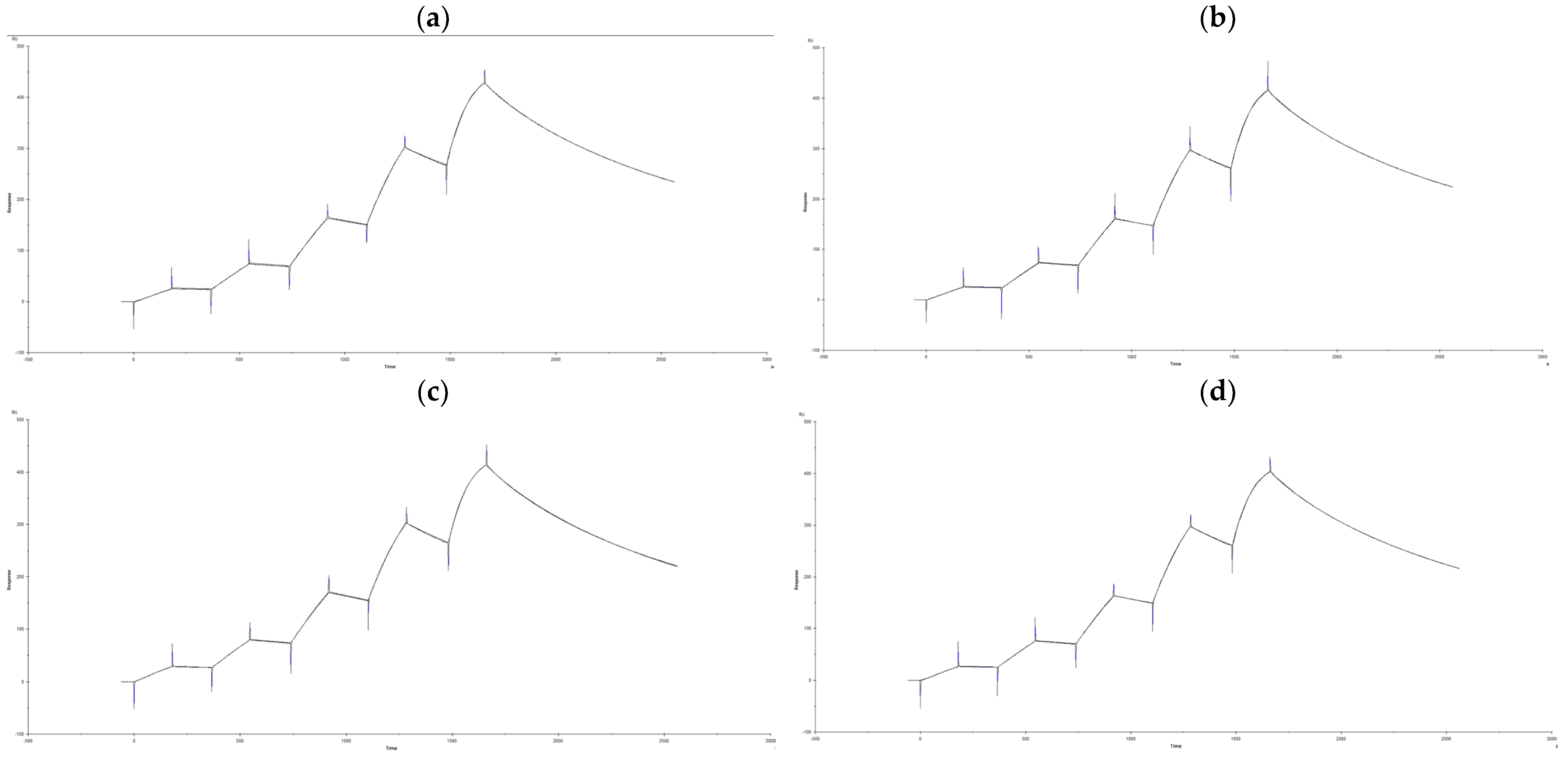
2.4. IgG Binding Capacity of a Single Protein Der f 2 Examination through Surface Plasmon Resonance (SPR) Experiments
2.5. In Silico Binding Sites and Affinities of Compounds in Todomatsu Oil on Der f 2
2.6. Statistical Analysis
3. Results
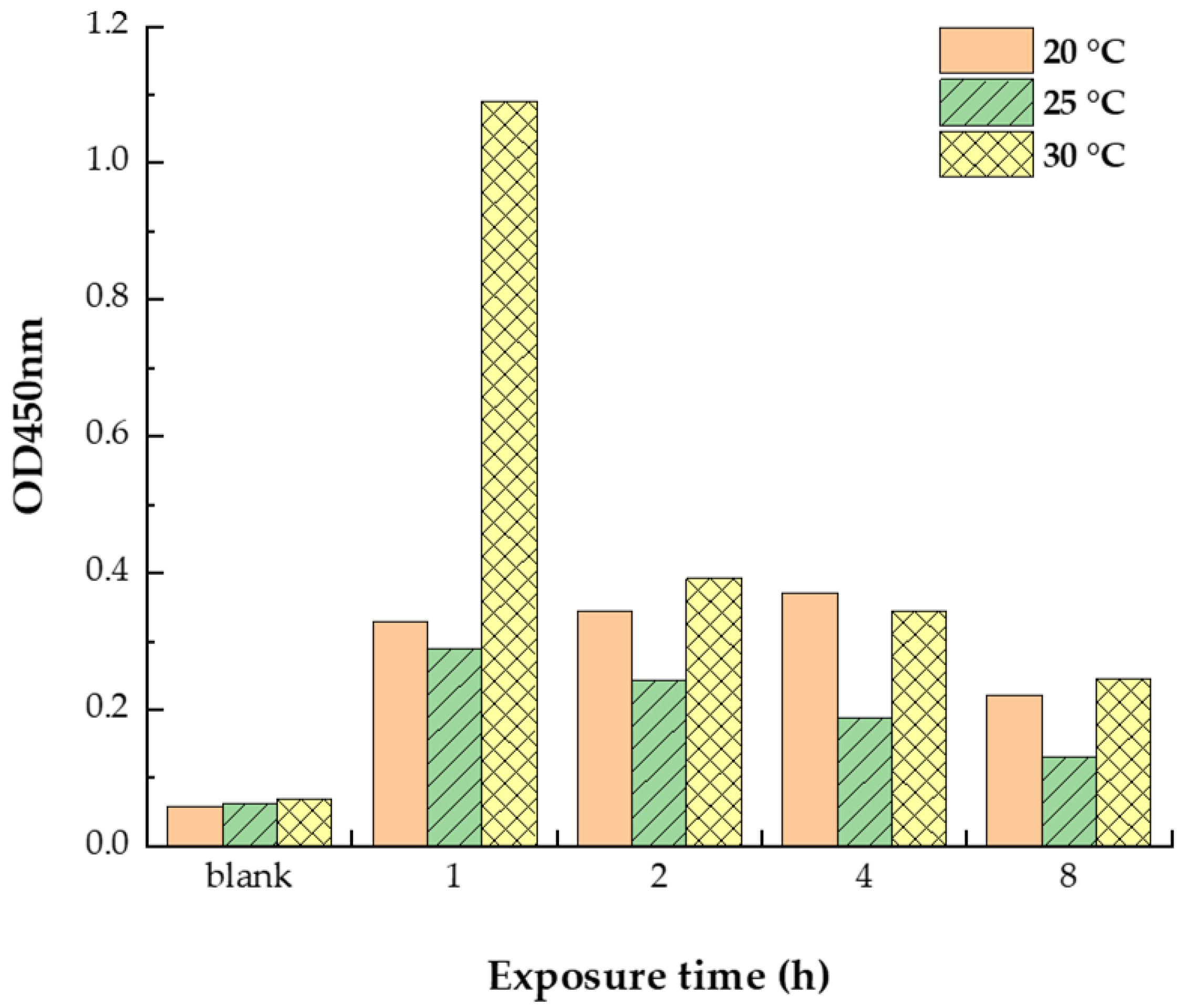
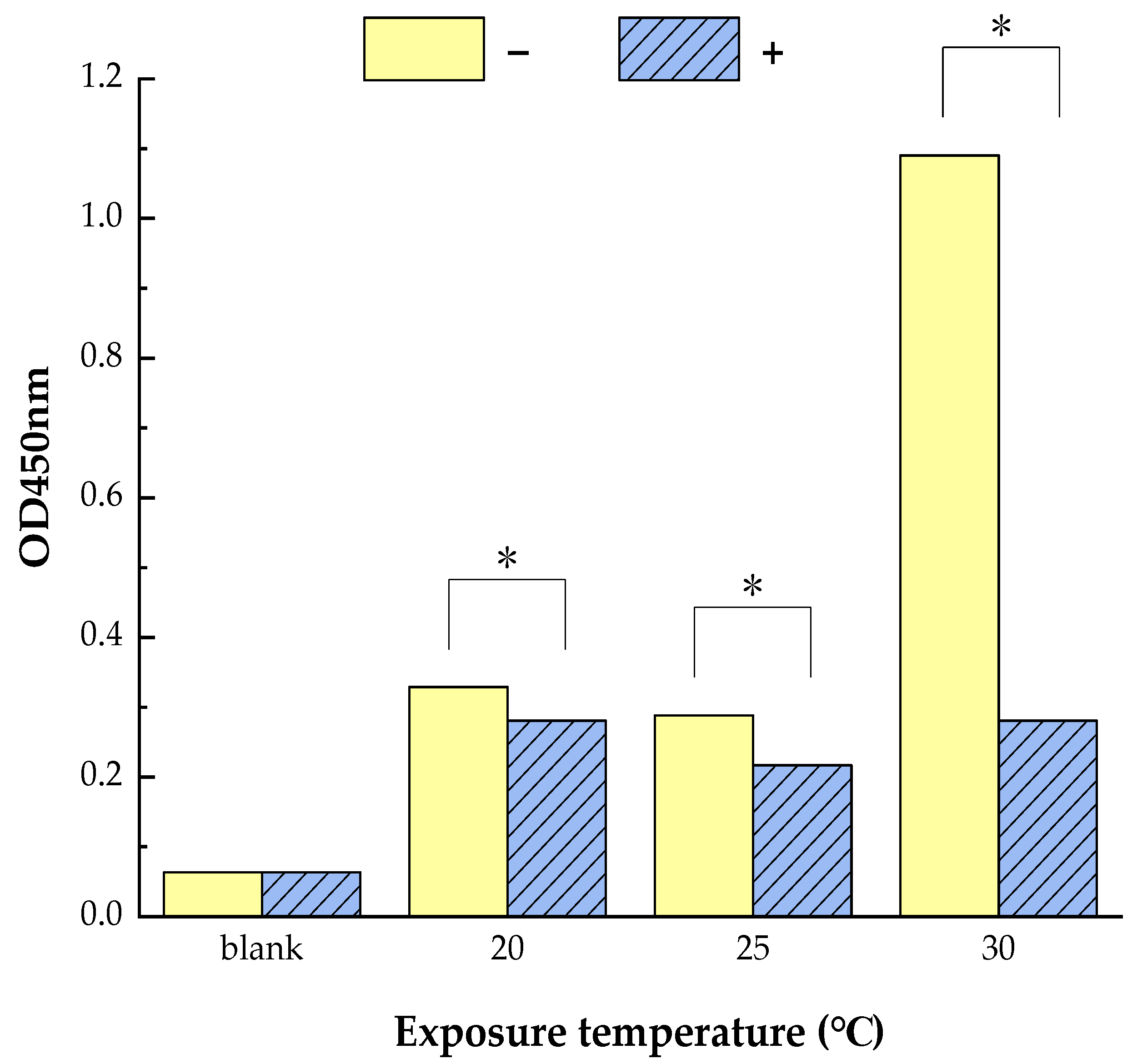
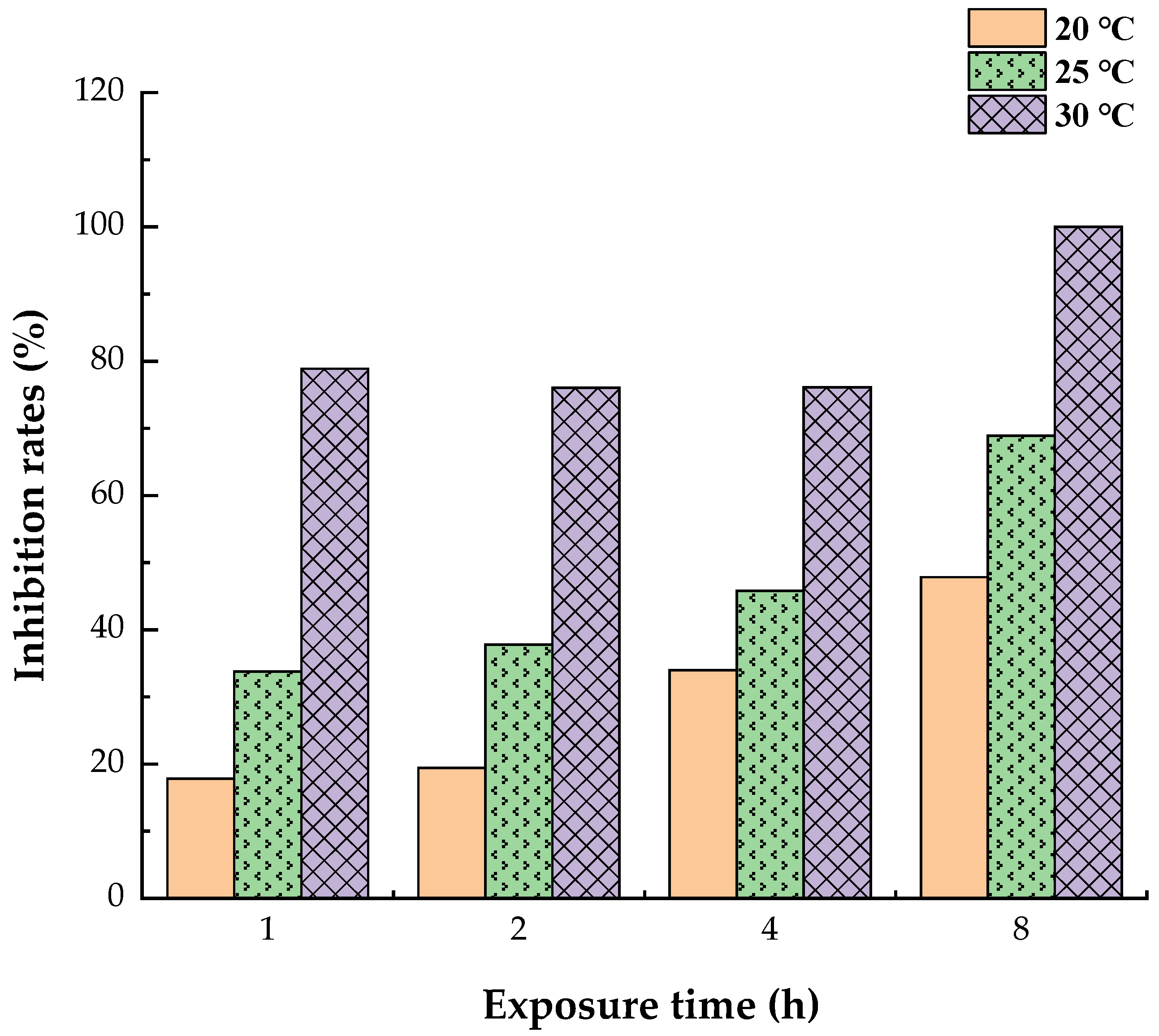
3.1. Reduced Allergen Der f 2 Caused by Todomatsu Oil
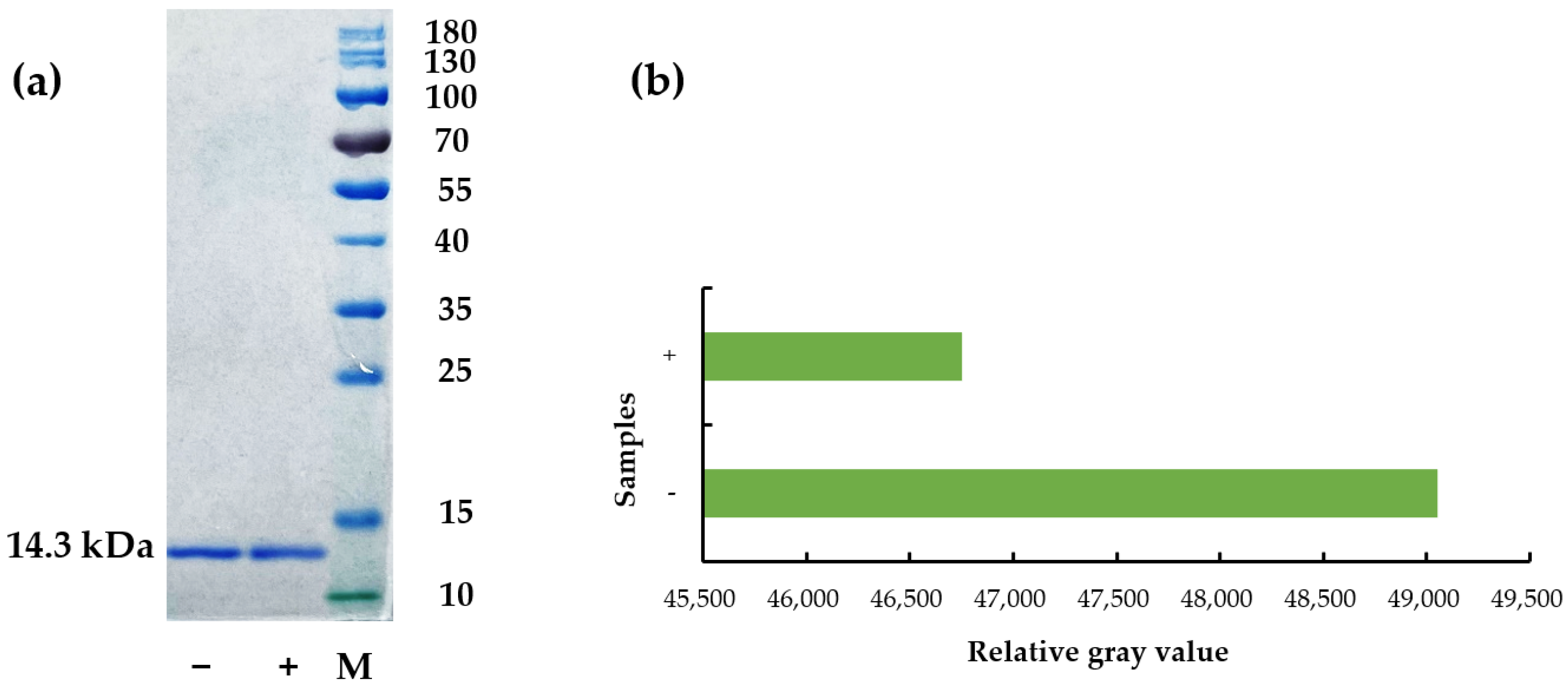
3.2. Differences in IgG Binding Capacity of a Single Protein Der f 2
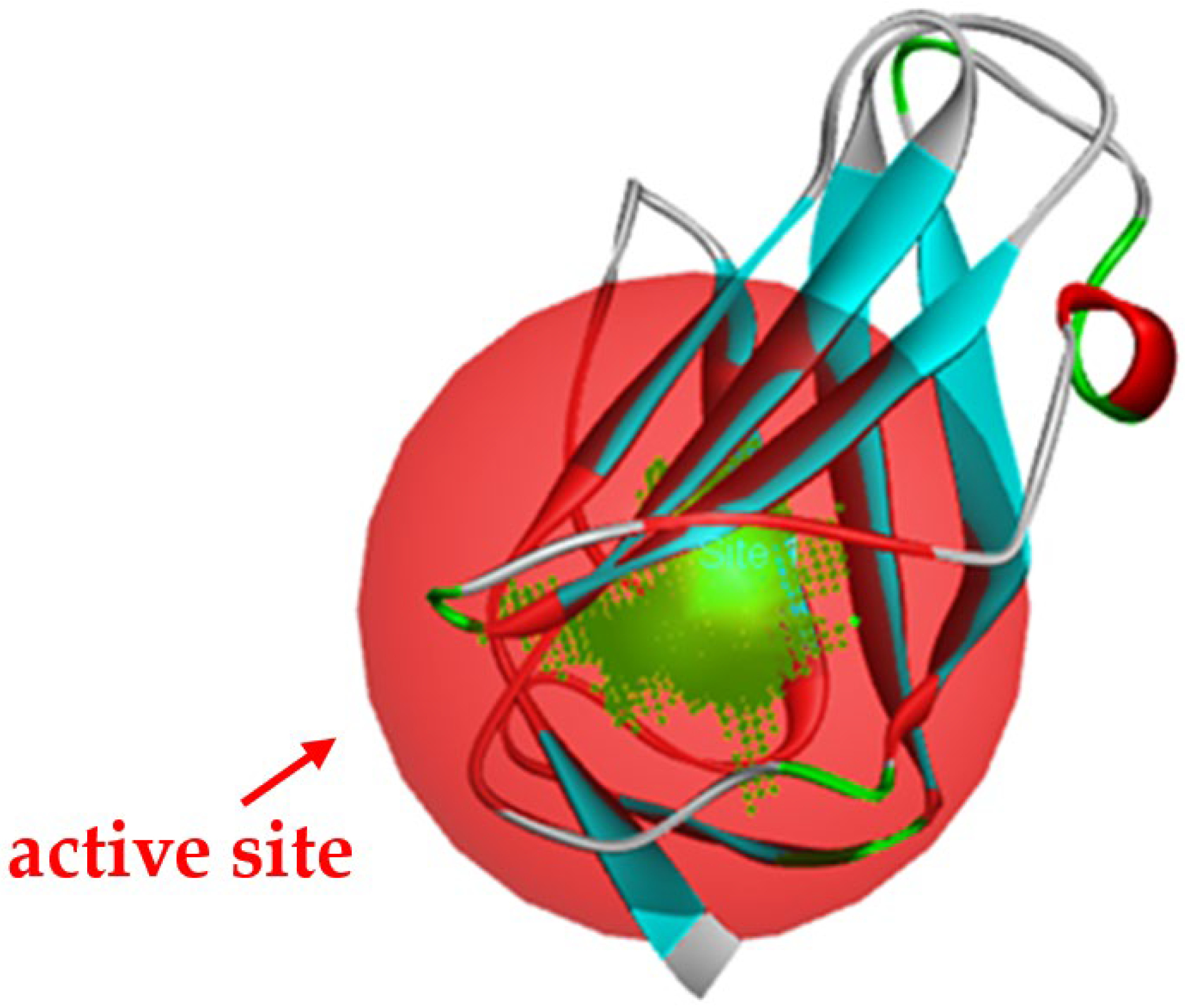
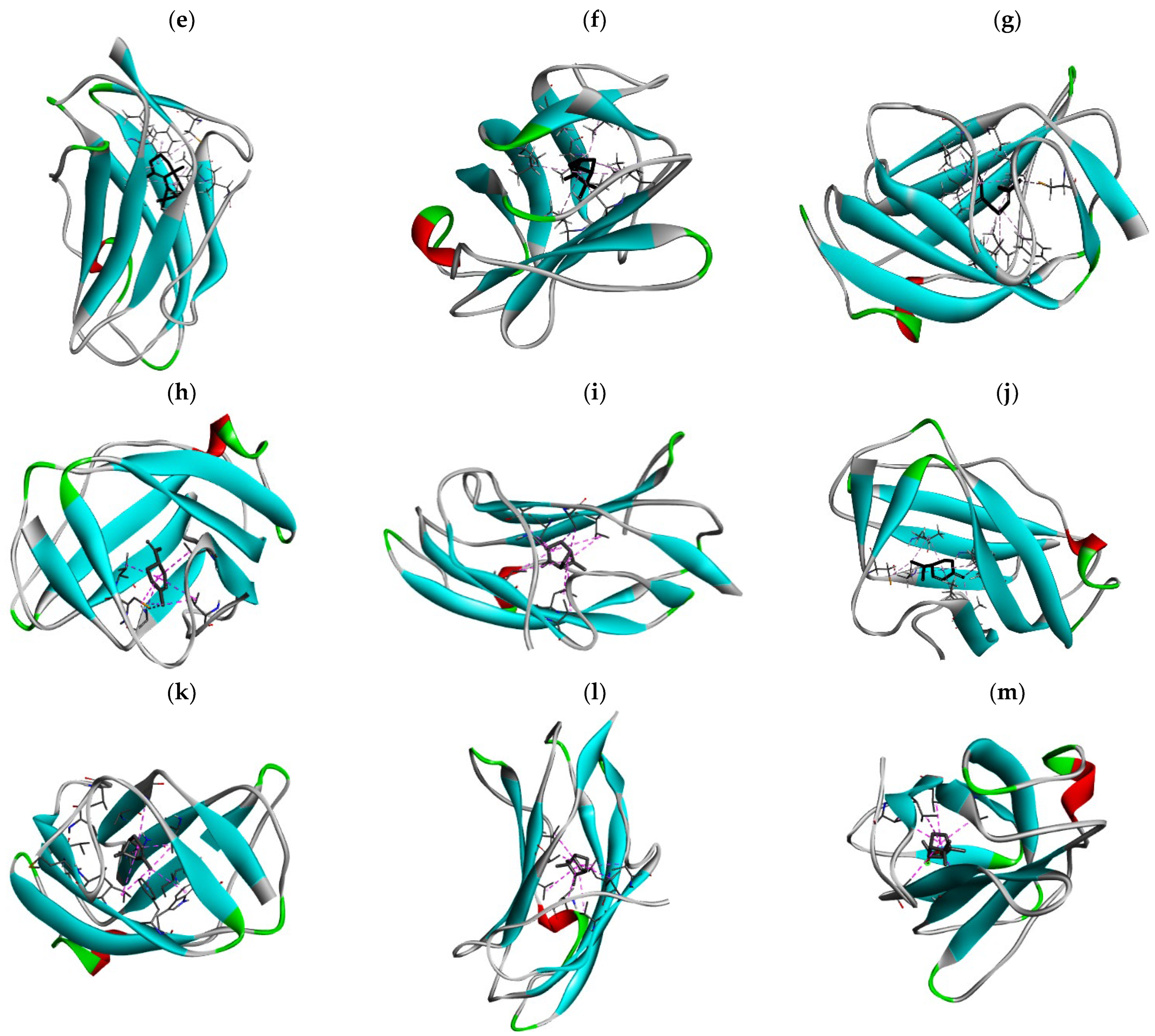
3.3. The Alkyl Hydrophobic Interactions between Todomatsu Oil and Der f 2
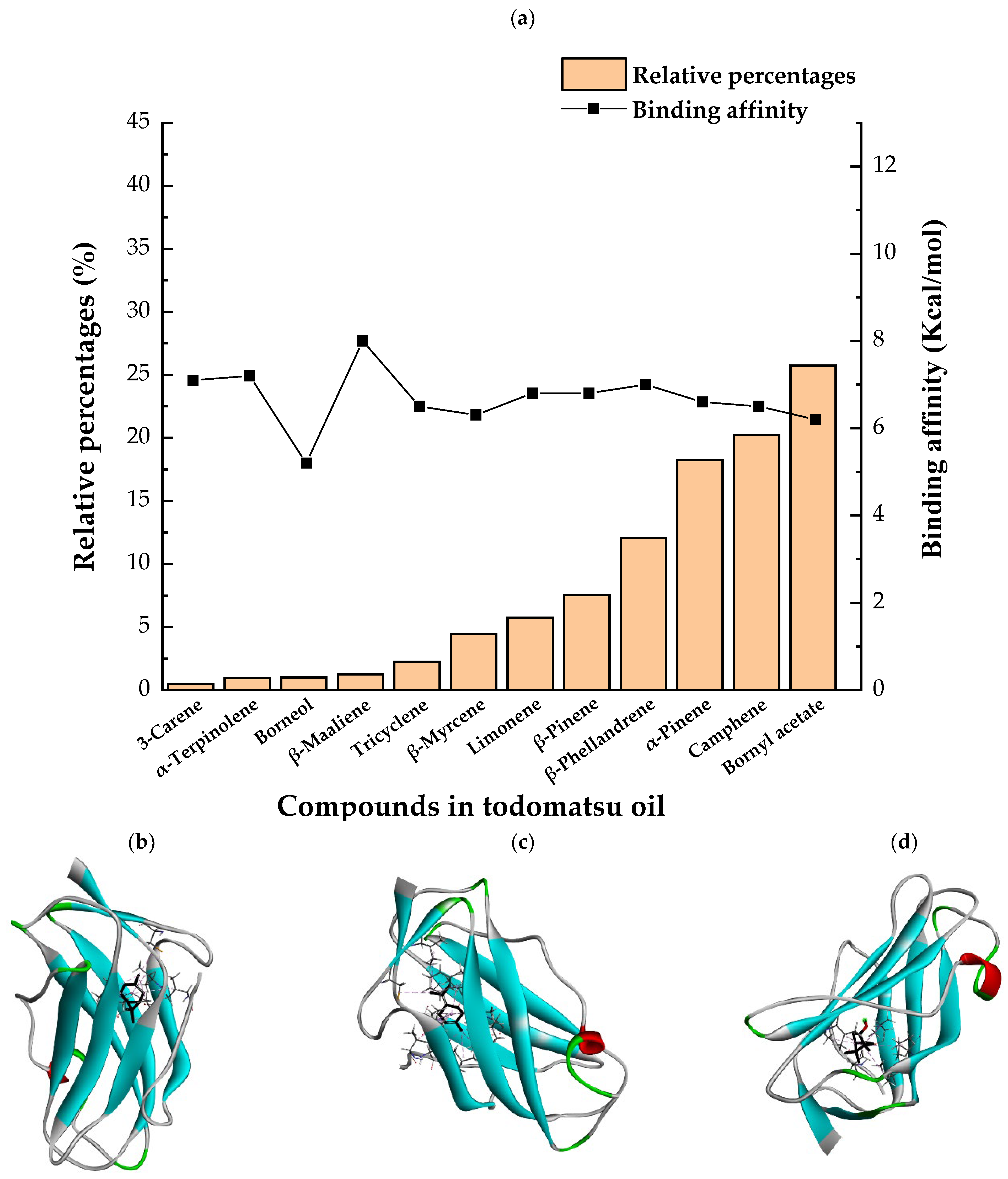
3.4. The Binding Affinity of Compounds in Todomatsu Oil on Der f 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhang, J.; Tao, A. Antigenicity, Immunogenicity, Allergenicity. Allergy Bioinform. 2015, 8, 175–186. [Google Scholar] [CrossRef]
- Huang, F.L.; Liao, E.C.; Yu, S.J. House Dust Mite Allergy: Its Innate Immune Response and Immunotherapy. Immunobiology 2018, 223, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, X.; Li, P.; He, L.; Xie, J.; Gao, X.; Wu, X.; Su, F.; Liang, Y. The Influence of House Dust Mite Sublingual Immunotherapy on the TSLP-OX40L Signaling Pathway in Patients with Allergic Rhinitis. Int. Forum Allergy Rhinol. 2016, 6, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.E.; Erwin, E.A.; Heymann, P.W.; Woodfolk, J.A. Pro: The Evidence for a Causal Role of Dust Mites in Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 109–113. [Google Scholar] [CrossRef]
- Dabbaghzadeh, A.; Ghaffari, J.; Feridoni, M.; Alipour, A. Research Paper: House Dust Mite Allergen Levels of Der p 1 and Der f 1 in Houses of Asthmatic Children. J. Pediatr. Rev. 2020, 8, 267–274. Available online: http://jpr.mazums.ac.ir/article-1-299-en.html (accessed on 20 August 2021). [CrossRef]
- Yasuda, Y.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Group 2 Innate Lymphoid Cells and the House Dust. Cells 2020, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Hernandez, D.; De Rojas, F.; Virchow, J.C.; Demoly, P.; Kingdom, U. Respiratory Allergy Caused by House Dust Mites: What Do We Really Know ? J. Allergy Clin. Immunol. 2015, 136, 38–48. [Google Scholar] [CrossRef]
- Ichikawa, S.; Takai, T.; Inoue, T.; Yuuki, T.; Okumura, Y.; Ogura, K.; Inagaki, F.; Hatanaka, H. NMR Study on the Major Mite Allergen Der f 2: Its Refined Tertiary Structure, Epitopes for Monoclonal Antibodies and Characteristics Shared by ML Protein Group Members. J. Biochem. 2005, 137, 255–263. [Google Scholar] [CrossRef]
- Inohara, N.; Nuez, G. ML—A Conserved Domain Involved in Innate Immunity and Lipid Metabolism. Trends Biochem. Sci. 2002, 27, 219–221. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Lee, I.Y.; Ree, H.I.; Hong, C.S.; Yong, T.S. Localization of Der f 2 in the Gut and Fecal Pellets of Dermatophagoides Farinae. Allergy 2002, 57, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, B.R.; Skov, L.K.; Kastrup, J.S.; Kristensen, O.; Bolwig, C.; Larsen, J.N.; Spangfort, M.; Lund, K.; Gajhede, M. Structure of the House Dust Mite Allergen Der f 2: Implications for Function and Molecular Basis of IgE Cross-Reactivity. FEBS Lett. 2005, 579, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, Z. Clinical Significance of Dust Mite Allergens. Mol. Biol. Rep. 2020, 47, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R.; Hales, B.J. T and B Cell Responses to HDM Allergens and Antigens. Immunol. Res. 2007, 37, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hirose, S.; Suzuki, K.; Hiroi, T.; Takaiwa, F. Expression of Hypoallergenic Der f 2 Derivatives with Altered Intramolecular Disulphide Bonds Induces the Formation of Novel ER-Derived Protein Bodies in Transgenic Rice Seeds. J. Exp. Bot. 2012, 63, 2947–2959. [Google Scholar] [CrossRef]
- Nishiyama, C.; Yuuki, T.; Takai, T.; Okumura, Y.; Okudaira, H. Determination of Three Disulfide Bonds in a Major House Dust Mite Allergen, Der f II. Int. Arch. Allergy Immunol. 1993, 101, 159–166. [Google Scholar] [CrossRef]
- Jung, J. Insecticidal Effect against House Dust Mite Using Ethanol Extract of Theobroma cacao L. Ann. Rom. Soc. Cell Biol. 2021, 25, 786–791. Available online: https://www.annalsofrscb.ro/index.php/journal/article/view/171 (accessed on 23 August 2021).
- Abidin, S.Z.; Ming, H.T. Effect of a Commercial Air Ionizer on Dust Mites Dermatophagoides Pteronyssinus and Dermatophagoides Farinae (Acari: Pyroglyphidae) in the Laboratory. Asian Pac. J. Trop. Biomed. 2012, 2, 156–158. [Google Scholar] [CrossRef]
- Murray, C.S.; Foden, P.; Sumner, H.; Shepley, E.; Custovic, A.; Simpson, A. Preventing Severe Asthma Exacerbations in Children a Randomized Trial of Mite-Impermeable Bedcovers. Am. J. Respir. Crit. Care Med. 2017, 196, 150–158. [Google Scholar] [CrossRef]
- Halken, S.; Høst, A.; Niklassen, U.; Hansen, L.G.; Nielsen, F.; Pedersen, S.; Østerballe, O.; Veggerby, C.; Poulsen, L.K. Effect of Mattress and Pillow Encasings on Children with Asthma and House Dust Mite Allergy. J. Allergy Clin. Immunol. 2003, 111, 169–176. [Google Scholar] [CrossRef]
- Terreehorst, I.; Hak, E.; Oosting, A.J.; Tempels-Pavlica, Z.; de Monchy, J.G.R.; Bruijnzeel-Koomen, C.A.F.M.; Aalberse, R.C.; van Wijk, R.G. Evaluation of Impermeable Covers for Bedding in Patients with Allergic Rhinitis. N. Engl. J. Med. 2003, 349, 237–246. [Google Scholar] [CrossRef]
- Van Den Bemt, L.; Van Knapen, L.; De Vries, M.P.; Jansen, M.; Cloosterman, S.; Van Schayck, C.P. Clinical Effectiveness of a Mite Allergen-Impermeable Bed-Covering System in Asthmatic Mite-Sensitive Patients. J. Allergy Clin. Immunol. 2004, 114, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Tsurikisawa, N.; Saito, A.; Oshikata, C.; Nakazawa, T.; Yasueda, H.; Akiyama, K. Encasing Bedding in Covers Made of Microfine Fibers Reduces Exposure to House Mite Allergens and Improves Disease Management in Adult Atopic Asthmatics. Allergy Asthma Clin. Immunol. 2013, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Rijssenbeek-Nouwens, L.H.M.; Oosting, A.J.; De Bruin-Weller, M.S.; Bregman, I.; De Monchy, J.G.R.; Postma, D.S. Clinical Evaluation of the Effect of Anti-Allergic Mattress Covers in Patients with Moderate to Severe Asthma and House Dust Mite Allergy: A Randomised Double Blind Placebo Controlled Study. Thorax 2002, 57, 784–790. [Google Scholar] [CrossRef]
- Gore, R.B.; Durrell, B.; Bishop, S.; Curbishley, L.; Woodcock, A.; Custovic, A. High-Efficiency Vacuum Cleaners Increase Personal Mite Allergen Exposure, but Only Slightly. Allergy Eur. J. Allergy Clin. Immunol. 2006, 61, 119–123. [Google Scholar] [CrossRef]
- Maloney, J.; Sicherer, S.H. Results of a Home-Based Environmental Intervention among Urban Children with Asthma. Pediatrics 2005, 116, 543. [Google Scholar] [CrossRef]
- Antonicelli, L.; Bilò, M.B.; Pucci, S.; Schou, C.; Bonifazi, F. Efficacy of an Air-cleaning Device Equipped with a High Efficiency Particulate Air Filter in House Dust Mite Respiratory Allergy. Allergy 1991, 46, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Reisman, R.E.; Mauriello, P.M.; Davis, G.B.; Georgitis, J.W.; DeMasi, J.M. A Double-Blind Study of the Effectiveness of a High-Efficiency Particulate Air (HEPA) Filter in the Treatment of Patients with Perennial Allergic Rhinitis and Asthma. J. Allergy Clin. Immunol. 1990, 85, 1050–1057. [Google Scholar] [CrossRef]
- Arlian, L.G.; Neal, J.S.; Vyszenski-Moher, D.A.L. Reducing Relative Humidity to Control the House Dust Mite Dermatophagoides Farinae. J. Allergy Clin. Immunol. 1999, 104, 852–856. [Google Scholar] [CrossRef]
- Hayden, M.L.; Rose, G.; Diduch, K.B.; Domson, P.; Chapman, M.D.; Heymann, P.W.; Platts-Mills, T.A.E. Benzyl Benzoate Moist Powder: Investigation of Acarical Activity in Cultures and Reduction of Dust Mite Allergens in Carpets. J. Allergy Clin. Immunol. 1992, 89, 536–545. [Google Scholar] [CrossRef]
- Lee, I.; Park, J. Insecticidal Effect of Dermatoohagoides Pteronyssinus Using Ginkgo Biloba Leaves Extracts. KSBB J. 2007, 22, 58–61. [Google Scholar]
- Hayes, W.J.; Laws, E.R. Handbook of Pesticide Toxicology; Classes of Pesticides Vol. 3; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Doungnapa, T.; Pumnuan, J.; Insung, A. Acaricidal Activity of Essential Oil Nanoemulsion against the African Red Mite (Eutetranychus Africanus). Chil. J. Agric. Res. 2021, 81, 228–236. [Google Scholar] [CrossRef]
- Huang, C.X.; Li, H.G.; Luo, H.Q.; Fu, Q.M.; He, B.S.; Bao, M.H. Essential Oils for the Treatment of Demodex. E3S Web Conf. 2021, 271, 4034. [Google Scholar] [CrossRef]
- Yu, H.; Ren, X.; Yang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Antimicrobial and Anti-Dust Mite Efficacy of Cinnamomum Camphora Chvar. Borneol Essential Oil Using Pilot-Plant Neutral Cellulase-Assisted Steam Distillation. Lett. Appl. Microbiol. 2021, 74, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula Angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef] [PubMed]
- Insung, A.; Pumnuan, J.; Mahakittikun, V.; Wangapai, T. Effectiveness of Essential Oils of Medicinal Plants at Reducing the Amounts of Allergen Produced by the European House Dust Mite, Dermatophagoides Pteronyssinus (Trouessart). J. Acarol. Soc. Jpn. 2016, 25, S179–S184. [Google Scholar] [CrossRef]
- Tatsuro, O.; Naoyuki, M.; Toshihiko, K.; Yuichi, T.M. Efficient extraction of essential oil from woody materials using vaccume microwave assisted steam distillation. Aroma Res. 2010, 11, 48–55. [Google Scholar]
- STC 2013. The Air Purifier “Clear Forest” Is Born!—Abies Sachalinensis Oil Cleans Odors and Dirt *! -New Release of 2 Types for Cars for Air Conditioner Louvers/for Under-Seat and Side Pockets. Available online: https://www.st-c.co.jp/news/newsrelease/2013/20131003_002911.html (accessed on 20 January 2022).
- Nishiyama, C.; Hatanaka, H.; Ichikawa, S.; Fukada, M.; Akagawa-Chihara, M.; Yuuki, T.; Yokota, T.; Inagaki, F.; Okumura, Y. Analysis of Human IgE Epitope of Der f 2 with Anti-Der f 2 Mouse Monoclonal Antibodies. Mol. Immunol. 1999, 36, 53–60. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Duru, I.A.; Duru, C.E.; Enyoh, C.E.; Umar, H.I. Computer-Aided Degradation Susceptibility Study of Crude Oil Compounds at Bacillus Subtilis Protein Target. Environ. Eng. Res. 2022, 28, 210565. [Google Scholar] [CrossRef]
- Cabanillas, B.; Pedrosa, M.M.; Rodríguez, J.; González, Á.; Muzquiz, M.; Cuadrado, C.; Crespo, J.F.; Burbano, C. Effects of Enzymatic Hydrolysis on Lentil Allergenicity. Mol. Nutr. Food Res. 2010, 54, 1266–1272. [Google Scholar] [CrossRef]
- Lv, L.; Qu, X.; Yang, N.; Ahmed, I. The Conformational Structural Change of β-Lactoglobulin via Acrolein Treatment Reduced the Allergenicity. Food Chem. X 2021, 10, 100120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhao, H.; Peng, J.; Hong, Q.; Xiao, K.; Shang, Y.; Lu, S.; Zhang, W.; Wu, M.; Li, S.; et al. Size Distribution of Platanus Acerifolia Allergen 3 (Pla A3) in Shanghai Ambient Size-Resolved Particles and Its Allergenic Effects. Atmos. Environ. 2019, 198, 324–334. [Google Scholar] [CrossRef]
- He, W.; He, K.; Sun, F.; Mu, L.; Liao, S.; Li, Q.; Yi, J.; Liu, Z.; Wu, X. Effect of Heat, Enzymatic Hydrolysis and Acid-Alkali Treatment on the Allergenicity of Silkworm Pupa Protein Extract. Food Chem. 2021, 343, 128461. [Google Scholar] [CrossRef]
- Pi, X.; Fu, G.; Dong, B.; Yang, Y.; Wan, Y.; Xie, M. Effects of Fermentation with Bacillus Natto on the Allergenicity of Peanut. Lwt 2021, 141, 110862. [Google Scholar] [CrossRef]
- Visentin, J.; Couzi, L.; Dromer, C.; Neau-Cransac, M.; Guidicelli, G.; Veniard, V.; Coniat, K.N.L.; Merville, P.; Di Primo, C.; Taupin, J.L. Overcoming Non-Specific Binding to Measure the Active Concentration and Kinetics of Serum Anti-HLA Antibodies by Surface Plasmon Resonance. Biosens. Bioelectron. 2018, 117, 191–200. [Google Scholar] [CrossRef]
- Visentin, J.; Minder, L.; Lee, J.H.; Taupin, J.L.; Di Primo, C. Calibration Free Concentration Analysis by Surface Plasmon Resonance in a Capture Mode. Talanta 2016, 148, 478–485. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Duru, C.E.; Umar, H.I.U.; Duru, I.A.; Enenebeaku, U.E.; Ngozi-Olehi, L.C.; Enyoh, C.E. Blocking the Interactions between Human Ace2 and Coronavirus Spike Glycoprotein by Selected Drugs: A Computational Perspective. Environ. Health Toxicol. 2021, 36, e2021010. [Google Scholar] [CrossRef]
- Duru, C.E.; Duru, I.A.; Enyoh, C.E. In Silico Binding Affinity Analysis of Microplastic Compounds on PET Hydrolase Enzyme Target of Ideonella Sakaiensis. Bull. Natl. Res. Cent. 2021, 45, 104. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Li, H.; Lin, Y.; Tong, S.; Li, Y. Assessing the Impact of Air Pollutants on Clinical Visits for Childhood Allergic Respiratory Disease Induced by House Dust Mite in Shanghai, China. Respir. Res. 2022, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Yucel, E.; Suleyman, A.; Demirkale, Z.H.; Guler, N.; Tamay, Z.U.; Ozdemir, C. ‘Stay at Home’: Is It Good or Not for House Dust Mite Sensitized Children with Respiratory Allergies? Pediatr. Allergy Immunol. 2021, 32, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Ichikawa, S.; Yokota, T.; Hatanaka, H.; Inagaki, F.; Okumura, Y. Unlocking the Allergenic Structure of the Major House Dust Mite Allergen Der f 2 by Elimination of Key Intramolecular Interactions. FEBS Lett. 2000, 484, 102–107. [Google Scholar] [CrossRef]
- STC 2018. New Brand of Health Care Products “MoriLabo” New Release of “MoriLabo Pollen Barrier Stick”, Which is a Scent to Prevent Pollen just by Applying It to the Mask. Available online: https://www.st-c.co.jp/news/newsrelease/2018/20181128_004349.html (accessed on 25 June 2022).
- Jakobsen, C.G.; Bodtger, U.; Poulsen, L.K.; Roggen, E.L. Vaccination for Birch Pollen Allergy: Comparison of the Affinities of Specific Immunoglobulins E, G1 and G4 Measured by Surface Plasmon Resonance. Clin. Exp. Allergy 2005, 35, 193–198. [Google Scholar] [CrossRef]
| Samples | Essential Oil (μL/cm3) | Allergen Der f 2 (ng/mL) | Exposure Temperature (°C) | Exposure Time (h) |
|---|---|---|---|---|
| Using for ELISA | − | 250 250 2000 | 20 25 30 | 1, 2, 4, 8 |
| 0.05 | 250 250 2000 | 20 25 30 | 1, 2, 4, 8 | |
| Using for SPR experiments | − | 500, 250, 125, 62.5, and 31.25 | 25 | 1 |
| 0.05 | 500, 250, 125, 62.5, and 31.25 | 25 | 1 | |
| Using for SDS-PAGE | − | 100,000 | 25 | 2 |
| 0.05 | 100,000 | 25 | 2 |
| Samples | KD (M) | Rmax (RU) | ||
|---|---|---|---|---|
| 1st Time | 2nd Time | 1st Time | 2nd Time | |
| Der f 2 without todomatsu oil | 2.217 × 10−9 | 2.255 × 10−9 | 476.2 | 456.5 |
| Der f 2 with todomatsu oil | 2.221 × 10−9 | 2.110 × 10−9 | 460.5 | 442.6 |
| Chemical Compounds | PubChem CID | Chemical Formula | Chemical 3D Structure | Chemical 2D Structure | ΔG Energy (kcal/mol) | Protein–Ligand Interaction | Interacting Amino Acid Residues |
|---|---|---|---|---|---|---|---|
| (a) 3-carene | 26049 | C10H16 |  | 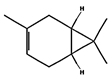 | −7.1 | 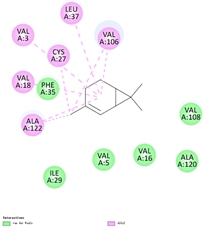 | VAL3; VAL18; CYS27; LEU37; VAL106; ALA122 |
| (b) α-terpinolene | 11463 | C10H16 |  | 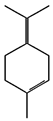 | −7.2 | 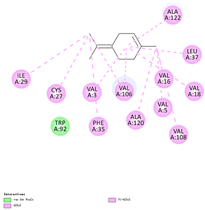 | VAL3; VAL5; VAL16; VAL18; CYS27; ILE29; PHE35; LEU37; VAL106; VAL108; ALA120; ALA122 |
| (c) borneol | 64685 | C10H18O |  | 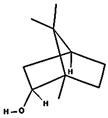 | −5.2 |  | VAL3; VAL16; VAL18; LEU37; VAL106; ALA122 |
| (d) β-maaliene | 101596917 | C15H24 |  | 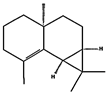 | −8.0 | 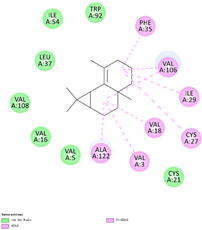 | VAL3; VAL18; CYS27; ILE29; PHE35; VAL106; ALA122 |
| (e) tricyclene | 79035 | C10H16 | 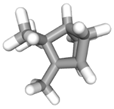 | 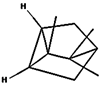 | −6.5 | 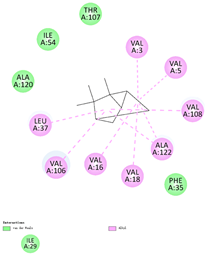 | VAL3; VAL5; VAL16; VAL18; LEU37; VAL106; VAL108; ALA122 |
| (f) β-myrcene | 31253 | C10H16 |  |  | −6.3 | 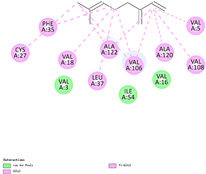 | VAL5; VAL18; CYS27; PHE35; LEU37; VAL106; VAL108; ALA120; ALA122 |
| (g) limonene | 22311 | C10H16 |  |  | −6.8 | 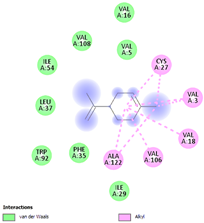 | VAL3; VAL18; CYS27; VAL106; ALA122 |
| (h) β-pinene | 14896 | C10H16 |  |  | −6.8 |  | LYS55; PRO66; ILE68 |
| (i) β-phellandrene | 11142 | C10H16 |  |  | −7.0 | 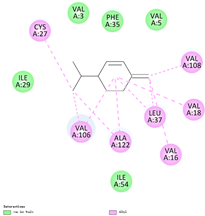 | VAL16; VAL18; CYS27; LEU37; VAL106; VAL108; ALA122 |
| (j) α-pinene | 6654 | C10H16 |  |  | −6.6 | 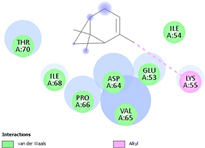 | LYS55 |
| (k) camphene | 6616 | C10H16 |  |  | −6.5 |  | VAL3; CYS27; ILE29; PHE35; VAL106; ALA122 |
| (l) bornyl acetate | 6448 | C12H20O2 |  |  | −6.2 | 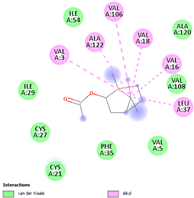 | VAL3; VAL16; VAL18; LEU37; VAL106; ALA122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Enyoh, C.E.; Wang, Q.; Lu, S.; Zhang, W.; Xiao, K.; Zhou, S.; Kaneko, T.; Seguchi, A.; Wang, W.; et al. Novel Approaches for Inhibiting the Indoor Allergen Der f 2 Excreted from House Dust Mites by Todomatsu Oil Produced from Woodland Residues. Int. J. Environ. Res. Public Health 2022, 19, 10881. https://doi.org/10.3390/ijerph191710881
Lin Y, Enyoh CE, Wang Q, Lu S, Zhang W, Xiao K, Zhou S, Kaneko T, Seguchi A, Wang W, et al. Novel Approaches for Inhibiting the Indoor Allergen Der f 2 Excreted from House Dust Mites by Todomatsu Oil Produced from Woodland Residues. International Journal of Environmental Research and Public Health. 2022; 19(17):10881. https://doi.org/10.3390/ijerph191710881
Chicago/Turabian StyleLin, Yichun, Christian Ebere Enyoh, Qingyue Wang, Senlin Lu, Wei Zhang, Kai Xiao, Shumin Zhou, Toshihiko Kaneko, Akifumi Seguchi, Weiqian Wang, and et al. 2022. "Novel Approaches for Inhibiting the Indoor Allergen Der f 2 Excreted from House Dust Mites by Todomatsu Oil Produced from Woodland Residues" International Journal of Environmental Research and Public Health 19, no. 17: 10881. https://doi.org/10.3390/ijerph191710881
APA StyleLin, Y., Enyoh, C. E., Wang, Q., Lu, S., Zhang, W., Xiao, K., Zhou, S., Kaneko, T., Seguchi, A., Wang, W., & Guo, Y. (2022). Novel Approaches for Inhibiting the Indoor Allergen Der f 2 Excreted from House Dust Mites by Todomatsu Oil Produced from Woodland Residues. International Journal of Environmental Research and Public Health, 19(17), 10881. https://doi.org/10.3390/ijerph191710881