Changes in Sulfur Metabolism in Mouse Brains following Radon Inhalation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Radon Exposure and Sample Preparation
2.3. Sulfur Metabolomics Analysis
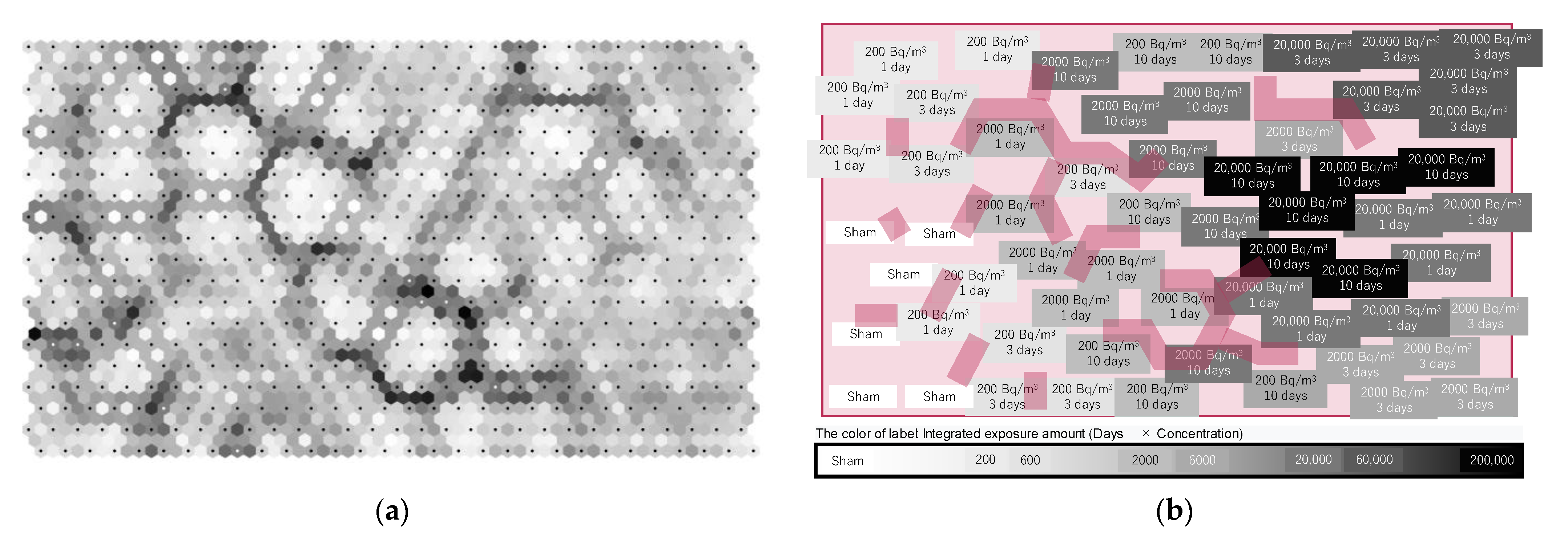
2.4. Statistical Analysis and Data Visualization Using Machine Learning
3. Results
3.1. Changes in Metabolites of the Brains of Mice Exposed to Radon
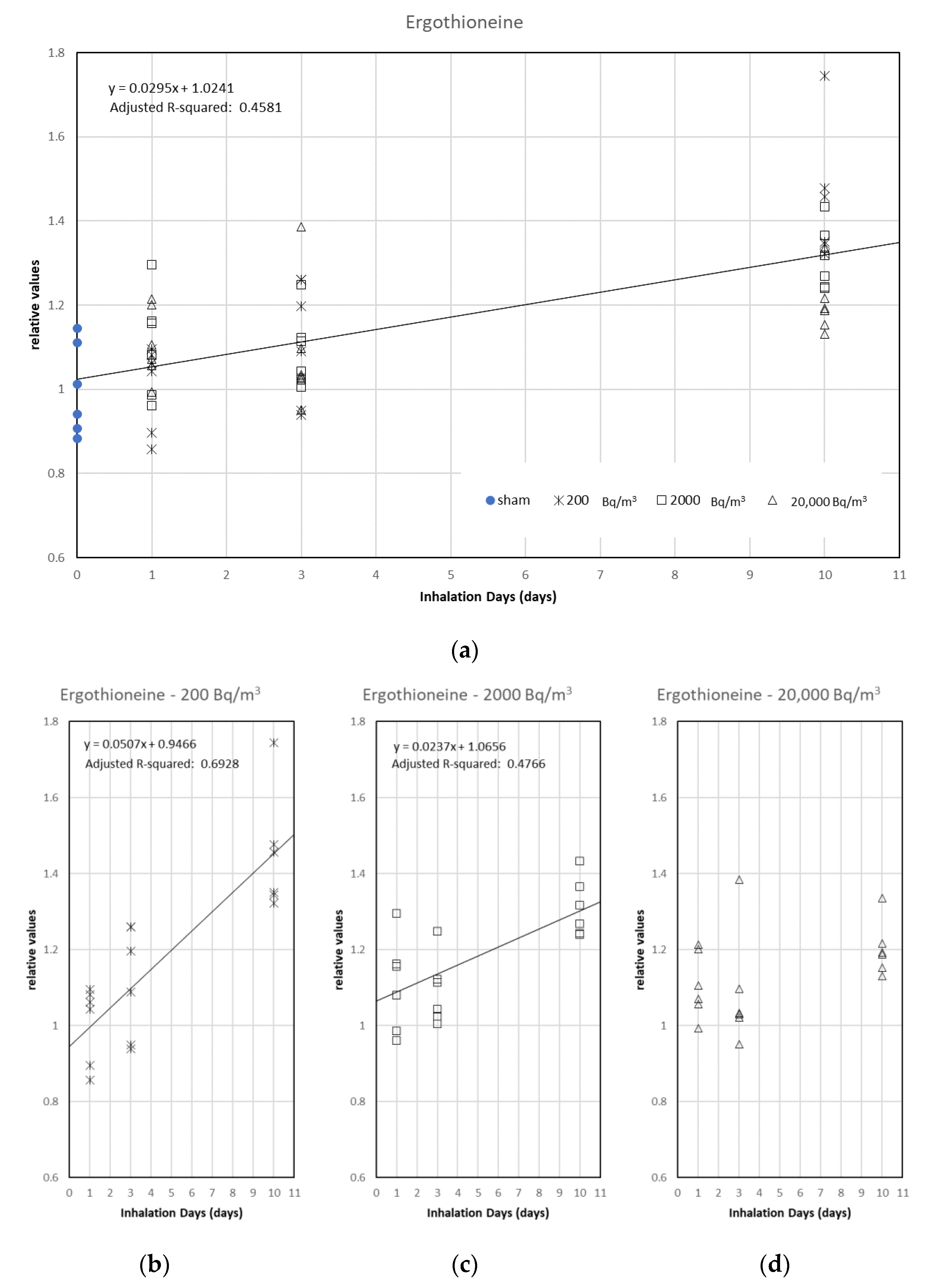
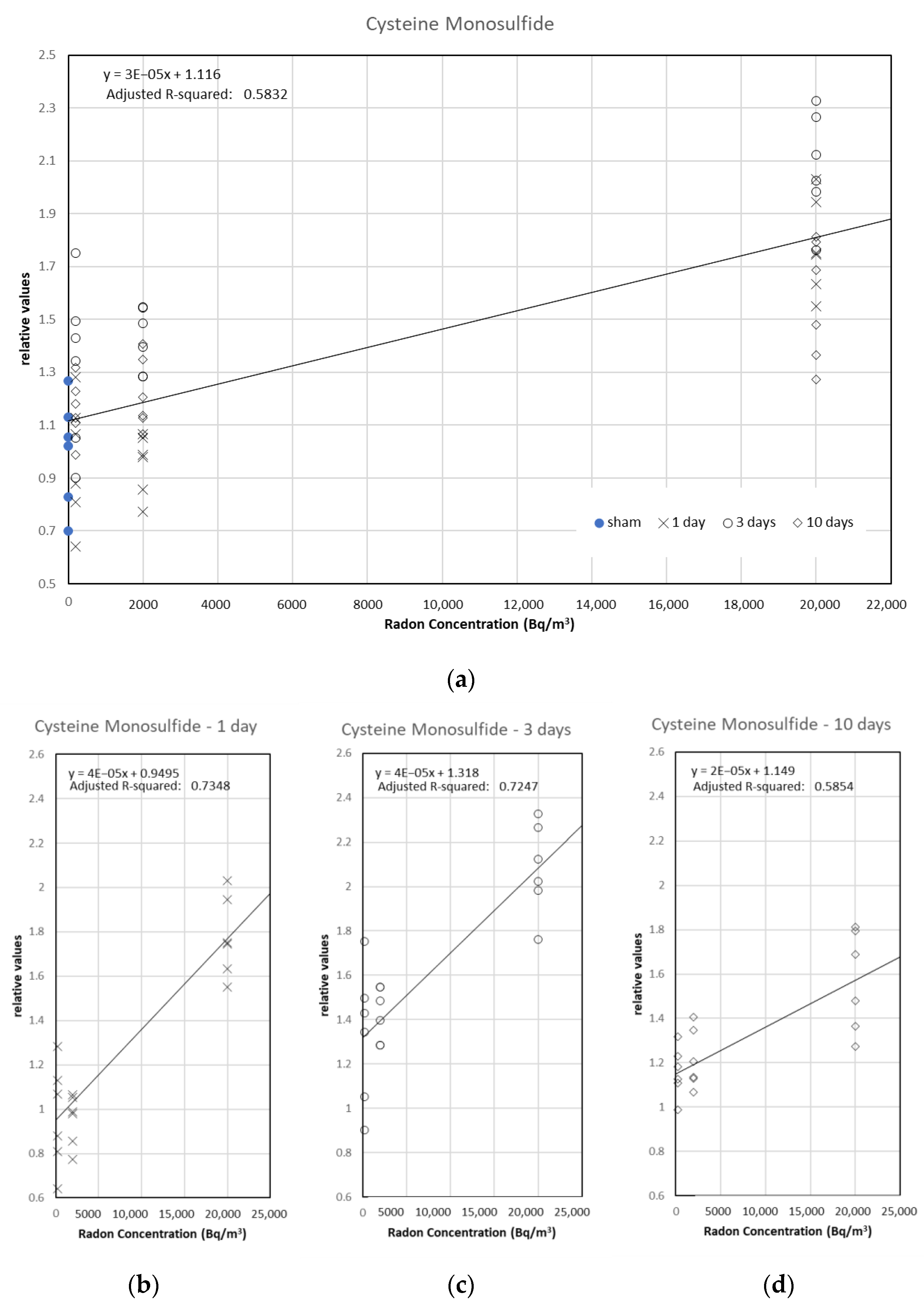
3.2. Analysis of the Relationship between Metabolites and Radon Inhalation Days or Radon Concentration
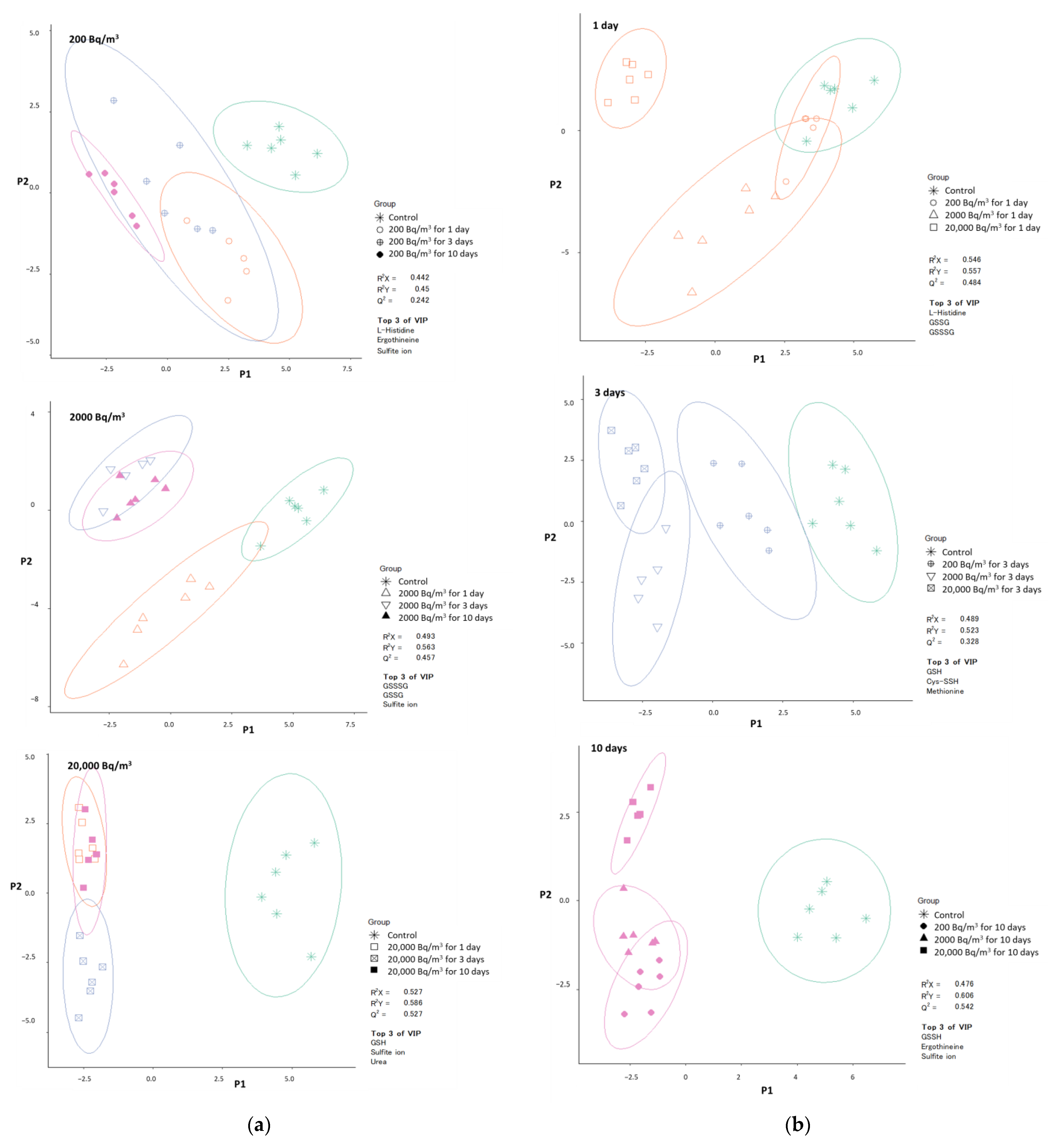
3.3. Visualization of the Effects of Radon Inhalation Using PLS-DA and ML
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreuzer, M.; Sobotzki, C.; Schnelzer, M.; Fenske, N. Factors Modifying the Radon-Related Lung Cancer Risk at Low Exposures and Exposure Rates among German Uranium Miners. Radiat. Res. 2017, 189, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C.; Yu, J.; Fan, Y.; Liu, D.; Zhou, W.; Shi, T. Residential Radon and Histological Types of Lung Cancer: A Meta-Analysis of Case—Control Studies. Int. J. Environ. Res. Public Health 2020, 17, 1457. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, F.; Yamaoka, K.; Hanamoto, K.; Kojima, S.; Hosaki, Y.; Ashida, K.; Sugita, K.; Tanizaki, Y. Elevation of Antioxidant Enzymes in the Clinical Effects of Radon and Thermal Therapy for Bronchial Asthma. J. Radiat. Res. 2003, 44, 95–99. [Google Scholar] [CrossRef]
- Falkenbach, A.; Kovacs, J.; Franke, A.; Jorgens, K.; Ammer, K. Radon therapy for the treatment of rheumatic diseases—Review and meta-analysis of controlled clinical trials. Rheumatol. Int. 2005, 25, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Wiedemann, J.; Rapp, F.; Papenfuß, F.; Rödel, F.; Hehlgans, S.; Gaipl, U.S.; Kraft, G.; Fournier, C.; Frey, B. Radon Exposure-Therapeutic Effect and Cancer Risk. Int. J. Mol. Sci. 2020, 22, 316. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Obrador, E.; Pellicer, J.A.; Asensi, M.; Vina, J.; Estrela, J.M. Blood Glutathione as an Index of Radiation-Induced Oxidative Stress in Mice and Humans. Free Radic. Biol. Med. 1997, 22, 1203–1209. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Sawa, T.; Motohashi, H.; Ihara, H.; Akaike, T. Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides. Biomolecules 2020, 10, 1245. [Google Scholar] [CrossRef]
- Etani, R.; Kataoka, T.; Kanzaki, N.; Sakoda, A.; Tanaka, H.; Ishimori, Y.; Mitsunobu, F.; Taguchi, T.; Yamaoka, K. Protective effects of hot spring water drinking and radon inhalation on ethanol-induced gastric mucosal injury in mice. J. Radiat. Res. 2017, 58, 614–625. [Google Scholar] [CrossRef]
- Kataoka, T.; Etani, R.; Kanzaki, N.; Sasaoka, K.; Kobashi, Y.; Hanamoto, K.; Taguchi, T.; Yamaoka, K. Evaluating the protective effects of radon inhalation or ascorbic acid treatment after transient global cerebral ischemic injury in gerbils. J. Nucl. Sci. Technol. 2016, 53, 1681–1685. [Google Scholar] [CrossRef][Green Version]
- Nishiyama, Y.; Kataoka, T.; Yamato, K.; Etani, R.; Taguchi, T.; Yamaoka, K. Radon inhalation suppresses nephropathy in streptozotocin-induced type-1 diabetic mice. J. Nucl. Sci. Technol. 2016, 53, 909–915. [Google Scholar] [CrossRef]
- Etani, R.; Kataoka, T.; Nishiyama, Y.; Takata, Y.; Yamaoka, K. Combined effects of radon inhalation and antioxidant vitamin administration on acute alcohol-induced hepatopathy in mice. J. Nucl. Sci. Technol. 2015, 52, 1512–1518. [Google Scholar] [CrossRef][Green Version]
- Kanzaki, N.; Kataoka, T.; Etani, R.; Yamaoka, K. Recent Studies on Anti-inflammatory Effects of Radon Inhalation in Mice. Inflamm. Cell Signal. 2015, 2, e601. [Google Scholar] [CrossRef]
- Kataoka, T.; Etani, R.; Kanzaki, N.; Kobashi, Y.; Yunoki, Y.; Ishida, T.; Sakoda, A.; Ishimori, Y.; Yamaoka, K. Radon inhalation induces manganese-superoxide dismutase in mouse brain via nuclear factor-κB activation. J. Radiat. Res. 2017, 58, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yonehara, H.; Ikebuchi, M.; Aoyama, T. Effect of Radon Exposure on Superoxide Dismutase (SOD) Activity in Rats. J. Radiat. Res. 1996, 37, 12–19. [Google Scholar] [CrossRef]
- Kataoka, T.; Sakoda, A.; Ishimori, Y.; Toyota, T.; Nishiyama, Y.; Tanaka, H.; Mitsunobu, F.; Yamaoka, K. Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J. Radiat. Res. 2011, 52, 775–781. [Google Scholar] [CrossRef][Green Version]
- Kojima, S.; Matsuki, O.; Nomura, T.; Shimura, N.; Kubodera, A.; Yamaoka, K.; Tanooka, H.; Wakasugi, H.; Honda, Y.; Honda, S.; et al. Localization of glutathione and induction of glutathione synthesis-related proteins in mouse brain by low doses of γ-rays. Brain Res. 1998, 808, 262–269. [Google Scholar] [CrossRef]
- Ishimori, Y.; Mitsunobu, F.; Yamaoka, K.; Tanaka, H.; Kataoka, T.; Sakoda, A. Performance of the first Japanese large-scale facility for radon inhalation experiments with small animals. Radiat. Prot. Dosim. 2011, 146, 31–33. [Google Scholar] [CrossRef]
- Ishimori, Y.; Mitsunobu, F.; Yamaoka, K.; Tanaka, H.; Kataoka, T.; Sakoda, A. Primary Functions of the First Japanese Large-Scale Facility for Exposing Small Animals to Radon. Jpn. J. Health. Phys. 2010, 45, 65–71. [Google Scholar] [CrossRef][Green Version]
- Kawano, Y.; Ohtsu, I.; Tamakoshi, A.; Shiroyama, M.; Tsuruoka, A.; Saiki, K.; Takumi, K.; Nonaka, G.; Nakanishi, T.; Hishiki, T.; et al. Involvement of the yciW gene in l-cysteine and l-methionine metabolism in Escherichia coli. J. Biosci. Bioeng. 2015, 119, 310–313. [Google Scholar] [CrossRef]
- Yamada, K.; Nitta, T.; Atsuji, K.; Shiroyama, M.; Inoue, K.; Higuchi, C.; Nitta, N.; Oshiro, S.; Mochida, K.; Iwata, O.; et al. Characterization of sulfur-compound metabolism underlying wax-ester fermentation in Euglena gracilis. Sci. Rep. 2019, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kawano, Y.; Satoh, Y.; Dairi, T.; Ohtsu, I. Gram-scale fermentative production of ergothioneine driven by overproduction of cysteine in Escherichia coli. Sci. Rep. 2019, 9, 1895. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Kawano, Y.; Ohtsu, I.; Maruyuama-Nakashita, A.; Allahham, A.; Sato, M.; Sawada, Y.; Hirai, M.Y.; Yokoyama, T.; Ohkama-Ohtsu, N. Effects of Thiosulfate as a Sulfur Source on Plant Growth, Metabolites Accumulation and Gene Expression in Arabidopsis and Rice. Plant Cell Physiol. 2019, 60, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Shiroyama, M.; Kanazawa, K.; Suzuki, Y.A.; Ohtsu, I. Development of high-throughput quantitative analytical method for l-cysteine-containing dipeptides by LC–MS/MS toward its fermentative production. AMB Express 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Gottfries, J.; Blennow, K.; Wallin, A.; Gottfries, C. Diagnosis of Dementias Using Partial Least Squares Discriminant Analysis. Dement. Geriatr. Cogn. Disord. 1995, 6, 83–88. [Google Scholar] [CrossRef]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Boulesteix, A.-L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Briefings Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Nishida, M.; Kumagai, Y.; Ihara, H.; Fujii, S.; Motohashi, H.; Akaike, T. Redox signaling regulated by electrophiles and reactive sulfur species. J. Clin. Biochem. Nutr. 2016, 58, 91–98. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ono, K.; Tsutsuki, H.; Ihara, H.; Islam, W.; Akaike, T.; Sawa, T. Enhanced Cellular Polysulfides Negatively Regulate TLR4 Signaling and Mitigate Lethal Endotoxin Shock. Cell Chem. Biol. 2019, 26, 686–698.e4. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, A.; Ishimori, Y.; Kawabe, A.; Kataoka, T.; Hanamoto, K.; Yamaoka, K. Physiologically Based Pharmacokinetic Modeling of Inhaled Radon to Calculate Absorbed Doses in Mice, Rats, and Humans. J. Nucl. Sci. Technol. 2010, 47, 731–738. [Google Scholar] [CrossRef]
- Kanzaki, N.; Kataoka, T.; Etani, R.; Sasaoka, K.; Kanagawa, A.; Yamaoka, K. Analysis of liver damage from radon, X-ray, or alcohol treatments in mice using a self-organizing map. J. Radiat. Res. 2017, 58, 33–40. [Google Scholar] [CrossRef]

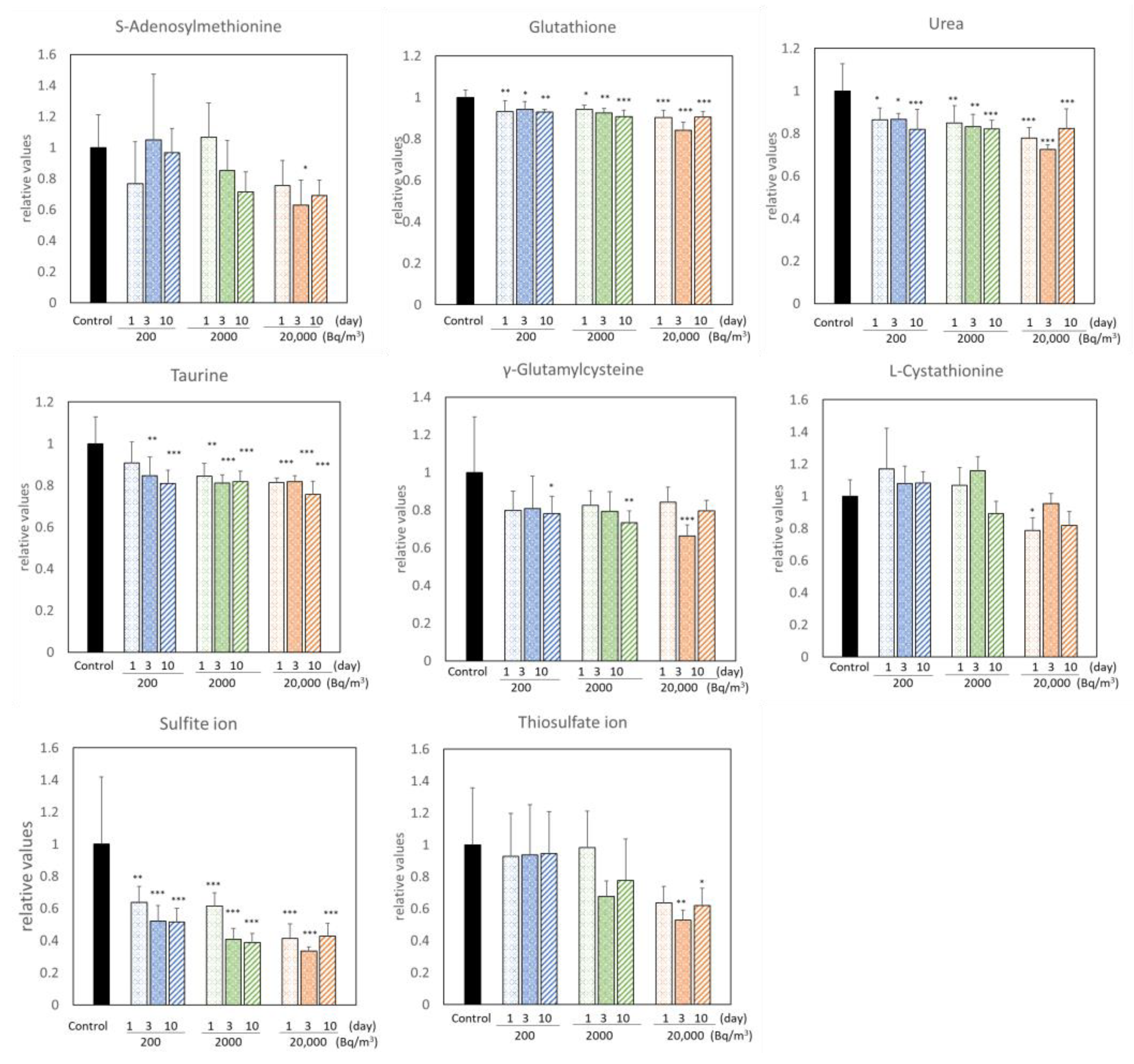

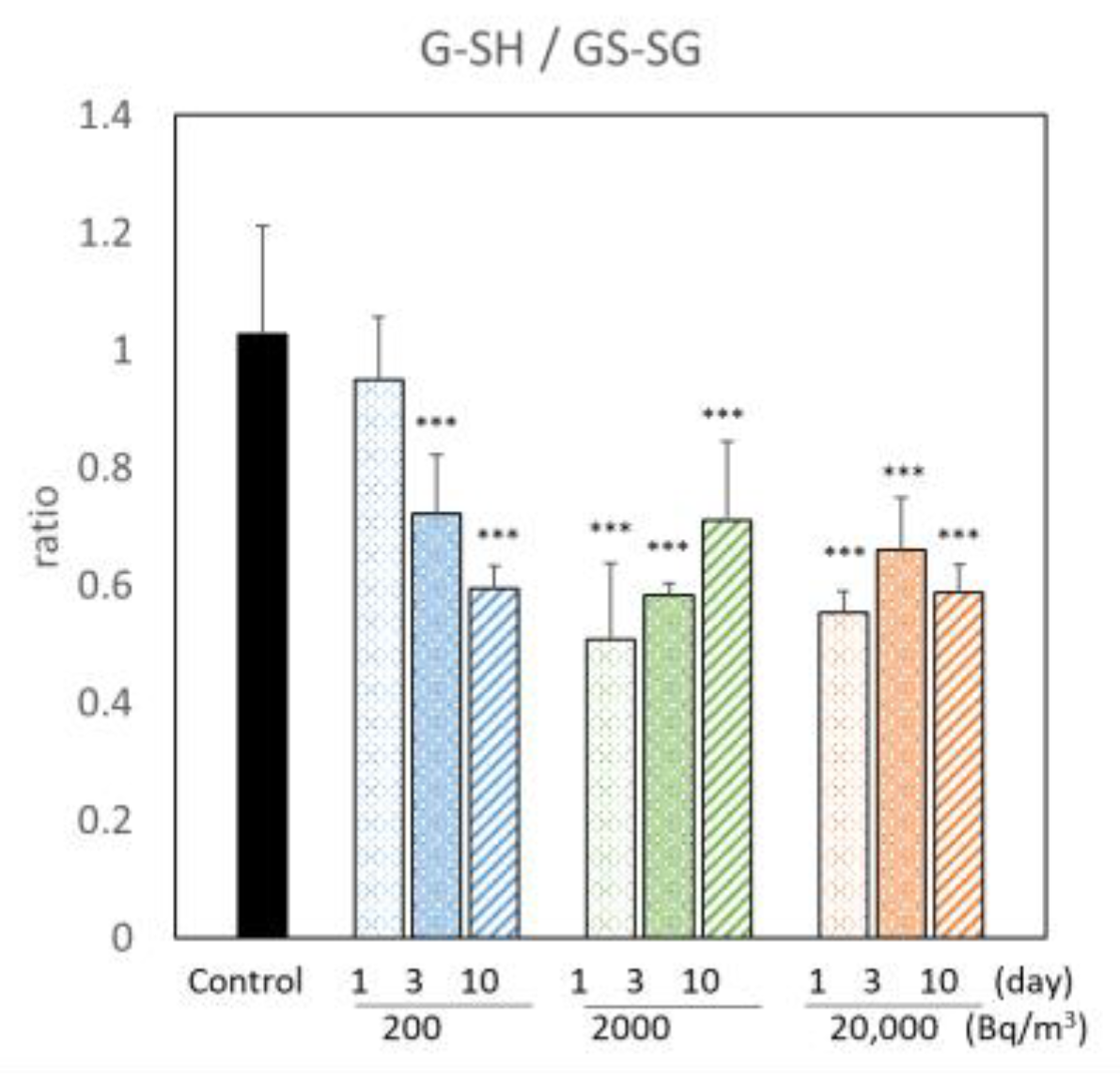
 indicates an increase,
indicates an increase,  indicates a decrease, and
indicates a decrease, and  indicates no change in radon exposure groups compared with the control.
indicates no change in radon exposure groups compared with the control.
 indicates an increase,
indicates an increase,  indicates a decrease, and
indicates a decrease, and  indicates no change in radon exposure groups compared with the control.
indicates no change in radon exposure groups compared with the control.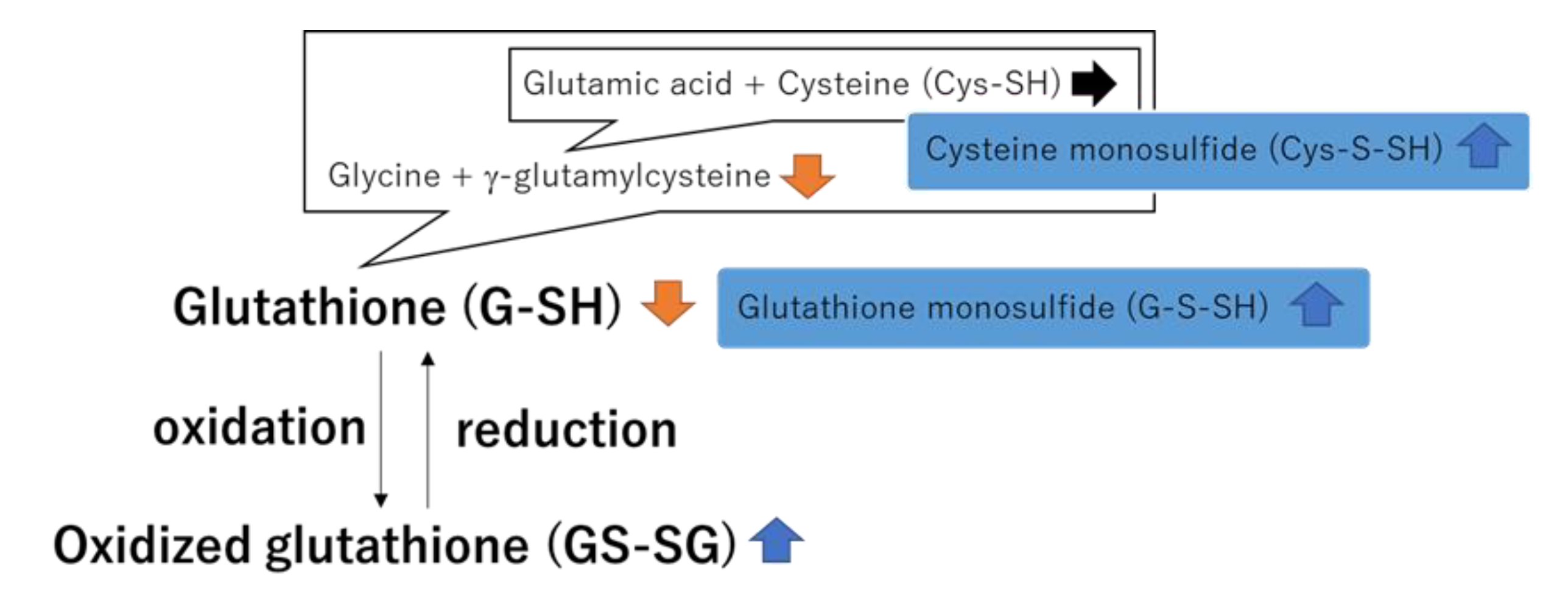
| Metabolites | ||
|---|---|---|
| Adenosine 3′,5′-diphosphate | L-Histidine | O-Acetylserine |
| Cysteine monosulfide (Cys-S-SH) | L-Homoserine | S-Adenosylhomocysteine |
| Cysteinylglycine | L-Lactic acid | S-Adenosylmethionine |
| Ergothioneine | L-Methionine | Sulfide ion |
| Glutathione (G-SH) | L-Serine | Sulfite ion |
| Glutathione monosulfide (G-S-SH) | Methylcysteine | Taurine |
| γ-Glutamylcysteine | Oxidized glutathione (GS-SG) | Thiamine |
| L-Cystathionine | Oxidized glutathione disulfide (GS-SS-SG) | Thiosulfate ion |
| L-Cysteine (Cys-SH) | Oxidized glutathione monosulfide (GS-S-SG) | Urea |
| (a) | ||
|---|---|---|
| Metabolites | Correlation Coefficient | |
| Ergothioneine | 0.65 | *** |
| Cycteinylglycine | 0.56 | *** |
| (b) | ||
| Metabolites | Correlation Coefficient | |
| Cysteine monosulfide | 0.77 | *** |
| S-Adenosylhomosyvteine | 0.55 | *** |
| O-Acetylserine | 0.53 | ** |
| Methylcysteine | 0.48 | * |
| Thiosulfate ion | −0.53 | ** |
| Glutathione | −0.56 | *** |
| L-Cystathionine | −0.59 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanzaki, N.; Sakoda, A.; Kataoka, T.; Sun, L.; Tanaka, H.; Ohtsu, I.; Yamaoka, K. Changes in Sulfur Metabolism in Mouse Brains following Radon Inhalation. Int. J. Environ. Res. Public Health 2022, 19, 10750. https://doi.org/10.3390/ijerph191710750
Kanzaki N, Sakoda A, Kataoka T, Sun L, Tanaka H, Ohtsu I, Yamaoka K. Changes in Sulfur Metabolism in Mouse Brains following Radon Inhalation. International Journal of Environmental Research and Public Health. 2022; 19(17):10750. https://doi.org/10.3390/ijerph191710750
Chicago/Turabian StyleKanzaki, Norie, Akihiro Sakoda, Takahiro Kataoka, Lue Sun, Hiroshi Tanaka, Iwao Ohtsu, and Kiyonori Yamaoka. 2022. "Changes in Sulfur Metabolism in Mouse Brains following Radon Inhalation" International Journal of Environmental Research and Public Health 19, no. 17: 10750. https://doi.org/10.3390/ijerph191710750
APA StyleKanzaki, N., Sakoda, A., Kataoka, T., Sun, L., Tanaka, H., Ohtsu, I., & Yamaoka, K. (2022). Changes in Sulfur Metabolism in Mouse Brains following Radon Inhalation. International Journal of Environmental Research and Public Health, 19(17), 10750. https://doi.org/10.3390/ijerph191710750






