Prevalence of Static Balance Impairment and Associated Factors of University Student Smartphone Users with Subclinical Neck Pain: Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
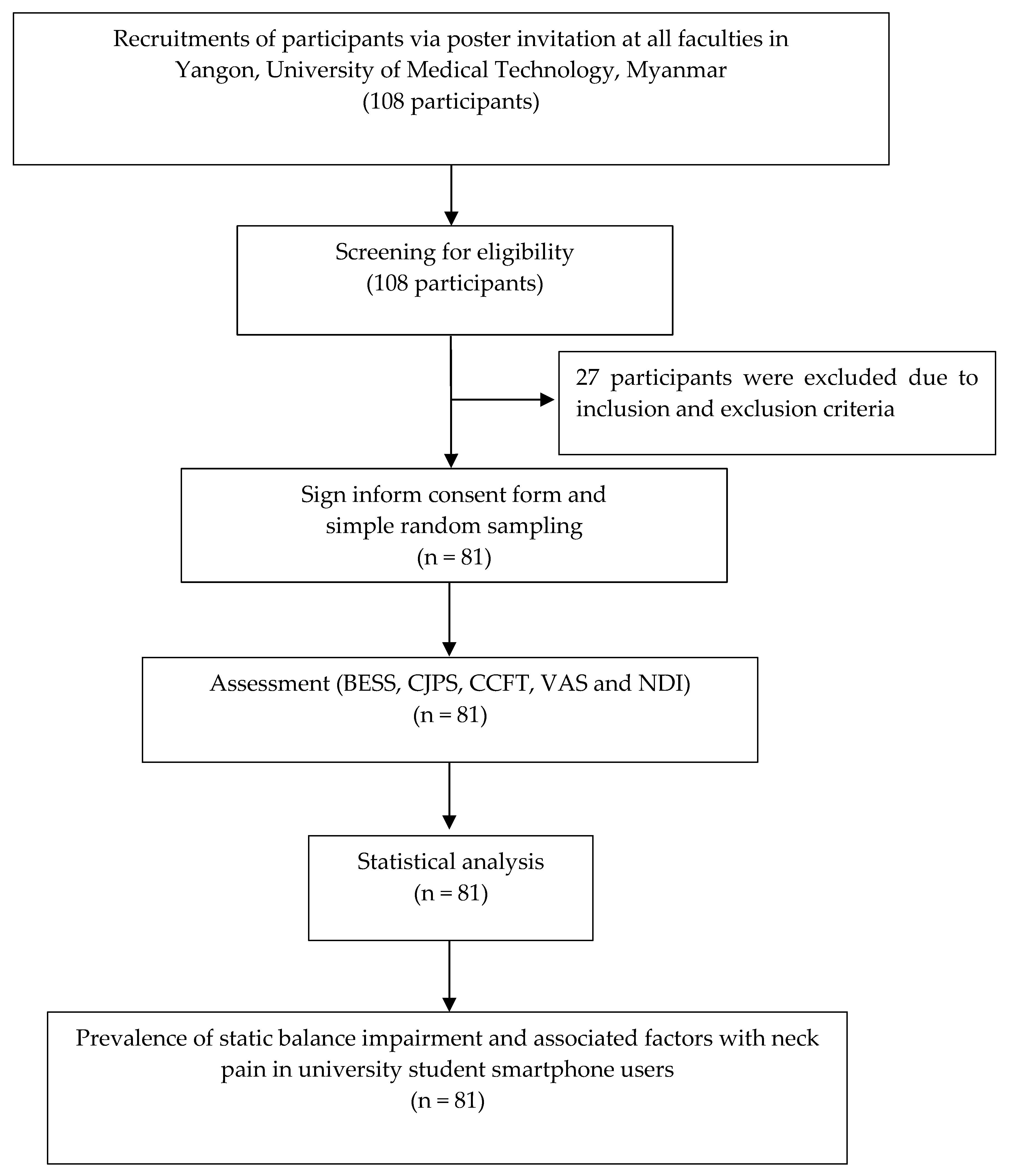
2.3. Screening and Experimental Process
2.4. Outcome Measurements
2.4.1. Static Balance
2.4.2. Cervical Joint Position Sense
2.4.3. Craniocervical Flexors Function Test
2.4.4. Visual Analogue Scale
2.4.5. Neck Disability Inventory Score
- 0–4 points: no disability;
- 5–14 points: mild disability;
- 15–24 points: moderate disability;
- 25–34 points: severe disability;
- 35–50 points: complete disability.
2.4.6. Dizziness Handicap Inventory
2.4.7. Beck Depression Inventory
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Impairment: Balance, CJPS and Muscle Function
3.3. Regression Analysis: Prevalence, AOR, and 95% CI of Balance Impairment with Risk Factors
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haug, S.; Castro, R.P.; Kwon, M.; Filler, A.; Kowatsch, T.; Schaub, M.P. Smartphone use and smartphone addiction among young people in Switzerland. J. Behav. Addict. 2015, 4, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Cheung, R.; Duong, S.; Paynter, R.; Asper, L. Viewing distance and eyestrain symptoms with prolonged viewing of smartphones. Clin. Exp. Optom. 2017, 100, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Kim, J.; Kim, K.; Kim, N.; Choi, I.; Lee, S.; Yim, J. The effects of heavy smartphone use on the cervical angle, pain threshold of neck muscles and depression. Adv. Sci. Technol. Lett. 2015, 91, 12–17. [Google Scholar]
- Hyong, I.H. The effects on dynamic balance of dual-tasking using smartphone functions. J. Phys. Ther. Sci. 2015, 27, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Choi, M.-H.; Goo, B.-O. Effect of smart phone use on dynamic postural balance. J. Phys. Ther. Sci. 2014, 26, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Azab, D.R.E.; Amin, D.I.; Mohamed, G.I. Effect of smart phone using duration and gender on dynamic balance. Int. J. Med. Res. Health Sci. 2017, 6, 42–49. [Google Scholar]
- Lee, J.; Seo, K. The comparison of cervical repositioning errors according to smartphone addiction grades. J. Phys. Ther. Sci. 2014, 26, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Do You Consume Media the Same Way as Your Parents? What about Your Peers or Children? The Fact Is … Q1 2017 The Nielsen Total Audience Report. 2017. Available online: https://www.nielsen.com/insights/2017/the-nielsen-total-audience-report-q1-2017/ (accessed on 20 May 2022).
- Woo, E.H.C.; White, P.; Lai, C.W.K. Effects of electronic device overuse by university students in relation to clinical status and anatomical variations of the median nerve and transverse carpal ligament. Muscle Nerve 2017, 56, 873–880. [Google Scholar] [CrossRef]
- Namwongsa, S.; Puntumetakul, R.; Neubert, M.S.; Chaiklieng, S.; Boucaut, R. Ergonomic risk assessment of smartphone users using the Rapid Upper Limb Assessment (RULA) tool. PLoS ONE 2018, 13, e0203394. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Wang, J.-D.; Yao, G.; Wang, S.-F. Association between cervicocephalic kinesthetic sensibility and frequency of subclinical neck pain. Man. Ther. 2008, 13, 419–425. [Google Scholar] [CrossRef]
- Lee, H.; Nicholoson, L.L.; Adams, R.D.; Bae, S.-S. Body Chart Pain Location and Side-Specific Physical Impairment in Subclinical Neck Pain. J. Manip. Physiol. Ther. 2005, 28, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Nicholson, L.L.; Adams, R.D. Cervical Range of Motion Associations With Subclinical Neck Pain. Spine 2004, 29, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, J.-S. The relationship between smartphone use and subjective musculoskeletal symptoms and university students. J. Phys. Ther. Sci. 2015, 27, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.E.; Driban, J.B.; Thomas, N.; Chakravarty, T.; Channell, V.; Komaroff, E. Postures, typing strategies, and gender differences in mobile device usage: An observational study. Appl. Ergon. 2012, 43, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Hansraj, K.K. Assessment of stresses in the cervical spine caused by posture and position of the head. Surg. Technol. Int. 2014, 25, 277–279. [Google Scholar]
- Eom, S.-H.; Choi, S.-Y.; Park, D.-H. An empirical study on relationship between symptoms of musculoskeletal disorders and amount of smartphone usage. J. Korea Saf. Manag. Sci. 2013, 15, 113–120. [Google Scholar]
- Shan, Z.; Deng, G.; Li, J.; Li, Y.; Zhang, Y.; Zhao, Q. Correlational analysis of neck/shoulder pain and low back pain with the use of digital products, physical activity and psychological status among adolescents in Shanghai. PLoS ONE 2013, 8, e78109. [Google Scholar] [CrossRef]
- Berolo, S.; Wells, R.P.; Amick, B.C. Musculoskeletal symptoms among mobile hand-held device users and their relationship to device use: A preliminary study in a Canadian university population. Appl. Ergon. 2011, 42, 371–378. [Google Scholar] [CrossRef]
- Xie, Y.; Szeto, G.; Dai, J. Prevalence and risk factors associated with musculoskeletal complaints among users of mobile handheld devices: A systematic review. Appl. Ergon. 2017, 59, 132–142. [Google Scholar] [CrossRef]
- Wright, K.E.; Lyons, T.S.; Navalta, J.W. Effects of exercise-induced fatigue on postural balance: A comparison of treadmill versus cycle fatiguing protocols. Eur. J. Appl. Physiol. 2013, 113, 1303–1309. [Google Scholar] [CrossRef]
- Kellis, E.; Kouvelioti, V. Agonist versus antagonist muscle fatigue effects on thigh muscle activity and vertical ground reaction during drop landing. J. Electromyogr. Kinesiol. 2009, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Juul-Kristensen, B.; Clausen, B.; Ris, I.; Jensen, R.V.; Steffensen, R.F.; Chreiteh, S.S.; Jørgensen, M.B.; Søgaard, K. Increased neck muscle activity and impaired balance among females with whiplash-related chronic neck pain: A cross-sectional study. J. Rehabil. Med. 2013, 45, 376–384. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; Schmitz, T.; Fulk, G. Physical Rehabilitation; FA Davis: Philadelphia, PA, USA, 2013; p. 10. [Google Scholar]
- Tabrizi, H.B.; Abbasi, A.; Sarvestani, H.J. Comparing the static and dynamic balances and their relationship with the anthropometrical characteristics in the athletes of selected sports. Middle East J. Sci. Res. 2013, 15, 216–221. [Google Scholar]
- Kim, Y.-G.; Kang, M.-H.; Kim, J.-W.; Jang, J.-H.; Oh, J.-S. Influence of the duration of smartphone usage on flexion angles of the cervical and lumbar spine and on reposition error in the cervical spine. Phys. Ther. Korea 2013, 20, 10–17. [Google Scholar] [CrossRef]
- Suh, Y.W.; Kim, K.H.; Kang, S.Y.; Kim, S.W.; Oh, J.R.; Kim, H.M.; Song, J.S. The Objective Methods to Evaluate Ocular Fatigue Associated With Computer Work. J. Korean Ophthalmol. Soc. 2010, 51, 1327–1332. [Google Scholar] [CrossRef][Green Version]
- Park, Y.-H.; An, C.-M.; Moon, S.-J. Effects of visual fatigue caused by smartphones on balance function in healthy adults. J. Phys. Ther. Sci. 2017, 29, 221–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.Y.; Lee, D.H.; Han, S.K. The effects of posture on neck flexion angle while using a smartphone according to duration. Korean Soc. Phys. Med. 2016, 11, 35–39. [Google Scholar] [CrossRef]
- Ning, X.; Huang, Y.; Hu, B.; Nimbarte, A.D. Neck kinematics and muscle activity during mobile device operations. Int. J. Ind. Ergon. 2015, 48, 10–15. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.; Shin, G. Head flexion angle while using a smartphone. Ergonomics 2015, 58, 220–226. [Google Scholar] [CrossRef]
- Carral, J.M.C.; Ayán, C.; Sturzinger, L.; Gonzalez, G. Relationships between body mass index and static and dynamic balance in active and inactive older adults. J. Geriatr. Phys. Ther. 2019, 42, E85–E90. [Google Scholar] [CrossRef]
- Wah, S.W.; Puntumetakul, R.; Boucaut, R. Effects of proprioceptive and craniocervical flexor training on static balance in university student smartphone users with balance impairment: A randomized controlled trial. J. Pain Res. 2021, 14, 1935. [Google Scholar] [CrossRef] [PubMed]
- Paulus, I.; Brumagne, S. Altered interpretation of neck proprioceptive signals in persons with subclinical recurrent neck pain. J. Rehabil. Med. 2008, 40, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Kaarto, M.L.; Koehle, M.S. Normative data for the balance error scoring system: Implications for brain injury evaluations. Brain Inj. 2008, 22, 147–152. [Google Scholar] [CrossRef]
- Erkmen, N.; Taşkın, H.; Kaplan, T.; Sanioǧlu, A. The effect of fatiguing exercise on balance performance as measured by the balance error scoring system. Isokinet. Exerc. Sci. 2009, 17, 121–127. [Google Scholar] [CrossRef]
- Bell, D.R.; Guskiewicz, K.M.; Clark, M.A.; Padua, D.A. Systematic review of the balance error scoring system. Sports Health 2011, 3, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Guskiewicz, K.M. Balance assessment in the management of sport-related concussion. Clin. Sports Med. 2011, 30, 89–102. [Google Scholar] [CrossRef]
- Burke, S.; Lynch, K.; Moghul, Z.; Young, C.; Saviola, K.; Schenk, R. The reliability of the cervical relocation test on people with and without a history of neck pain. J. Man. Manip. Ther. 2016, 24, 210–214. [Google Scholar] [CrossRef]
- Audette, I.; Dumas, J.P.; Côté, J.N.; De Serres, S.J. Validity and between-day reliability of the cervical range of motion (CROM) device. J. Orthop. Sports Phys. Ther. 2010, 40, 318–323. [Google Scholar] [CrossRef]
- Inokuchi, H.; Tojima, M.; Mano, H.; Ishikawa, Y.; Ogata, N.; Haga, N. Neck range of motion measurements using a new three-dimensional motion analysis system: Validity and repeatability. Eur. Spine J. 2015, 24, 2807–2815. [Google Scholar] [CrossRef]
- Revel, M.; Andre-Deshays, C.; Minguet, M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch. Phys. Med. Rehabil. 1991, 72, 288–291. [Google Scholar]
- Reddy, R.S.; Alahmari, K.A.; Silvian, P.S. Test-retest reliability of assessing cervical proprioception using cervical range of motion device. Saudi J. Sports Med. 2016, 16, 118. [Google Scholar] [CrossRef]
- Pinsault, N.; Vuillerme, N.; Pavan, P. Cervicocephalic relocation test to the neutral head position: Assessment in bilateral labyrinthine-defective and chronic, nontraumatic neck pain patients. Arch. Phys. Med. Rehabil. 2008, 89, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Jull, G.A.; O’Leary, S.P.; Falla, D.L. Clinical Assessment of the Deep Cervical Flexor Muscles: The Craniocervical Flexion Test. J. Manip. Physiol. Ther. 2008, 31, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Jull, G.; Barrett, C.; Magee, R.; Ho, P. Further clinical clarification of the muscle dysfunction in cervical headache. Cephalalgia 1999, 19, 179–185. [Google Scholar] [CrossRef]
- James, G.; Doe, T. The craniocervical flexion test: Intra-tester reliability in asymptomatic subjects. Physiother. Res. Int. 2010, 15, 144–149. [Google Scholar] [CrossRef]
- Wing Chiu, T.T.; Law, E.Y.H.; Chiu, T.H.F. Performance of the craniocervical flexion test in subjects with and without chronic neck pain. J. Orthop. Sports Phys. Ther. 2005, 35, 567–571. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar]
- Jensen, M.P.; Chen, C.; Brugger, A.M. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J. Pain 2003, 4, 407–414. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Deloach, J.; Porucznik, C.A.; Powell, A.P. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J. Shoulder Elb. Surg. 2009, 18, 927–932. [Google Scholar] [CrossRef]
- Burckhardt, C.S.; Jones, K.D. Adult measures of pain: The McGill pain questionnaire (MPQ), rheumatoid arthritis pain scale (RAPS), short-form mcgill pain questionnaire (SF-MPQ), verbal descriptive scale (VDS), visual analog scale (VAS), and west haven-yale multidisciplinary pain inventory (WHYMPI). Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2003, 49, S96–S104. [Google Scholar]
- Ailliet, L.; Knol, D.L.; Rubinstein, S.M.; de Vet, H.C.; van Tulder, M.W.; Terwee, C.B. Definition of the construct to be measured is a prerequisite for the assessment of validity. The Neck Disability Index as an example. J. Clin. Epidemiol. 2013, 66, 775–782.e2. [Google Scholar] [CrossRef] [PubMed]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; Mcalpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: A systematic review. J. Orthop. Sports Phys. Ther. 2009, 39, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Young, I.A.; Cleland, J.A.; Michener, L.A.; Brown, C. Reliability, construct validity, and responsiveness of the neck disability index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am. J. Phys. Med. Rehabil. 2010, 89, 831–839. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.J.H.; Grevitt, M.P.; Silcocks, P.; Hobbs, G. The reliability of the Vernon and Mior neck disability index, and its validity compared with the short form-36 health survey questionnaire. Eur. Spine J. 2007, 16, 2111–2117. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W. The Development of the Dizziness Handicap Inventory. JAMA Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Kaufman, L.J.; Brangwynne, C.P.; Kasza, K.E.; Filippidi, E.; Gordon, V.D.; Deisboeck, T.S.; Weitz, D.A. Glioma Expansion in Collagen I Matrices: Analyzing Collagen Concentration-Dependent Growth and Motility Patterns. Biophys. J. 2005, 89, 635–650. [Google Scholar] [CrossRef]
- Treleaven, J.; Jull, G.; LowChoy, N. Smooth pursuit neck torsion test in whiplash-associated disorders: Relationship to self-reports of neck pain and disability, dizziness and anxiety. J. Rehabil. Med. 2005, 37, 219–223. [Google Scholar] [CrossRef]
- Whitney, S.L.; Wrisley, D.M.; Brown, K.E.; Furman, J.M. Is Perception of Handicap Related to Functional Performance in Persons with Vestibular Dysfunction? Otol. Neurotol. 2004, 25, 139–143. [Google Scholar] [CrossRef]
- Enloe, L.J.; Shields, R.K. Evaluation of Health-Related Quality of Life in Individuals with Vestibular Disease Using Disease-Specific and General Outcome Measures. Phys. Ther. 1997, 77, 890–903. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Groth-Marnat, G. Financial efficacy of clinical assessment: Rational guidelines and issues for future research. J. Clin. Psychol. 1999, 55, 813–824. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14. [Google Scholar]
- Naing, L. Practical Issues in Calculating the Sample Size for Prevalence Studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Puntumetakul, R.; Chatprem, T.; Saiklang, P.; Phadungkit, S.; Kamruecha, W.; Sae-Jung, S. Prevalence and Associated Factors of Clinical Myelopathy Signs in Smartphone-Using University Students with Neck Pain. Int. J. Environ. Res. Public Health 2022, 19, 4890. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398. [Google Scholar]
- Won, M.; Kim, M.; Kim, S.; Lee, J. The effect of visual information provision on the changes of electromyogram activity in trunk and lower leg muscles during dynamic balance control. Korean J. Sports Med. 2014, 32, 44–54. [Google Scholar] [CrossRef]
- The Ministry of Transport and Communications. 85 Percent of Myanmar Internet Use Is on Smartphones: Survey. Coconuts: Yangon, Myanmar, 2018. [Google Scholar]
- Shen, J.; Reingold, E.M.; Pomplun, M. Guidance of eye movements during conjunctive visual search: The distractor-ratio effect. Can. J. Exp. Psychol. /Rev. Can. Psychol. Exp. 2003, 57, 76. [Google Scholar] [CrossRef]
- Dornan, J.; Fernie, G.R.; Holliday, P.J. Visual input: Its importance in the control of postural sway. Arch. Phys. Med. Rehabil. 1978, 59, 586–591. [Google Scholar]
- Boucher, P.; Descarreaux, M.; Normand, M.C. Postural control in people with osteoarthritis of the cervical spine. J. Manip. Physiol. Ther. 2008, 31, 184–190. [Google Scholar]
- Field, S.; Treleaven, J.; Jull, G. Standing balance: A comparison between idiopathic and whiplash-induced neck pain. Man. Ther. 2008, 13, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Cruz, A.L. Standing balance in patients with whiplash-associated neck pain and idiopathic neck pain when compared with asymptomatic participants: A systematic review. Physiother. Theory Pract. 2013, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.; Treleaven, J.; Jull, G. The influence of neck pain on balance and gait parameters in community-dwelling elders. Man. Ther. 2008, 13, 317–324. [Google Scholar] [CrossRef] [PubMed]
- AlAbdulwahab, S.S.; Kachanathu, S.J.; AlMotairi, M.S. Smartphone use addiction can cause neck disability. Musculoskelet. Care 2017, 15, 10–12. [Google Scholar] [CrossRef]
- Ylinen, J.; Salo, P.; Nykänen, M.; Kautiainen, H.; Häkkinen, A. Decreased isometric neck strength in women with chronic neck pain and the repeatability of neck strength measurements. Arch. Phys. Med. Rehabil. 2004, 85, 1303–1308. [Google Scholar] [CrossRef]
- SurESh, A.; SudhAn, S.G.; MohAn, P.; Ramalingam, A.T. Impact of Smartphone Addiction on Neck Pain and Disability in University Students. J. Clin. Diagn. Res. 2021, 15, YC01–YC03. [Google Scholar]
- Kim, E.-Y.; Kim, K.-J.; Park, H.-R. Comparison of the effects of deep neck flexor strengthening exercises and Mackenzie neck exercises on head forward postures due to the use of smartphones. Indian J. Sci. Technol. 2015, 8, 569–575. [Google Scholar] [CrossRef]
- Capra, N.F.; Ro, J.Y. Experimental muscle pain produces central modulation of proprioceptive signals arising from jaw muscle spindles. Pain 2000, 86, 151–162. [Google Scholar] [CrossRef]
- Le Pera, D.; Graven-Nielsen, T.; Valeriani, M.; Oliviero, A.; Di Lazzaro, V.; Tonali, P.A.; Arendt-Nielsen, L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin. Neurophysiol. 2001, 112, 1633–1641. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; Thé, D.J.; Doldo, N.A.; Ploutz-Snyder, L.L. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral com-pression. J. Appl. Physiol. 2003, 94, 2263–2272. [Google Scholar] [CrossRef]
- Hrysomallis, C. Relationship between balance ability, training and sports injury risk. Sports Med. 2007, 37, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Salavati, M.; Moghadam, M.; Ebrahimi, I.; Arab, A.M. Changes in postural stability with fatigue of lower extremity frontal and sagittal plane movers. Gait Posture 2007, 26, 214–218. [Google Scholar] [CrossRef] [PubMed]

| Factors | Total Participants, n (%) | Mean (SD) | Min-Max | Participants without BESS Impairment, n (%) | Participants with BESS Impairment, n (%) |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years) | |||||
| <20 ≥20 | 45 (55.56) 36 (44.44) | 19 (0.51) 21 (1.64) | 18–19 20–25 | 9 (20) 12 (33) | 36 (80) 24 (67) |
| Gender | |||||
| Male Female | 10 (12.35) 71 (87.65) | - | - | 4 (40) 17 (24) | 6 (60) 54 (76) |
| BMI (kg/m2) | |||||
| <20 ≥20 | 49 (60.49) 32 (39.51) | 18.17 (1.27) 22.45 (2.11) | 15.9–19.95 20.3–28.38 | 10 (20) 11 (34) | 39 (80) 21 (66) |
| BDI (score) | |||||
| <4 ≥4 | 44 (54.32) 37 (45.68) | 1.72 (1.93) 8.29 (8.38) | 0–8 4–17 | 14 (32) 7 (19) | 30 (68) 30 (81) |
| DHI (score) | |||||
| <7 ≥7 | 43 (53.09) 38 (46.91) | 0.67 (0.07) 0.82 (0.06) | 0–6 8–28 | 14 (33) 7 (18) | 29 (67) 31 (82) |
| Smart phone and other visual display terminal use | |||||
| Daily hours of smartphone use | |||||
| <4 ≥4 | 24 (29.63) 57 (70.37) | 2.88 (0.27) 4.83 (1.70) | 2.5–3.5 2.5–8.0 | 16 (67) 5 (9) | 8 (33) 52 (91) |
| Years of smartphone use | |||||
| <4 ≥4 | 33 (40.74) 48 (59.26) | 2.67 (0.48) 4.88 (0.90) | 2–3 4–7 | 15 (45) 6 (13) | 18 (55) 42 (88) |
| Posture when using smartphone | |||||
| Sitting Lying | 33 (40.74) 48 (59.26) | - | - | 11 (33) 10 (21) | 22 (67) 38 (79) |
| Daily hours of other visual display terminal use | |||||
| <2 ≥2 | 60 (74.07) 21 (25.93) | 0.38 (0.48) 3.29 (1.82) | 0–1 2–9 | 18 (30) 3 (14) | 42 (70) 18 (86) |
| Neck pain status | |||||
| Visual analogue scale (mm) | |||||
| <44 ≥44 | 60 (74.07) 21 (25.93) | 33.83 (4.37) 50.19 (4.75) | 30–44 45–64 | 21 (35) 0 (0) | 39 (65) 21 (100) |
| NDI (score) | |||||
| <7 ≥7 | 43 (53.09) 38 (46.91) | 5.30 (0.46) 9.29 (1.74) | 5–6 7–14 | 19 (44) 2 (5) | 24 (56) 36 (95) |
| Site of neck pain | |||||
| Central pain Right or left pain | 29 (35.80) 52 (64.20) | - | - | 9 (17) 11 (52) | 43 (83) 10 (48) |
| Stage of neck pain | |||||
| Subacute Chronic | 54 (66.67) 27 (33.33) | - | - | 17 (31) 4 (15) | 37 (69) 23 (85) |
| Episode | |||||
| <3 ≥3 | 14 (17.28) 67 (82.72) | - | - | 10 (71) 11 (16) | 4 (29) 56 (84) |
| Cervical joint position sense error (degrees) | |||||
| Right rotation | |||||
| <4.5 ≥4.5 | 52 (64.20) 29 (35.80) | 3.05 (0.88) 6.29 (1.84) | 1.33–4 3.33–11.3 | 20 (35) 1 (4) | 37 (65) 23 (96) |
| Left rotation | |||||
| <4.5 ≥4.5 | 55 (67.90) 26 (32.10) | 3.03 (0.85) 6.09(1.80) | 1.33–4 4.5–11.3 | 21 (40) 0 (0) | 32 (60) 28 (100) |
| Craniocervical flexors test (mmHg) | |||||
| <24 ≥24 | 59 (72.84) 22 (27.16) | 26.47 (2.39) 17.64 (3.06) | 24–30 12–20 | 2 (9) 19 (32) | 20 (91) 40 (68) |
| Impairment | Number | Prevalence | 95% CI |
|---|---|---|---|
| Static balance impairment | 60 | 74.07 | 64.32 to 83.82 |
| Cervical joint position sense impairment | |||
| Right rotation | 29 | 35.80 | 25.14 to 46.47 |
| Left rotation | 26 | 32.10 | 21.71 to 42.49 |
| Craniocervical flexors function test impairment | 59 | 72.84 | 55.87 to 79.72 |
| Factors | Neck Pain with Static Balance Impairment | |
|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Demographic | ||
| Gender | ||
| Male | 1.00 | - |
| Female | 2.12 (0.53 to 8.40) | |
| Age (years) | ||
| <20 | 1.00 | - |
| ≥20 | 0.50 (0.53 to 8.40) * | |
| BMI (kg/m2) | ||
| <20 | 1.00 | - |
| ≥20 | 0.49 (0.81 to 1.34) * | |
| Beck Depression Inventory or BDI (scores) | ||
| <4 | 1.00 | - |
| ≥4 | 2.00 (0.71 to 5.65) * | |
| Dizziness Handicap Inventory or DHI (scores) | ||
| <7 | 1.00 | 1.00 |
| ≥7 | 2.14 (0.76 to 6.04) * | 2.02 (0.29 to 14.23) |
| Smart phoneand other visual display terminaluse | ||
| Daily hours of smartphone use (hours) | ||
| <4 | 1.00 | 1.00 |
| ≥4 | 20.8 (5.96 to 72.60) * | 19.24 (4.72 to 78.48) ** |
| Years of smartphone use (years) | ||
| <4 | 1.00 | 1.00 |
| ≥4 | 5.83 (1.95 to 17.45) * | 5.01 (1.12 to 22.38) ** |
| Posture when using smartphone | ||
| Sitting | 1.00 | - |
| Lying | 1.9 (0.70 to 5.19) | |
| Daily hours of other visual display terminal use (hours) | ||
| <2 | 1.00 | 1.00 |
| ≥2 | 2.57 (0.67 to 9.83) * | 2.56 (0.34 to 19.12) |
| Neck pain status | ||
| Neck pain or VAS (mm) | ||
| mild pain (30–44 mm) | 1.00 | 1.00 |
| moderate pain (45–74 mm) | 10.00 (1.25 to 79.95) * | 7.66 (0.33 to 177.14) |
| Neck disability index or NDI (scores) | ||
| <7 | 1.00 | 1.00 |
| ≥7 | 14.25 (3.04 to 66.86) * | 12.91 (2.24 to 74.45) ** |
| Pain regions (group) | ||
| Right or left pain | 1.00 | 1.00 |
| Central pain | 3.37 (1.20 to 9.45) * | 1.80 (0.29 to 11.16) |
| Stage of neck pain (stage) | ||
| Sub-acute | 1.00 | - |
| Chronic | 2.64 (0.79 to 8.83) * | |
| Episode of neck pain (episode) | ||
| <3 | 1.00 | 1.00 |
| ≥3 | 12.73 (3.37 to 48.00) * | 0.61 (0.03 to 11.99) |
| Cervical joint position sense error (degrees) | ||
| Right rotation | ||
| <4.5 | 1.00 | 1.00 |
| ≥4.5 | 7.77 (1.66 to 36.37) * | 5.65 (0.88 to 36.43) |
| Left rotation | ||
| <4.5 | 1.00 | - |
| ≥4.5 | 6.33 (1.35 to 29.72) * | |
| Craniocervical flexors test (mmHG) | ||
| <24 | 1.00 | 1.00 |
| ≥24 | 4.75 (1.00 to 22.44) * | 3.70 (0.53 to 25.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wah, S.W.; Chatchawan, U.; Chatprem, T.; Puntumetakul, R. Prevalence of Static Balance Impairment and Associated Factors of University Student Smartphone Users with Subclinical Neck Pain: Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 10723. https://doi.org/10.3390/ijerph191710723
Wah SW, Chatchawan U, Chatprem T, Puntumetakul R. Prevalence of Static Balance Impairment and Associated Factors of University Student Smartphone Users with Subclinical Neck Pain: Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(17):10723. https://doi.org/10.3390/ijerph191710723
Chicago/Turabian StyleWah, Saw Wah, Uraiwan Chatchawan, Thiwaphon Chatprem, and Rungthip Puntumetakul. 2022. "Prevalence of Static Balance Impairment and Associated Factors of University Student Smartphone Users with Subclinical Neck Pain: Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 17: 10723. https://doi.org/10.3390/ijerph191710723
APA StyleWah, S. W., Chatchawan, U., Chatprem, T., & Puntumetakul, R. (2022). Prevalence of Static Balance Impairment and Associated Factors of University Student Smartphone Users with Subclinical Neck Pain: Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(17), 10723. https://doi.org/10.3390/ijerph191710723





