Cannabidiol (CBD) Protects Adipose-Derived Mesenchymal Stem Cells (ASCs) against Endoplasmic Reticulum Stress Development and Its Complications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Model Setting
2.2. Proliferation Rate Assay
2.3. Visualization of Cell Organelles
2.4. Visualization of Ki-67
2.5. Evaluation of β-Galactosidase Activation
2.6. Red Oil Staining
2.7. Gene Expression Analysis
2.8. Western Blot Analysis
2.9. Evaluation of Oxidative Stress Factors
2.10. Evaluation of Nictric Oxide
2.11. Cell Cycle Analysis
2.12. Evaluation of Apoptosis
2.13. Statistical Analysis
3. Results
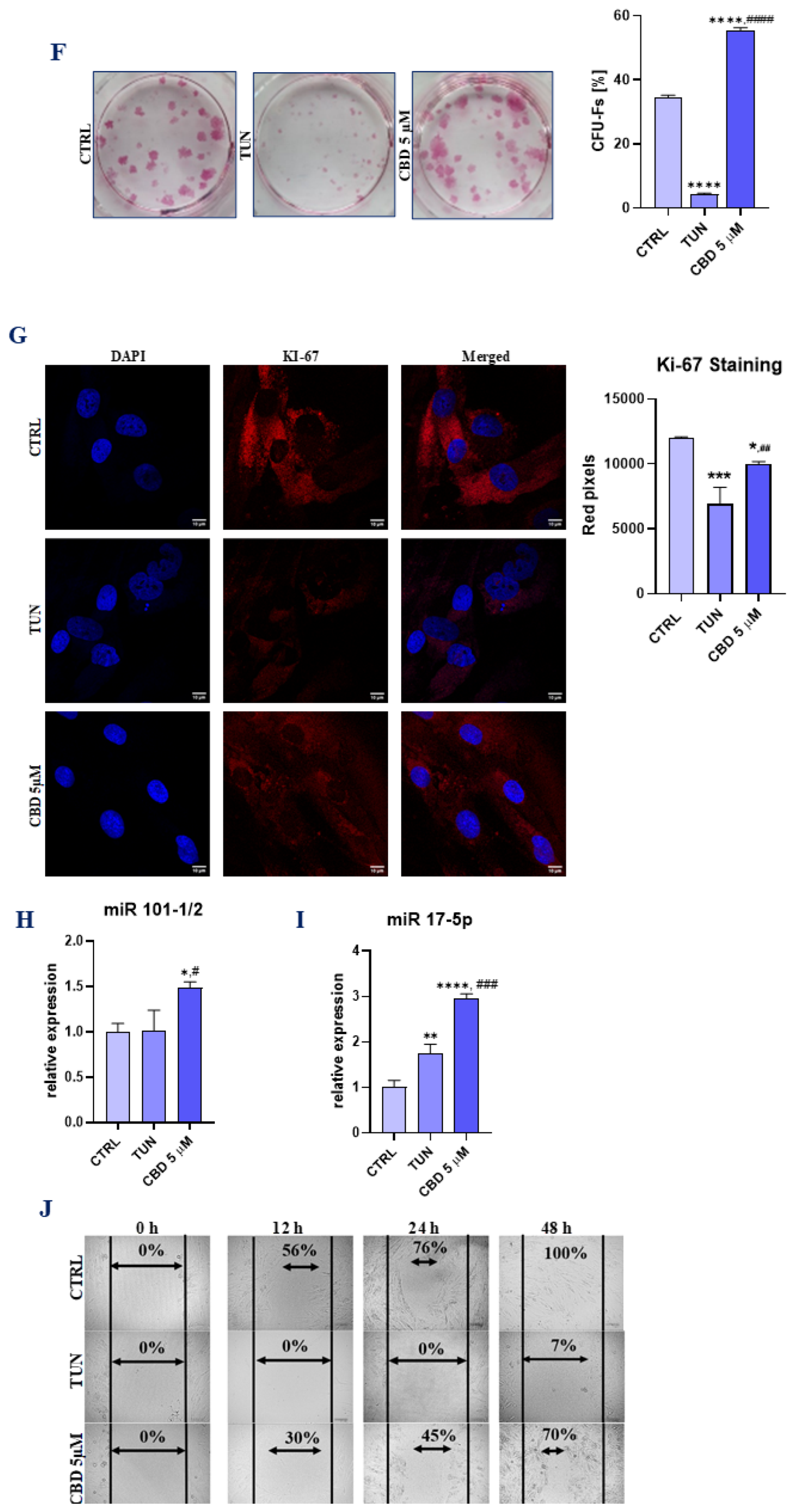
3.1. Evaluation of Morphology, Proliferation Rate, and Adipogenic Differentiation of Affected with Tunicamycin ASCs Treated with CBD
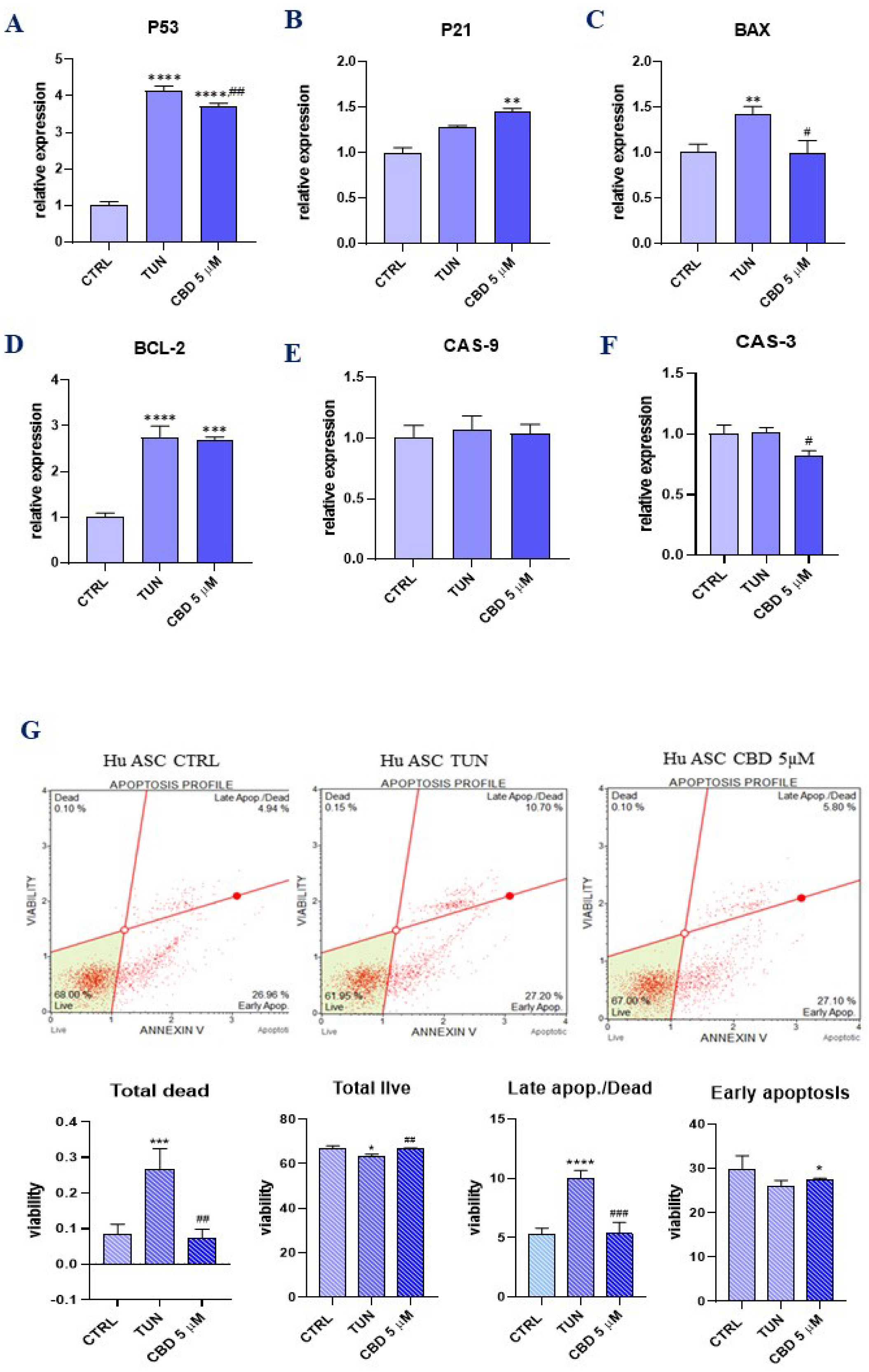
3.2. Evaluation of Apoptosis (CBD Protects ER Stress-Induced Apoptosis in ASCs)
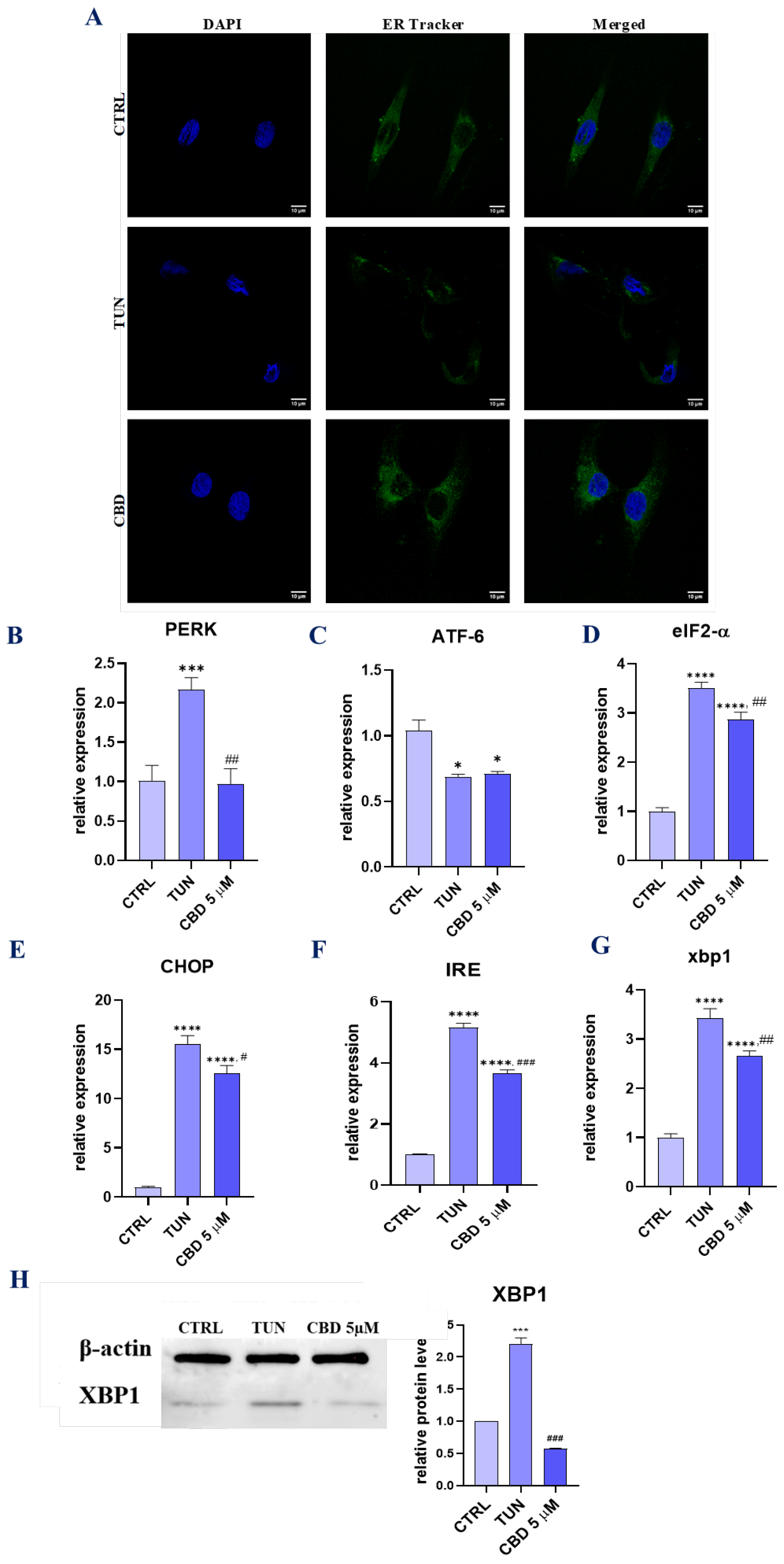
3.3. Evaluation of ER Stress (CBD Regulates PERK-, ATF6-, and IRE1-Mediated ER Stress Response)
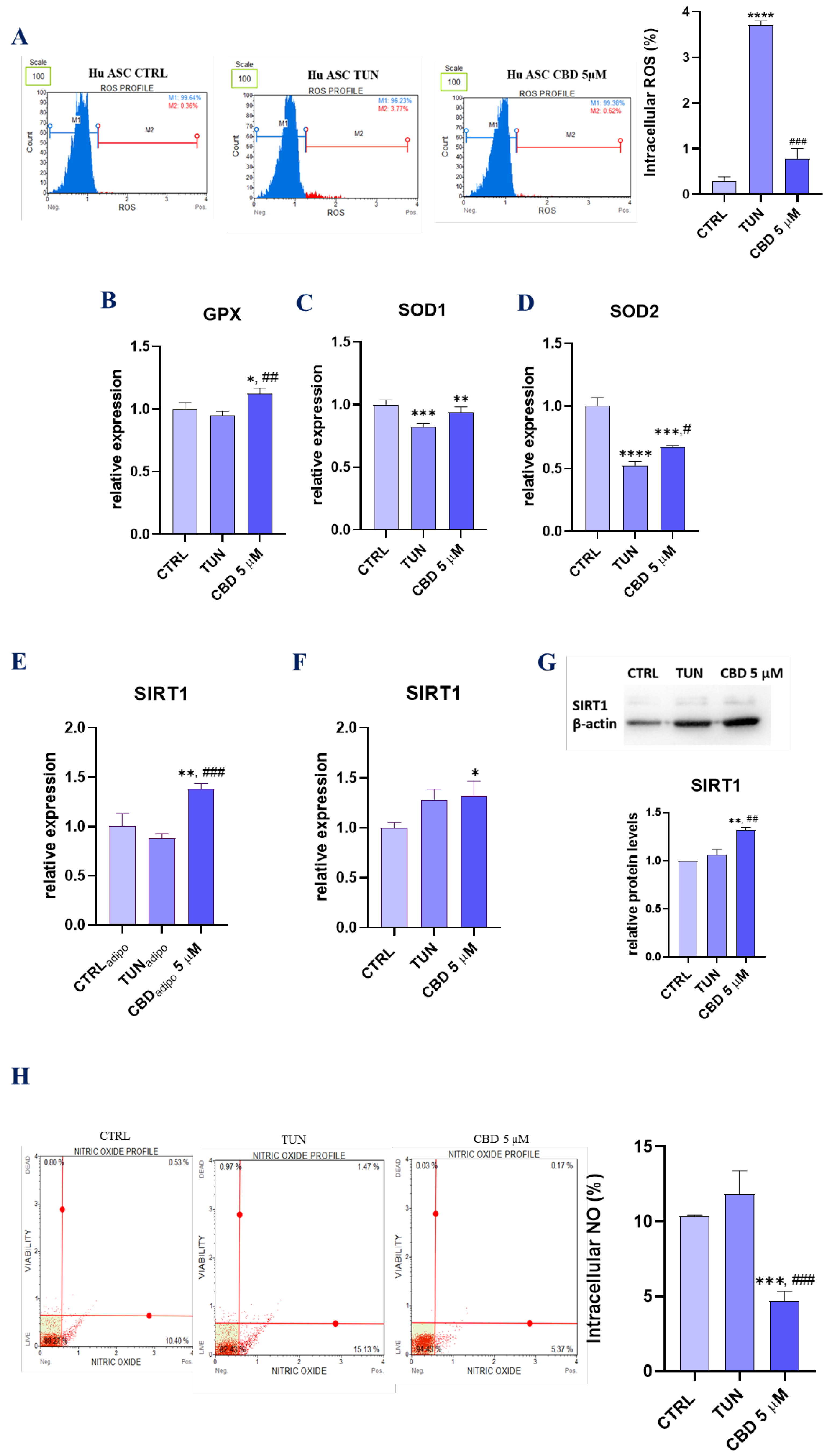
3.4. Assessment of Oxidative Stress (CBD Attenuates Oxidative Stress in Affected with Tunicamycin ASCs)
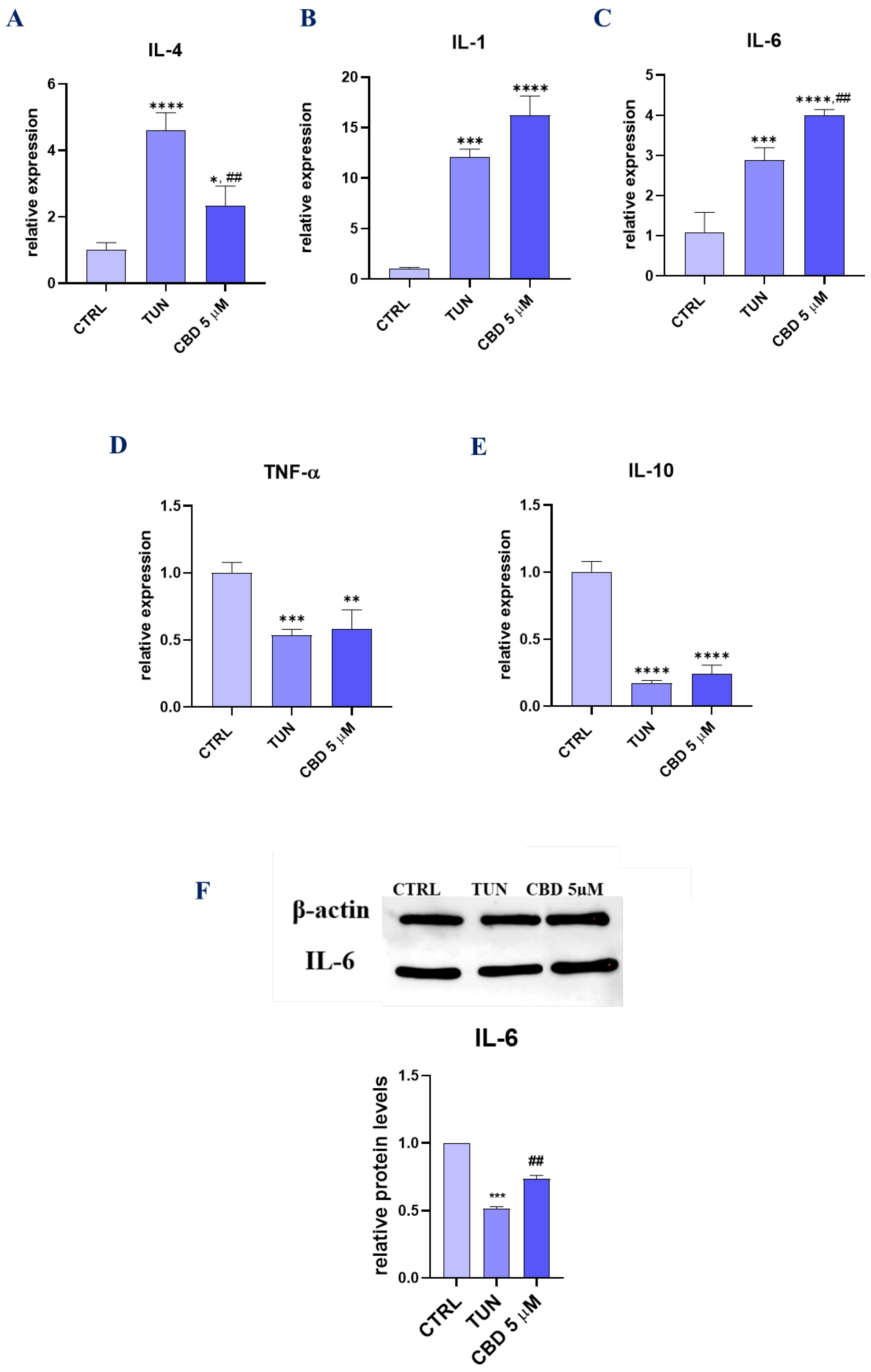
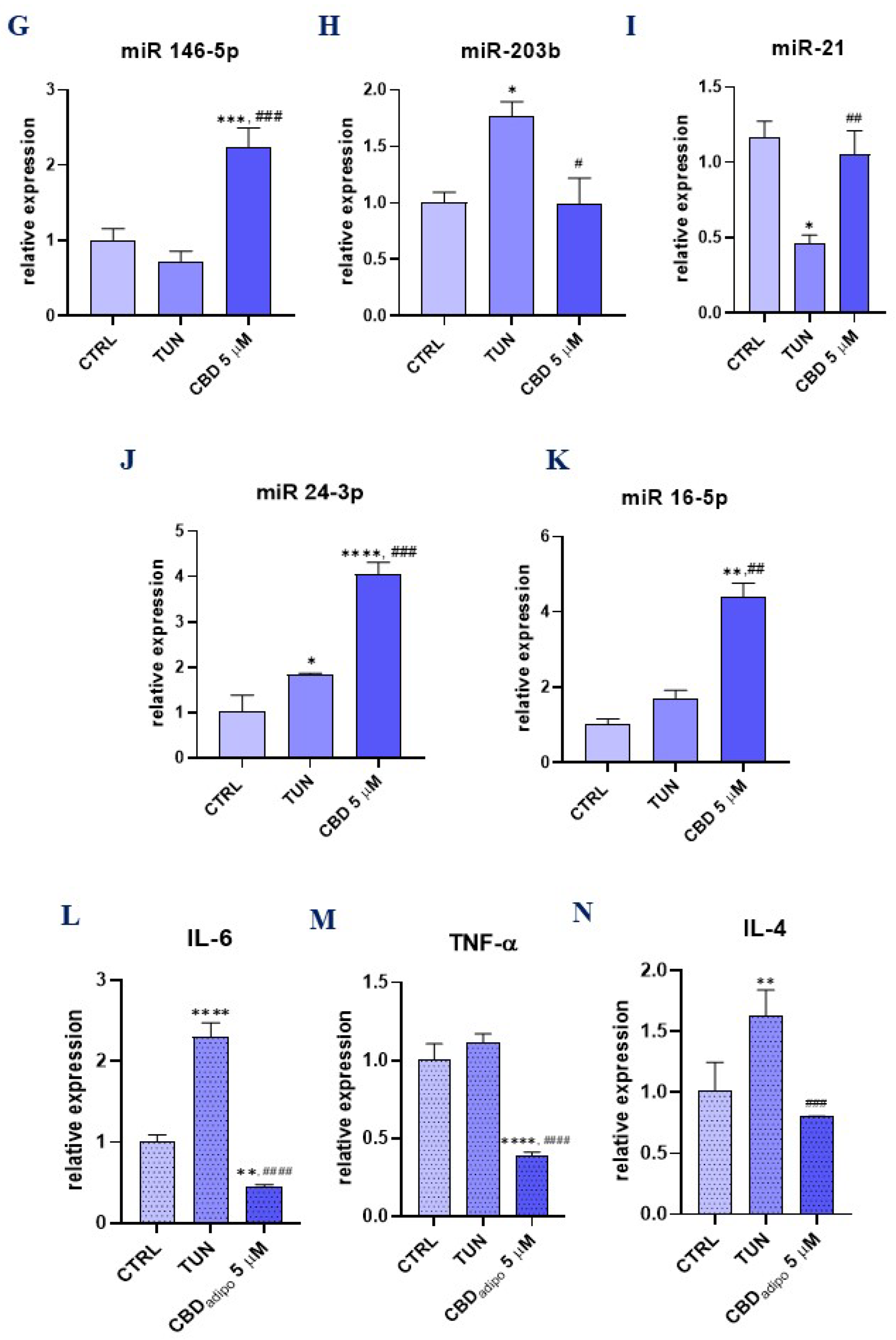
3.5. Assessment of the Inflammation (CBD Positively Modulates Inflammation during Stem Cell Adipogenesis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Cislo-Pakuluk, A.; Marycz, K. A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications. Stem Cell Rev. Rep. 2017, 13, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Geburek, F.; Röcken, M.; Marycz, K. Stem Cells in Equine Veterinary Practice-Current Trends, Risks, and Perspectives. J. Clin. Med. 2019, 8, 675. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxidative Med. Cell. Longev. 2016, 2016, 4710326. [Google Scholar] [CrossRef]
- Kornicka, K.; Szłapka-Kosarzewska, J.; Śmieszek, A.; Marycz, K. 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J. Cell. Mol. Med. 2018, 23, 237–259. [Google Scholar] [CrossRef]
- Kornicka, K.; Marycz, K.; Marędziak, M.; Tomaszewski, K.; Nicpoń, J. The effects of the DNA methyltranfserases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J. Cell. Mol. Med. 2017, 21, 387–401. [Google Scholar] [CrossRef]
- Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. [Google Scholar] [CrossRef] [Green Version]
- Krott, L.M.; Piscitelli, F.; Heine, M.; Borrino, S.; Scheja, L.; Silvestri, C.; Heeren, J.; Di Marzo, V. Endocannabinoid regulation in white and brown adipose tissue following thermogenic activation. J. Lipid Res. 2016, 57, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Sidibeh, C.O.; Pereira, M.; Börjesson, J.L.; Kamble, P.G.; Skrtic, S.; Katsogiannos, P.; Sundbom, M.; Svensson, M.K.; Eriksson, J.W. Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance. Endocrine 2017, 55, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; van’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef]
- Kumar, R.N.; Chambers, W.A.; Pertwee, R.G. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia 2001, 56, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-Dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int. Immunopharmacol. 2009, 9, 216–222. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef]
- Romero-Zerbo, S.Y.; García-Fernández, M.; Espinosa-Jiménez, V.; Pozo-Morales, M.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Lara, E.; Cobo-Vuilleumier, N.; Rafacho, A.; Olveira, G.; et al. The Atypical Cannabinoid Abn-CBD Reduces Inflammation and Protects Liver, Pancreas, and Adipose Tissue in a Mouse Model of Prediabetes and Non-alcoholic Fatty Liver Disease. Front. Endocrinol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Trillou, C.R.; Delgorge, C.; Menet, C.; Arnone, M.; Soubrié, P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Fellous, T.; De Maio, F.; Kalkan, H.; Carannante, B.; Boccella, S.; Petrosino, S.; Maione, S.; Di Marzo, V.; Iannotti, F.A. Phytocannabinoids promote viability and functional adipogenesis of bone marrow-derived mesenchymal stem cells through different molecular targets. Biochem. Pharmacol. 2020, 175, 113859. [Google Scholar] [CrossRef] [PubMed]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rossi, E.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Choublier, N.; Smirnova, M.; Glacial, F.; Perrière, N.; Bourdoulous, S.; et al. Cannabidiol Increases Proliferation, Migration, Tubulogenesis, and Integrity of Human Brain Endothelial Cells through TRPV2 Activation. Mol. Pharm. 2019, 16, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Cort, A.; Müller-Sánchez, C.; Espel, E. Anti-proliferative and cytotoxic effect of cannabidiol on human cancer cell lines in presence of serum. BMC Res. Notes 2020, 13, 389. [Google Scholar] [CrossRef]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G.; et al. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front. Physiol. 2016, 7, 559. [Google Scholar] [CrossRef]
- Gómez del Pulgar, T.; Velasco, G.; Guzmán, M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 2000, 347, 369–373. [Google Scholar] [CrossRef]
- Gowran, A.; McKayed, K.; Campbell, V.A. The cannabinoid receptor type 1 is essential for mesenchymal stem cell survival and differentiation: Implications for bone health. Stem Cells Int. 2013, 2013, 796715. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef]
- Tsao, C.-H.; Shiau, M.-Y.; Chuang, P.-H.; Chang, Y.-H.; Hwang, J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014, 55, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Velde, A.T.; Huijbens, R.; Heije, K.; De Vries, J.; Figdor, C. Interleukin-4 (IL-4) Inhibits Secretion of IL-1β, Tumor Necrosis Factor a, and IL-6 by Human Monocytes. Blood 1990, 76, 1392–1397. [Google Scholar] [CrossRef]
- Cheung, D.L.; Hart, P.H.; Vitti, G.F.; Whitty, A.G.; Hamilton, A.J. Contrasting effects of interferon-gamma and interleukin-4 on the interleukin-6 activity of stimulated human monocytes. Immunology 1990, 71, 70–75. [Google Scholar] [PubMed]
- Chang, Y.-H.; Ho, K.-T.; Lu, S.-H.; Huang, C.-N.; Shiau, M.-Y. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. 2012, 36, 993–998. [Google Scholar] [CrossRef]
- Makwana, R.; Gozzard, N.; Spina, D.; Page, C. TNF-α-induces airway hyperresponsiveness to cholinergic stimulation in guinea pig airways. Br. J. Pharmacol. 2012, 165, 1978–1991. [Google Scholar] [CrossRef]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef]
- Dudášová, A.; Keir, S.; Parsons, M.; Molleman, A.; Page, C. The effects of cannabidiol on the antigen-induced contraction of airways smooth muscle in the guinea-pig. Pulm. Pharmacol. Ther. 2013, 26, 373–379. [Google Scholar] [CrossRef]
- Makwana, R.; Venkatasamy, R.; Spina, D.; Page, C.P. The Effect of Phytocannabinoids on Airway Hyper-Responsiveness, Airway Inflammation, and Cough. J. Pharmacol. Exp. Ther. 2015, 353, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X. Downregulated miR-203 attenuates IL-β, IL-6, and TNF-α activation in TRAF6-treated human renal mesangial and tubular epithelial cells. Int. J. Clin. Exp. Pathol. 2020, 13, 324–331. [Google Scholar] [PubMed]
- Cai, Z.; Li, K.; Yang, K.; Luo, D.; Xu, H. Suppression of miR-203-3p inhibits lipopolysaccharide induced human intervertebral disc inflammation and degeneration through upregulating estrogen receptor α. Gene Ther. 2020, 27, 417–426. [Google Scholar] [CrossRef]
- Pua, H.H.; Steiner, D.F.; Patel, S.; Gonzalez, J.; Ortiz-Carpena, J.F.; Kageyama, R.; Chiou, N.-T.; Gallman, A.; de Kouchkovsky, D.; Jeker, L.; et al. MicroRNAs 24 and 27 suppress allergic inflammation and target a network of regulators of T helper-2 cell-associated cytokine production. Immunity 2016, 44, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y. MiR-24 inhibits inflammatory responses in LPS-induced acute lung injury of neonatal rats through targeting NLRP3. Pathol. Res. Pract. 2019, 215, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qian, X.; Ding, R. MiR-24-3p attenuates IL-1β-induced chondrocyte injury associated with osteoarthritis by targeting BCL2L12. J. Orthop. Surg. Res. 2021, 16, 371. [Google Scholar] [CrossRef]
- Yan, F.; Wufuer, D.; Ding, J.; Wang, J. MicroRNA miR-146a-5p inhibits the inflammatory response and injury of airway epithelial cells via targeting TNF receptor-associated factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef]
- Luly, F.R.; Lévêque, M.; Licursi, V.; Cimino, G.; Martin-Chouly, C.; Théret, N.; Negri, R.; Cavinato, L.; Ascenzioni, F.; Del Porto, P. MiR-146a is over-expressed and controls IL-6 production in cystic fibrosis macrophages. Sci. Rep. 2019, 9, 16259. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Z.; Yuan, M.; Zhang, Y.; Zhao, B.; Wang, J.; Zhang, A.; Li, G. MicroRNA-16 suppresses the activation of inflammatory macrophages in atherosclerosis by targeting PDCD4. Int. J. Mol. Med. 2016, 37, 967–975. [Google Scholar] [CrossRef]
- Nara, K.; Kawashima, N.; Noda, S.; Fujii, M.; Hashimoto, K.; Tazawa, K.; Okiji, T. Anti-inflammatory roles of microRNA 21 in lipopolysaccharide-stimulated human dental pulp cells. J. Cell. Physiol. 2019, 234, 21331–21341. [Google Scholar] [CrossRef]
- Liechty, C.; Hu, J.; Zhang, L.; Liechty, K.W.; Xu, J. Role of microRNA-21 and Its Underlying Mechanisms in Inflammatory Responses in Diabetic Wounds. Int. J. Mol. Sci. 2020, 21, 3328. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence (5′->3′) | Annieling Temperature [°C] |
|---|---|---|
| BAX | F: ACCAAGAAGCTGAGCGAGTGTC | 60.4 |
| R: ACAAAGATGGTCACGGTCTGC | ||
| BCL2 | F: ATCGCCCTGTGGATGACTGAG | 60.4 |
| R: CAGCCAGGAGAAATCAAACAGAGG | ||
| p21 | F: TGCCGAAGTCAGTTCCTTGT | 60.4 |
| R: GTTCTGACATGGCGCCTCC | ||
| p53 | F: AGTCACAGCACATGACGGAGG | 60.4 |
| R: GGAGTCTTCCAGTGTGATGATGG | ||
| Cas3 | F: GCGGTTGTAGAAGTTAATAAAGGT | 62.8 |
| R: CGACATCTGTACCAGACCGAG | ||
| Cas9 | F: TTGGTGATGTCGAGCAGAAAG | 61.4 |
| R: CCAGGGTCTCAACGTACCAG | ||
| GPX | F: CTCCGGAACAACAGCCTTCT | 51 |
| R: GGAAAGGGGTCTGTGATGGG | ||
| SOD1 | F: GACCATTGCATCATTGGCCG | 51 |
| R: CAAGCCAAACGACTTCCAGC | ||
| SOD2 | F: GGAGCGGCACTCGTGG | 52 |
| R: CAGATACCCCAAAGCCGGAG | ||
| SIRT1 | F: ACAGGTTGCGGGAATCCAAA | 66 |
| R: GTTCATCAGCTGGGCACCTA | ||
| IL-1β | F: AAACAGATGAAGTGCTCCTTCCAGG | 66 |
| R: TGGAGAACACCACTTGTTGCTCCA | ||
| IL-6 | F: TCCTTCTCCACAAACATGTAACAA | 66 |
| R: ATTTGTGGTTGGGTCAGGGG | ||
| TNF α | F: AGTGACAAGCCTGTAGCCCA | 62.4 |
| R: GTCTGGTAGGAGACGGCGAT | ||
| IL-4 | F: CTTTGCTGCCTCCAAGAACAC | 62.4 |
| R: GCGAGTGTCCTTCTCATGGT | ||
| IL-10 | F: AGACAGACTTGCAAAAGAAGGC | 65 |
| R: TCGAAGCATGTTAGGCAGGTT | ||
| PERK | F: TGCTCCCACCTCAGCGAC | 67 |
| R: TTTCAGGATCCAAGGCAGCA | ||
| eIF2-α | F: ATGTTTCAGCCAAGCCCAGA | 61.4 |
| R: ACCAGGGGATCTACCACCAA | ||
| CHOP | F: TAAAGATGAGCGGGTGGCAG | 64.5 |
| R: GGATAATGGGGAGTGGCTGG | ||
| ATF6 | F: ACCTCCTTGTCAGCCCCTAA | 65.9 |
| R: CACTCCCTGAGTTCCTGCTG | ||
| IRE1 | F: CGGCCTCGGGATTTTTGGA | 66.6 |
| R: AGAAAGGCAGGCTCTTCCAC | ||
| XBP1 | F: CGCGGATCCGAATGAAGTGAGGCCAGTG | 62.8 |
| R: GGGGCTTGG TATATATGTGG |
| Primer miRNAs | Primer Sequence (5′->3′) |
|---|---|
| miR101-1/2 | TACAGTACTGTGATAACTGAA |
| miR17-5p | CAAAGTGCTTACAGTGCAGGTAG |
| miR16-5p | TAGCAGCACGTAAATATTGGCG |
| miR-203b | TTGAACTGTTAAGAACCACTGGA |
| miR-21 | TAGCTTATCAGACTGATGTTGA |
| miR 24-3p | TGGCTCAGTTCAGCAGGAACAG |
| miR 146-5p | TGAGAACTGAATTCCATGGGTT |
| Antibodies | Concentrations | CAT Numbers | Company |
|---|---|---|---|
| β actin | 1:1000 | orb10033 | Biorbyt |
| SIRT1 | 1:1000 | ARP32386 | Aviva |
| IL-6 | 1:1000 | ab6672 | Abcam |
| XBP1 | 1:1000 | ARP31440_P050 | Aviva |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczuk, A.; Marycz, K.; Kornicka-Garbowska, K.; Kornicka, J.; Bujalska-Zadrożny, M.; Groborz, S. Cannabidiol (CBD) Protects Adipose-Derived Mesenchymal Stem Cells (ASCs) against Endoplasmic Reticulum Stress Development and Its Complications. Int. J. Environ. Res. Public Health 2022, 19, 10864. https://doi.org/10.3390/ijerph191710864
Kowalczuk A, Marycz K, Kornicka-Garbowska K, Kornicka J, Bujalska-Zadrożny M, Groborz S. Cannabidiol (CBD) Protects Adipose-Derived Mesenchymal Stem Cells (ASCs) against Endoplasmic Reticulum Stress Development and Its Complications. International Journal of Environmental Research and Public Health. 2022; 19(17):10864. https://doi.org/10.3390/ijerph191710864
Chicago/Turabian StyleKowalczuk, Anna, Krzysztof Marycz, Katarzyna Kornicka-Garbowska, Justyna Kornicka, Magdalena Bujalska-Zadrożny, and Sylwia Groborz. 2022. "Cannabidiol (CBD) Protects Adipose-Derived Mesenchymal Stem Cells (ASCs) against Endoplasmic Reticulum Stress Development and Its Complications" International Journal of Environmental Research and Public Health 19, no. 17: 10864. https://doi.org/10.3390/ijerph191710864
APA StyleKowalczuk, A., Marycz, K., Kornicka-Garbowska, K., Kornicka, J., Bujalska-Zadrożny, M., & Groborz, S. (2022). Cannabidiol (CBD) Protects Adipose-Derived Mesenchymal Stem Cells (ASCs) against Endoplasmic Reticulum Stress Development and Its Complications. International Journal of Environmental Research and Public Health, 19(17), 10864. https://doi.org/10.3390/ijerph191710864







