The Evolving Scenario of COVID-19 in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Presentation and Radiological Findings
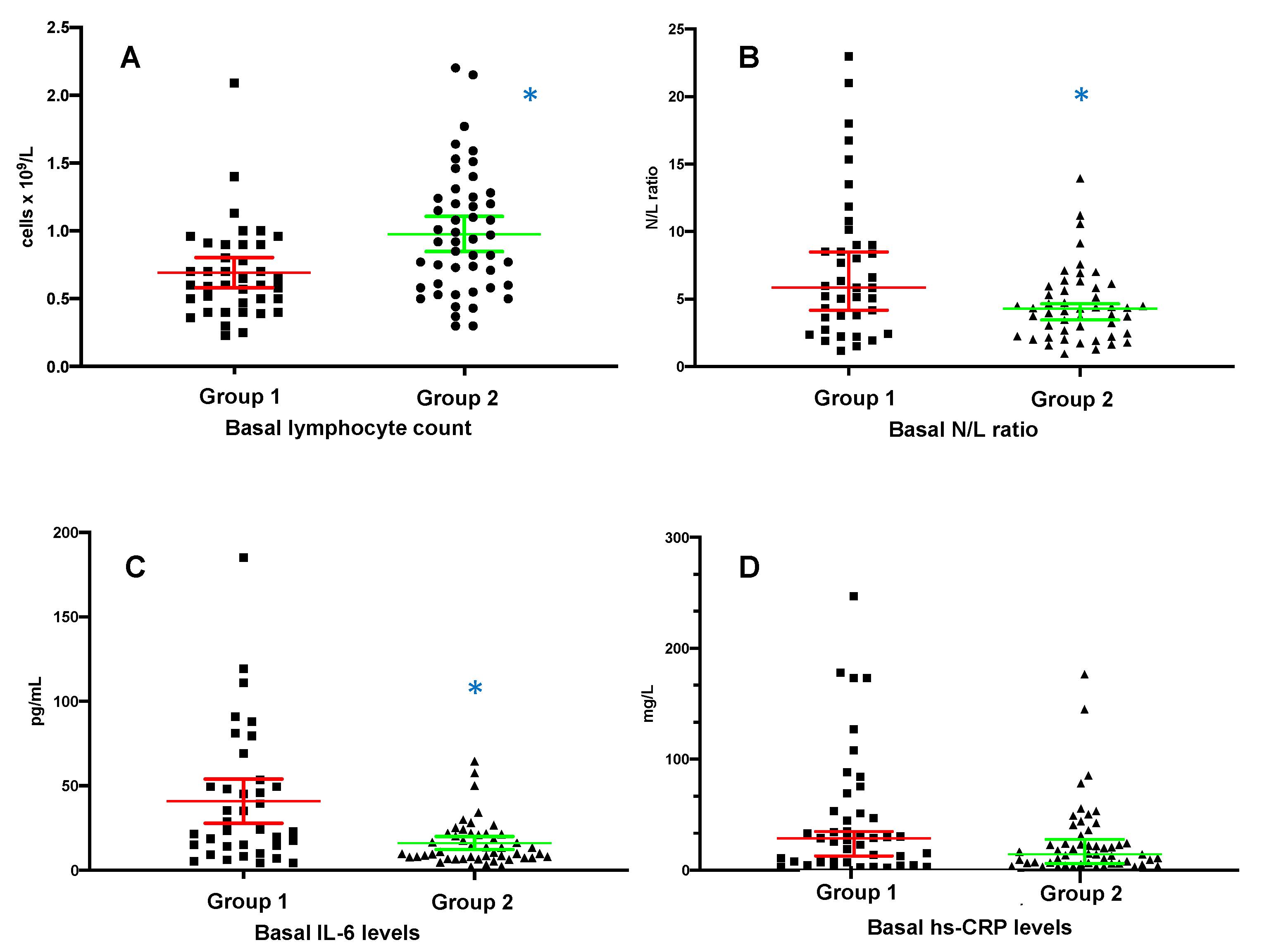
3.3. Basal Laboratory Characteristics
3.4. Time Evolution of Inflammatory Parameters
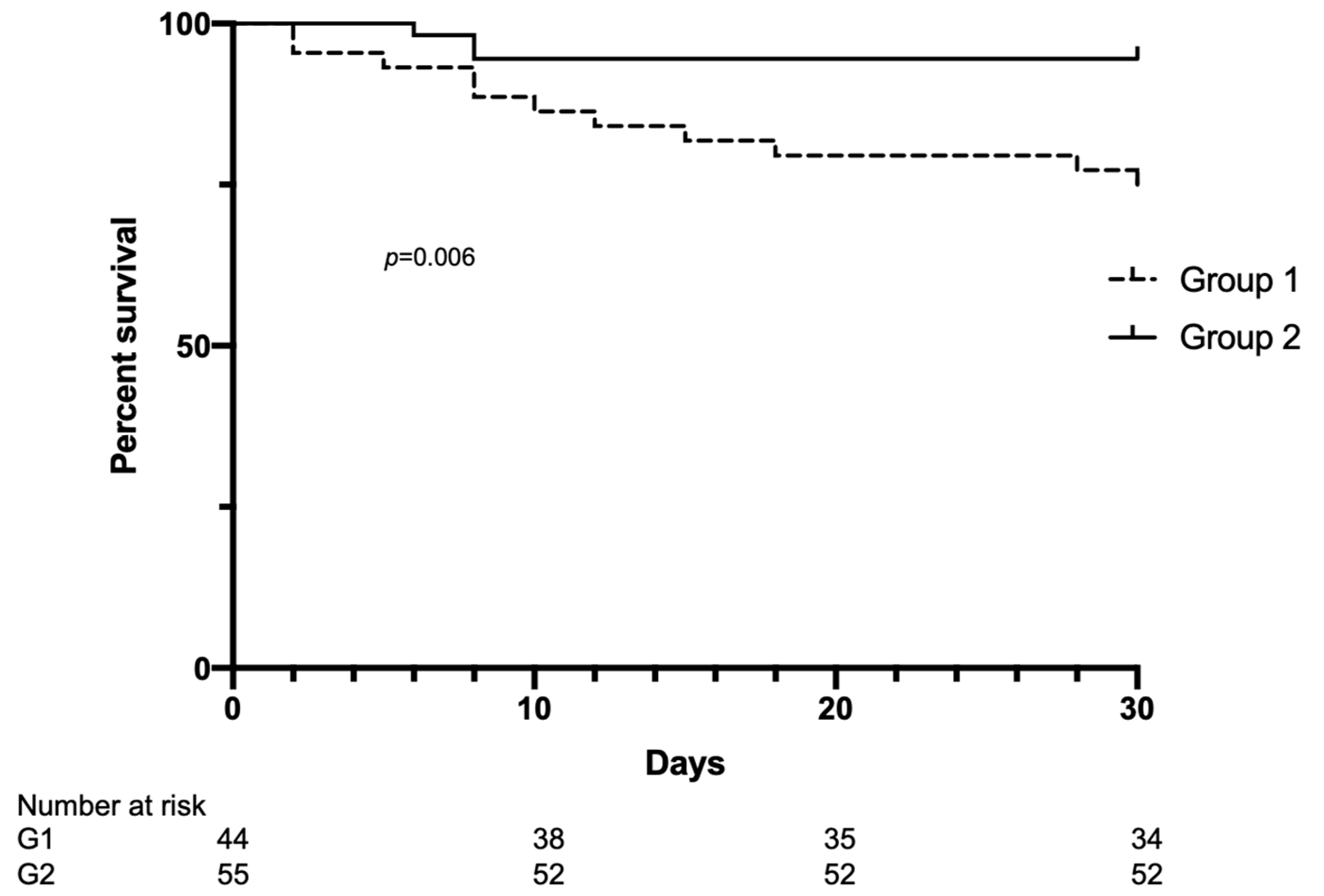
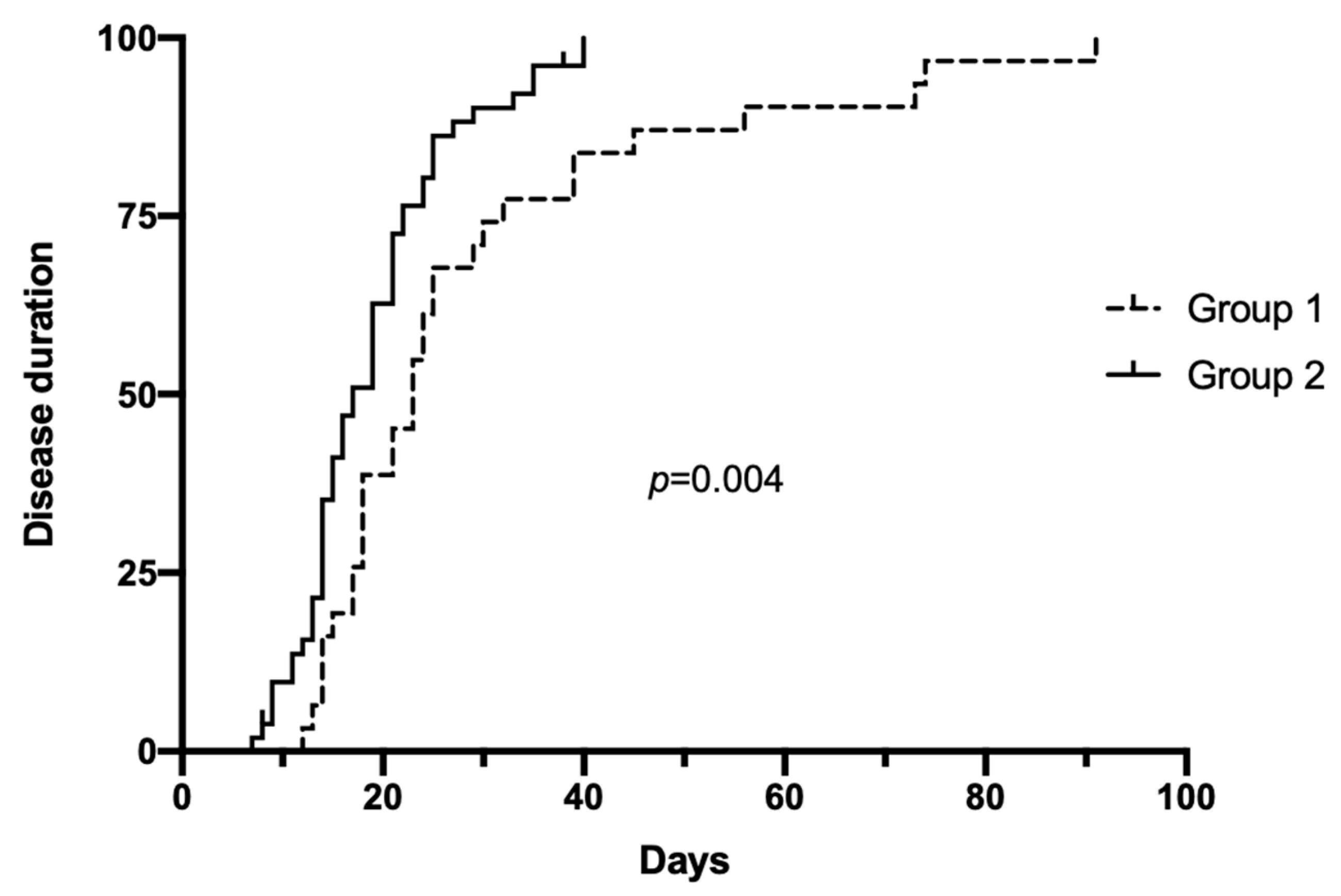
3.5. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vena, A.; Giacobbe, D.R.; Di Biagio, A.; Mikulska, M.; Taramasso, L.; De Maria, A.; Ball, L.; Brunetti, I.; Loconte, M.; Patroniti, N.A.; et al. Clinical characteristics, management, and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin. Microbiol. Infect. 2020, 26, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef]
- Noor, F.M.; Islam, M.M. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J. Community Health 2020, 45, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Peana, M.; Pivina, L.; Srinath, S.; Benahmed, A.G.; Semenova, Y.; Menzel, A.; Dadar, M.; Bjørklund, G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2021, 224, 108651. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Esposito, P.; Taramasso, L.; Magnasco, L.; Saio, M.; Briano, F.; Russo, C.; Dettori, S.; Vena, A.; Di Biagio, A.; et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J. Nephrol. 2021, 34, 173–183. [Google Scholar] [CrossRef]
- Goicoechea, M.; Cámara, L.A.S.; Macías, N.; de Morales, A.M.; Rojas, G.; Bascuñana, A.; Arroyo, D.; Vega, A.; Abad, S.; Verde, E.; et al. COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020, 98, 27–34. [Google Scholar] [CrossRef]
- Goffin, E.; Candellier, A.; Vart, P.; Noordzij, M.; Arnol, M.; Covic, A.; Lentini, P.; Malik, S.; Reichert, L.J.; Sever, M.S.; et al. COVID-19-related mortality in kidney transplant and haemodialysis patients, a comparative, prospective registry-based study. Nephrol. Dial. Transplant. 2021, 36, 2094–2105. [Google Scholar] [CrossRef]
- Cao, X. COVID-19, immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.M.; Ison, M.G.; Ghossein, C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am. J. Kidney Dis. 2020, 75, 417–425. [Google Scholar] [CrossRef] [PubMed]
- El Karoui, K.; Hourmant, M.; Ayav, C.; Glowacki, F.; Couchoud, C.; Lapidus, N.; REIN Registry. Vaccination and COVID-19 Dynamics in Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2022, 17, 395–402. [Google Scholar] [CrossRef]
- Angel-Korman, A.; Peres, E.; Bryk, G.; Lustig, Y.; Indenbaum, V.; Amit, S.; Rappoport, V.; Katzir, Z.; Yagil, Y.; Iaina, N.L.; et al. Diminished and waning immunity to COVID-19 vaccination among hemodialysis patients in Israel, the case for a third vaccine dose. Clin. Kidney J. 2021, 15, 226–234. [Google Scholar] [CrossRef]
- Aleem, A.; Samad, A.B.A.; Slenker, A.K. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19). Available online: https://www.ncbi.nlm.nih.gov/books/NBK570580/ (accessed on 6 July 2022).
- Esposito, P.; Russo, R.; Conti, N.; Falqui, V.; Massarino, F.; Moriero, E.; Peloso, G.; Traverso, G.B.; Garibotto, G.; Viazzi, F. Management of COVID-19 in hemodialysis patients, The Genoa experience. Hemodial. Int. 2020, 24, 423–427. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 Antibody. Available online: https://www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed on 6 July 2022).
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kalantar-Zadeh, K.; Lee, G.H. The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin. J. Am. Soc. Nephrol. 2006, 1 (Suppl. 1), S9–S18. [Google Scholar] [CrossRef]
- Ouyang, J.; Bajracharya, S.; John, S.; Wagner, J.; Xu, J.; Luo, Y.; Thaxton, M.; Salifu, M.; Yap, E.; Mallappallil, M. Clotting of Hemodialysis Access in Patients with COVID-19 in an Inner-City Hospital. Nephron Exp. Nephrol. 2022, 146, 179–184. [Google Scholar] [CrossRef]
- Shabaka, A.; Gruss, E.; Landaluce-Triska, E.; Gallego-Valcarce, E.; Cases-Corona, C.; Ocaña, J.; Tato-Ribera, A.; Lopez-Revuelta, K.; Furaz-Czerpak, K.R.; Fernández-Juárez, G. Late thrombotic complications after SARS-CoV-2 infection in hemodialysis patients. Hemodial. Int. 2021, 25, 507–514. [Google Scholar] [CrossRef]
- Salerno, S.; Messana, J.M.; Gremel, G.W.; Dahlerus, C.; Hirth, R.A.; Han, P.; Segal, J.H.; Xu, T.; Shaffer, D.; Jiao, A.; et al. COVID-19 Risk Factors and Mortality Outcomes Among Medicare Patients Receiving Long-term Dialysis. JAMA Netw. Open 2021, 4, e2135379. [Google Scholar] [CrossRef]
- Swan, D.A.; Bracis, C.; Janes, H.; Moore, M.; Matrajt, L.; Reeves, D.B.; Burns, E.; Donnell, D.; Cohen, M.S.; Schiffer, J.T.; et al. COVID-19 vaccines that reduce symptoms but do not block infection need higher coverage and faster rollout to achieve population impact. Sci. Rep. 2021, 11, 15531. [Google Scholar] [CrossRef]
- Galmiche, S.; Nguyen, L.B.L.; Tartour, E.; de Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations, a systematic review. Clin. Microbiol. Infect. 2022, 28, 163–177. [Google Scholar] [CrossRef]
- Espi, M.; Charmetant, X.; Barba, T.; Mathieu, C.; Pelletier, C.; Koppe, L.; Chalencon, E.; Kalbacher, E.; Mathias, V.; Ovize, A.; et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022, 101, 390–402. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- ECDC-European Center for Disease Control. SARS-CoV-2 Variants of Concern as of 6 May 2021. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 6 July 2022).
- Lai, A.; Bergna, A.; Menzo, S.; Zehender, G.; Caucci, S.; Ghisetti, V.; Rizzo, F.; Maggi, F.; Cerutti, F.; Giurato, G.; et al. Circulating SARS-CoV-2 variants in Italy, October 2020–March 2021. Virol. J. 2021, 18, 168. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. 2021. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_28-dicembre-2021.pdf (accessed on 18 April 2022).
- Boscolo-Rizzo, P.; Tirelli, G.; Meloni, P.; Hopkins, C.; Madeddu, G.; De Vito, A.; Gardenal, N.; Valentinotti, R.; Tofanelli, M.; Borsetto, D.; et al. Coronavirus disease 2019 (COVID-19)-related smell and taste impairment with widespread diffusion of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) Omicron variant. Int. Forum. Allergy Rhinol. 2022. [Google Scholar] [CrossRef]
- Hui, K.P.; Ho, J.C.; Cheung, M.C.; Ng, K.C.; Ching, R.H.; Lai, K.L.; Kam, T.T.; Gu, H.; Sit, K.Y.; Hsin, M.K.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J.; et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with COVID-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef]
- European Agency of Drugs. Available online: https://www.ema.europa.eu/en/documents/product-information/paxlovid-epar-product-information_en.pdf (accessed on 6 July 2022).
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf (accessed on 6 July 2022).
- Available online: https://www.aifa.gov.it/documents/20142/1475526/report_n.41_monitoraggio_monoclonali_13.01.2022.pdf/e387c303-8dcb-0df3-5241-fd421d7105be (accessed on 6 July 2022).
- El Karoui, K.; De Vriese, A.S. COVID-19 in dialysis: Clinical impact, immune response, prevention, and treatment. Kidney Int. 2022, 101, 883–894. [Google Scholar] [CrossRef]
| Group 1 | Group 2 | p | |
|---|---|---|---|
| N | 44 | 55 | |
| Age, yrs | 69.3 ± 14.6 | 67.4 ± 15.3 | 0.6 |
| Men, n (%) | 24 (54.5) | 39 (70.9) | 0.09 |
| BMI, kg/m2 | 24.1 ± 4.9 | 25 ± 5.2 | 0.4 |
| Length of time on dialysis, months | 67 ± 58.4 | 39 ± 29 | 0.015 |
| Vascular access (arteriovenous fistula, n%) | 16 (36) | 29 (52) | 0.1 |
| Comorbidities | |||
| Hypertension, n (%) | 34 (77) | 48 (87.2) | 0.3 |
| Diabetes mellitus, n (%) | 12 (27.3) | 23 (41.8) | 0.03 |
| Cardiovascular disease, n (%) | 30 (68.2) | 22 (40) | 0.008 |
| Autoimmune disorders, n (%) | 1 (2) | 5 (9) | 0.2 |
| Prior transplant, n (%) | 4 (9) | 8 (14.5) | 0.5 |
| History of COVID-19, n (%) | - | 6 (11) | - |
| Vaccinated patients, n (%) | - | 52 (94.5) | - |
| Vaccinated with booster dose, n (%) | - | 43 (78.2) | - |
| Responders after the second dose of vaccine, n (%) * | - | 39 (80) |
| COVID-19 Presentation, n (%) | Group 1 (n, 44) | Group 2 (n, 55) | p |
|---|---|---|---|
| Patients symptomatic at diagnosis | 40 (90) | 35 (62) | 0.002 |
| Fever | 28 (63.3) | 17 (31) | 0.002 |
| Cough | 17 (38.6) | 17 (31) | 0.5 |
| Dyspnea (exertional or rest) | 14 (32) | 4 (7) | 0.003 |
| Dysgeusia/anosmia | 10 (23) | 2 (3.6) | 0.005 |
| Fatigue/malaise | 3 (6.8) | 1 (1.8) | 0.8 |
| Gastrointestinal | 5 (11.4) | 1 (1.8) | 0.08 |
| Myalgia | 6 (13.6) | 0 | 0.006 |
| Arterial blood oxygen saturation, % | 0.95 ± 0.03 | 0.96 ± 0.03 | 0.16 |
| Systolic blood pressure, mmHg | 124.7 ± 28 | 138 ± 25 | 0.018 |
| Diastolic blood pressure, mmHg | 62.7 ± 13 | 67.7 ± 14 | 0.17 |
| Chest X-ray (clinical indication), n (%) | 17 (38.6) | 6 (10.9) | 0.001 * |
| Lung consolidations | 14 (82) | 5 (83) | 1 |
| Pleural effusion | 2 (11.8) | 2 (33) | 0.2 |
| Group 1 | Group 2 | p | |
|---|---|---|---|
| N | 44 | 55 | |
| WBC count, ×109/L | 5.5 ± 3.2 | 5.5 ± 1.8 | 0.2 |
| Neutrophil count, ×109/L | 4.3 ± 3.2 | 3.6 ± 1.4 | 0.5 |
| Neutrophils, (% of WBC) | 72.2 ± 17.7 | 66.7 ± 10.9 | 0.003 |
| Lymphocyte count, ×109/L | 0.69 ± 0.35 | 0.97 ± 0.45 | 0.008 |
| Lymphocytes, (% of WBC) | 14.7 ± 8.9 | 18.2 ± 8.1 | 0.024 |
| Neutrophil/lymphocyte ratio | 5.8 (5.4) | 4.2 (3.5) | 0.009 |
| Platelet count, ×109/L | 200 ± 127 | 194 ± 70 | 0.3 |
| Hemoglobin, mmol/L | 6.7 ± 0.99 | 6.95 ± 0.8 | 0.19 |
| Albumin, g/L | 34 ± 4 | 36 ± 7 | 0.07 |
| LDH, U/L | 254 ± 98 | 220 ± 173 | 0.003 |
| Procalcitonin, μg/L | 1.81 (3.3) | 1.40 (1.5) | 0.25 |
| hs-CRP, mg/L | 28.8 (45.6) | 14.4 (16.5) | 0.08 |
| Interleukin-6, pg/mL | 41 ± 39.4 | 16 ± 13.3 | 0.002 |
| Ferritin, μg/L | 552.5 (903) | 242 (407) | 0.02 |
| Group 1 | Group 2 | p | |
|---|---|---|---|
| N | 44 | 55 | |
| Illness duration, days * | 29.2 ± 19.5 | 18.8 ± 7.7 | 0.005 |
| Thrombotic events, n (%) | 2 (4.5) | 1 (1.8) | 0.5 |
| Hospitalized patients, n (%) | 17 (38) | 7 (12.7) | 0.004 |
| Deaths, n (%) | 11 (25) | 3 (5.4) | 0.008 |
| Time from diagnosis to death, days | 13 ± 10.5 | 7.3 ± 1.1 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, P.; Picciotto, D.; Cappadona, F.; Russo, E.; Falqui, V.; Conti, N.E.; Parodi, A.; Mallia, L.; Cavagnaro, S.; Battaglia, Y.; et al. The Evolving Scenario of COVID-19 in Hemodialysis Patients. Int. J. Environ. Res. Public Health 2022, 19, 10836. https://doi.org/10.3390/ijerph191710836
Esposito P, Picciotto D, Cappadona F, Russo E, Falqui V, Conti NE, Parodi A, Mallia L, Cavagnaro S, Battaglia Y, et al. The Evolving Scenario of COVID-19 in Hemodialysis Patients. International Journal of Environmental Research and Public Health. 2022; 19(17):10836. https://doi.org/10.3390/ijerph191710836
Chicago/Turabian StyleEsposito, Pasquale, Daniela Picciotto, Francesca Cappadona, Elisa Russo, Valeria Falqui, Novella Evelina Conti, Angelica Parodi, Laura Mallia, Sara Cavagnaro, Yuri Battaglia, and et al. 2022. "The Evolving Scenario of COVID-19 in Hemodialysis Patients" International Journal of Environmental Research and Public Health 19, no. 17: 10836. https://doi.org/10.3390/ijerph191710836
APA StyleEsposito, P., Picciotto, D., Cappadona, F., Russo, E., Falqui, V., Conti, N. E., Parodi, A., Mallia, L., Cavagnaro, S., Battaglia, Y., & Viazzi, F. (2022). The Evolving Scenario of COVID-19 in Hemodialysis Patients. International Journal of Environmental Research and Public Health, 19(17), 10836. https://doi.org/10.3390/ijerph191710836







