Immunomodulatory Effects of Radon Inhalation on Lipopolysaccharide-Induced Inflammation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Radon Inhalation
2.4. LPS Administration
2.5. Sample Preparation
2.6. Cytokine Assay
2.7. Antioxidant Assay
2.8. Statistical Analyses
3. Results
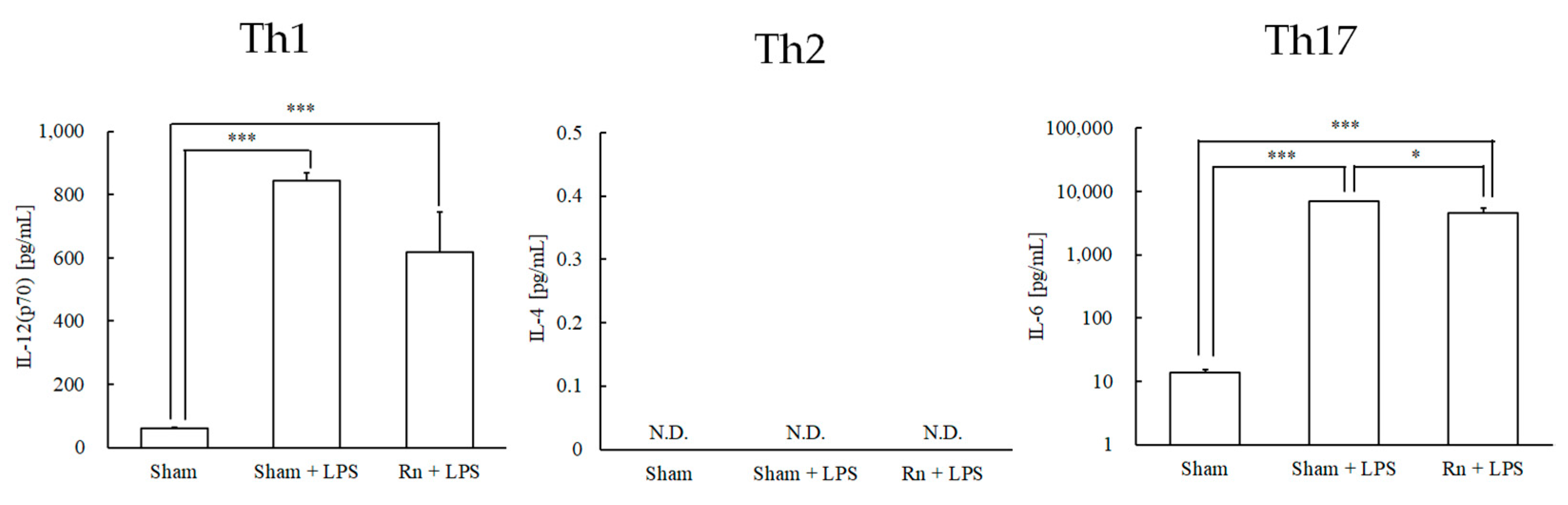
3.1. Effects of Radon Inhalation on LPS-Induced Differentiation-Inducing Cytokines in Serum
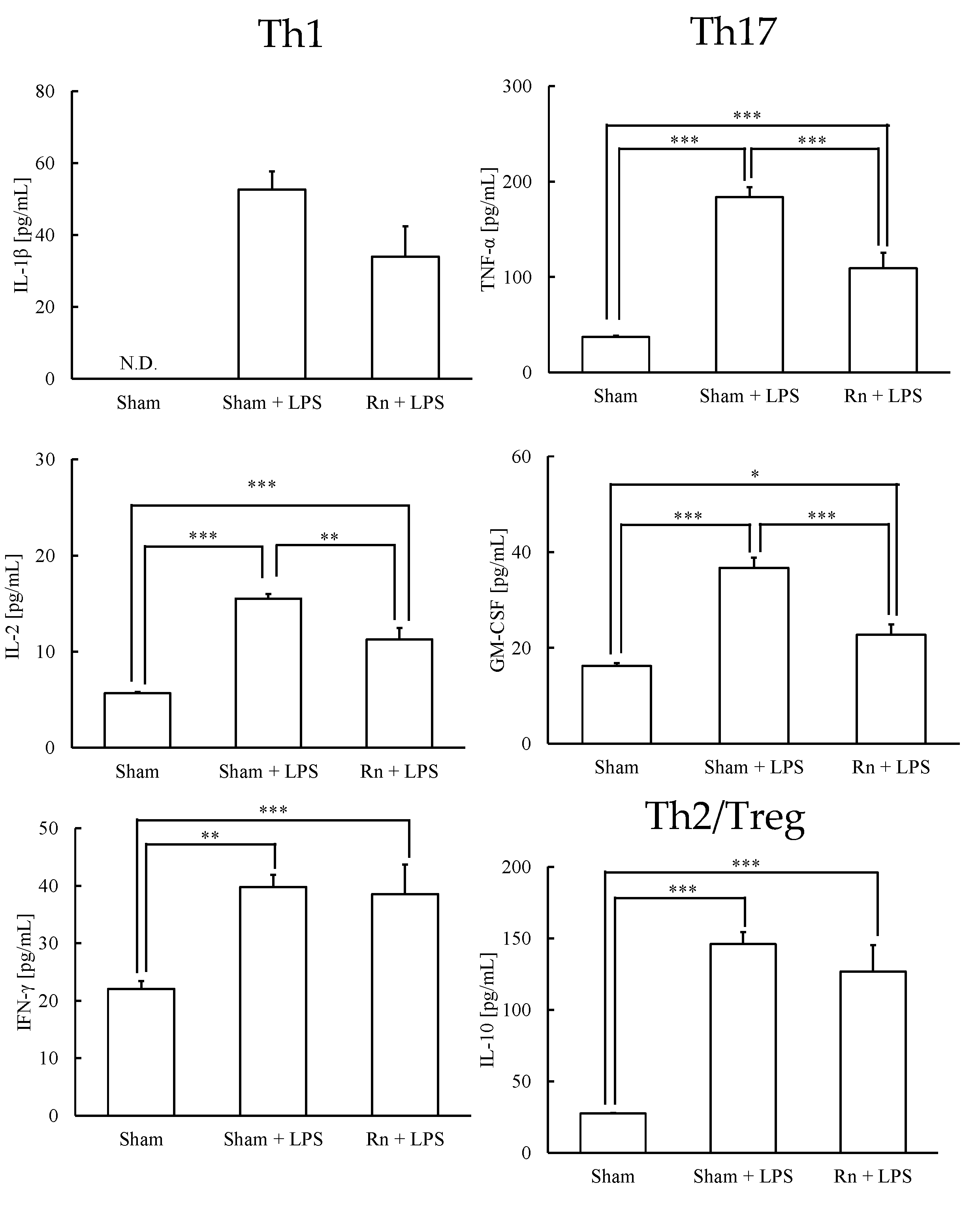
3.2. Effects of Radon Inhalation on LPS-Induced Th1-, Th17-, and Treg-Prone Cytokines in Serum
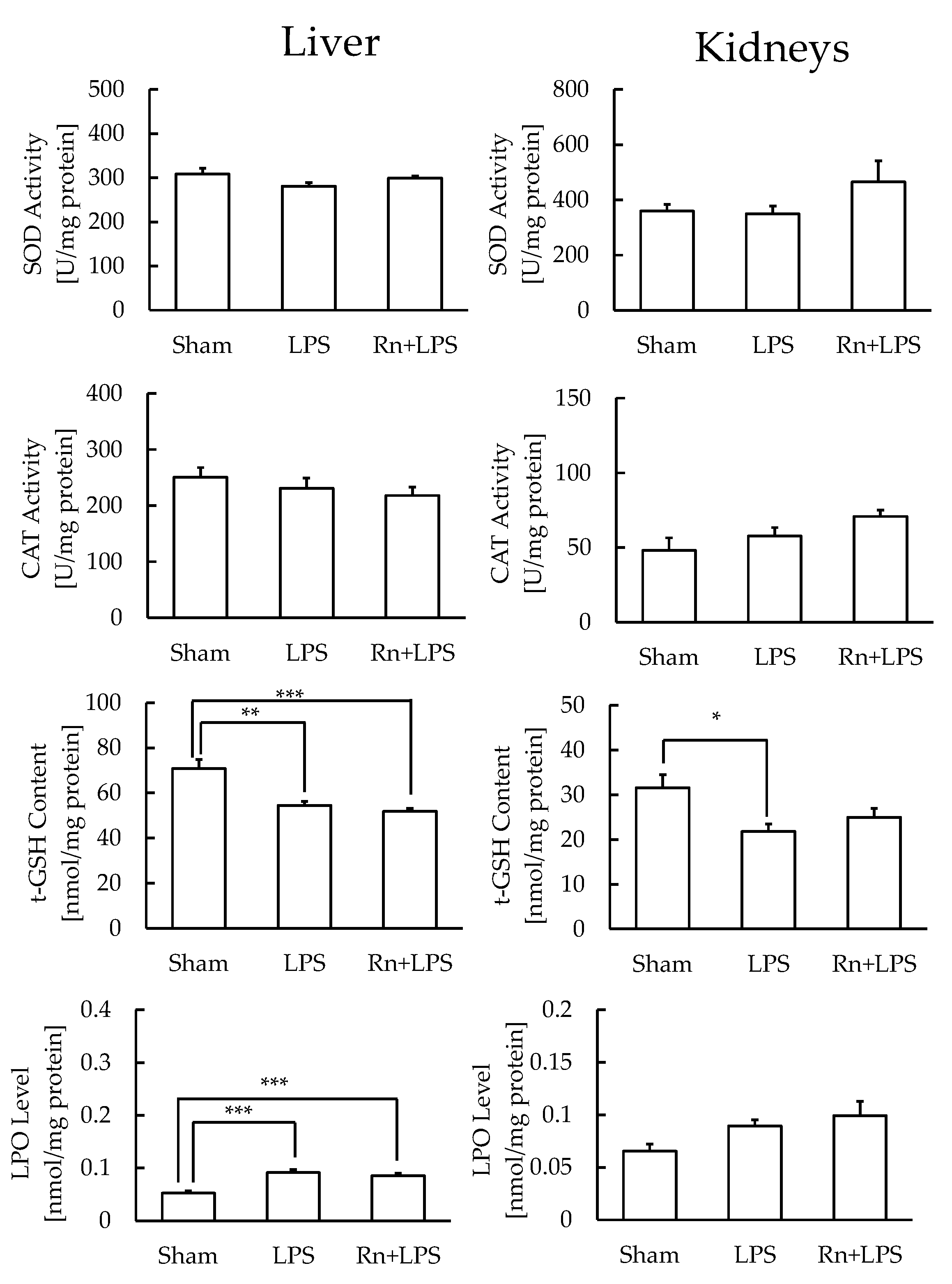
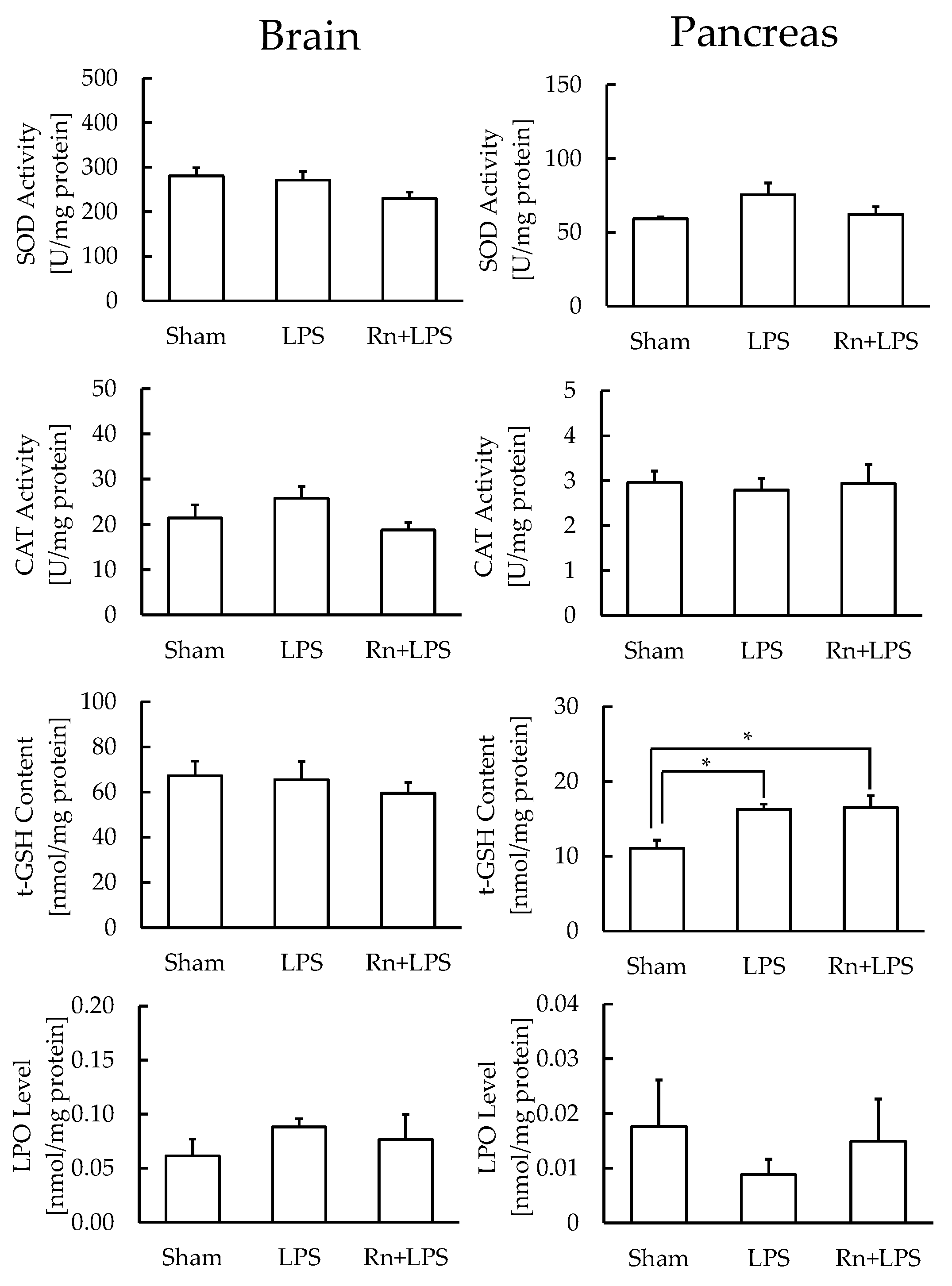
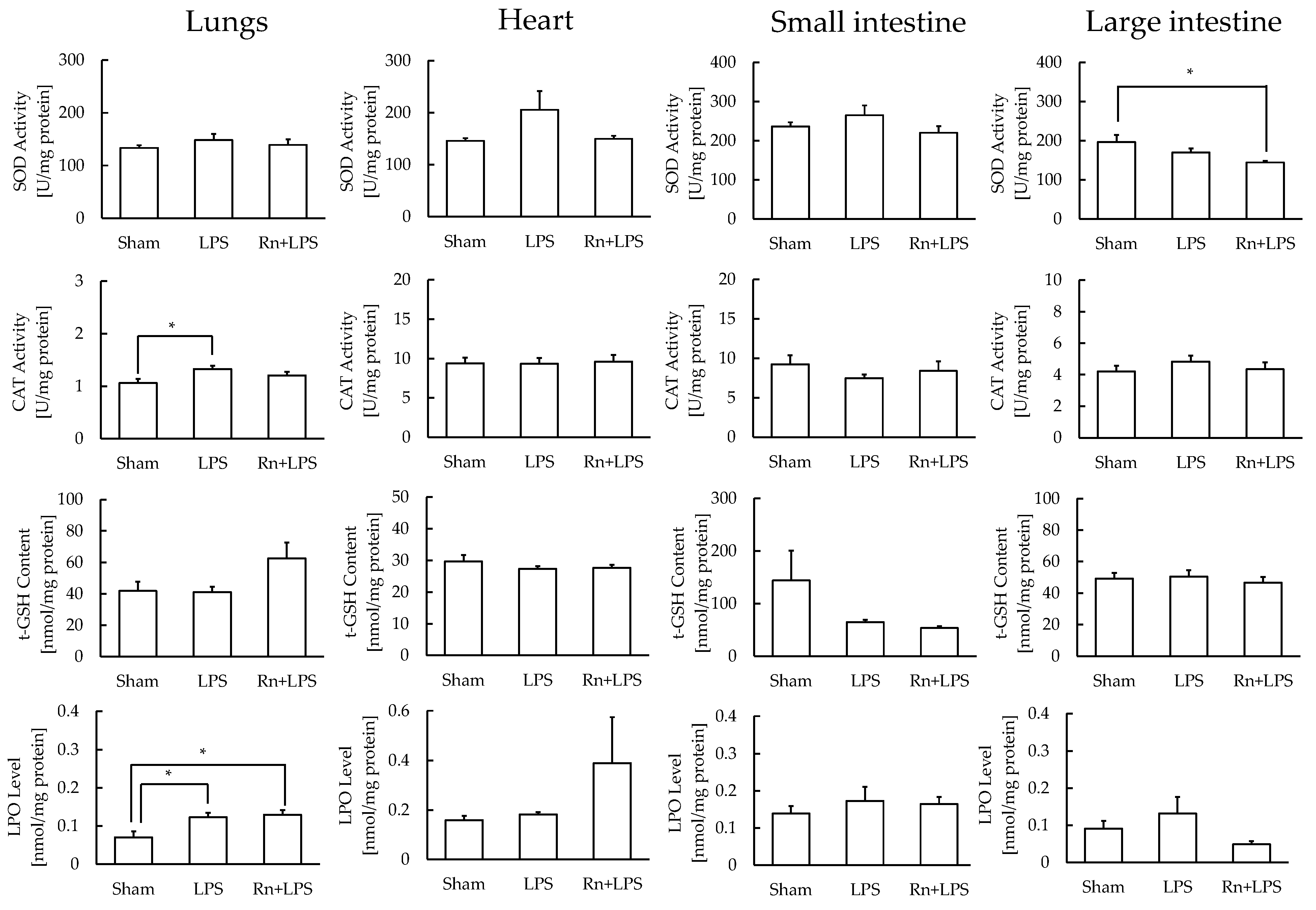
3.3. Effect of Radon Inhalation on Oxidative Stress Associated with LPS Administration in Various Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Franke, A.; Reiner, L.; Pratzel, H.; Franke, T.; Resch, K. Long-term efficacy of radon spa therapy in rheumatoid arthritis—A randomized, sham-controlled study and follow-up. Rheumatology 2000, 39, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Reiner, L.; Resch, K.L. Long-term benefit of radon spa therapy in the rehabilitation of rheumatoid arthritis: A randomised, double-blinded trial. Rheumatol. Int. 2007, 27, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Falkenbach, A.; Kovacs, J.; Franke, A.; Jörgens, K.; Ammer, K. Radon therapy for the treatment of rheumatic diseases—Review and meta-analysis of controlled clinical trials. Rheumatol. Int. 2005, 25, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Wiedemann, J.; Rapp, F.; Papenfuß, F.; Rödel, F.; Hehlgans, S.; Gaipl, U.S.; Kraft, G.; Fournier, C.; Frey, B. Radon exposure-therapeutic effect and cancer risk. Int. J. Mol. Sci. 2020, 22, 316. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T. Study of Antioxidative effects and anti-inflammatory effects in mice due to low-dose X-irradiation or radon inhalation. J. Radiat. Res. 2013, 54, 587–596. [Google Scholar] [CrossRef]
- Kataoka, T.; Kanzaki, N.; Sakoda, A.; Shuto, H.; Yano, J.; Naoe, S.; Tanaka, H.; Hanamoto, K.; Terato, H.; Mitsunobu, F.; et al. Evaluation of the redox state in mouse organs following radon inhalation. J. Radiat. Res. 2021, 62, 206–216. [Google Scholar] [CrossRef]
- Kataoka, T.; Teraoka, J.; Sakoda, A.; Nishiyama, Y.; Yamato, K.; Monden, M.; Ishimori, Y.; Nomura, T.; Taguchi, T.; Yamaoka, K. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation 2012, 35, 713–722. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kataoka, T.; Yamato, K.; Taguchi, T.; Yamaoka, K. Suppression of dextran sulfate sodium-induced colitis in mice by radon inhalation. Mediat. Inflamm. 2012, 2012, 239617. [Google Scholar] [CrossRef]
- Yamato, K.; Kataoka, T.; Nishiyama, Y.; Taguchi, T.; Yamaoka, K. Antinociceptive effects of radon inhalation on formalin-induced inflammatory pain in mice. Inflammation 2013, 36, 355–363. [Google Scholar] [CrossRef]
- Niu, Q.; Cai, B.; Huang, Z.C.; Shi, Y.Y.; Wang, L.L. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2731–2736. [Google Scholar] [CrossRef]
- Kataoka, T.; Naoe, S.; Murakami, K.; Yukimine, R.; Fujimoto, Y.; Kanzaki, N.; Sakoda, A.; Mitsunobu, F.; Yamaoka, K. Mechanisms of action of radon therapy on cytokine levels in normal mice and rheumatoid arthritis mouse model. J. Clin. Biochem. Nutri. 2022, 70, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, M.; Weis, J.J.; Toshchakov, V.; Salkowski, C.A.; Cody, M.J.; Ward, D.C.; Qureshi, N.; Michalek, S.M.; Vogel, S.N. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 2001, 69, 14771482. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, S.Y.; Choi, A.J.; Kim, S.J. Lipopolysaccharide directly stimulates Th17 differentiation in vitro modulating phosphorylation of RelB and NF-κB1. Immunol. Lett. 2015, 165, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C.; Piggott, D.A.; Huleatt, J.W.; Visintin, I.; Herrick, C.A.; Bottomly, K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002, 196, 1645–1651. [Google Scholar] [CrossRef]
- Khoshkhouy, F.; Farshbaf, A.; Mahmoudabady, M.; Gholamnezhad, Z. Effects of moderate exercise on lipopolysaccharide-induced inflammatory responses in rat’s cardiac tissue. Cytokine 2021, 138, 155409. [Google Scholar] [CrossRef]
- Kataoka, T.; Shuto, H.; Yano, J.; Naoe, S.; Ishida, T.; Nakada, T.; Yamato, K.; Hanamoto, K.; Nomura, T.; Yamaoka, K. X-irradiation at 0.5 Gy after the forced swim test reduces forced swimming-induced immobility in mice. J. Radiat. Res. 2020, 61, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Baehner, R.L.; Murrmann, S.K.; Davis, J.; Johnston, R.B., Jr. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J. Clin. Investig. 1975, 56, 571–576. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shehata, M.; Schwarzmeier, J.D.; Hilgarth, M.; Demirtas, D.; Richter, D.; Hubmann, R.; Boeck, P.; Leiner, G.; Falkenbach, A. Effect of combined spa-exercise therapy on circulating TGF- 1 levels in patients with ankylosing spondylitis. Wien. Klin. Wochenschr. 2006, 118, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Cucu, A.; Shreder, K.; Kraft, D.; Rühle, P.F.; Klein, G.; Thiel, G.; Frey, B.; Gaipl, U.S.; Fournier, C. Decrease of markers related to bone erosion in serum of patients with musculoskeletal disorders after serial low-dose radon spa therapy. Front. Immunol. 2017, 8, 882. [Google Scholar] [CrossRef] [PubMed]
- Rühle, P.F.; Wunderlich, R.; Deloch, L.; Fournier, C.; Maier, A.; Klein, G.; Fietkau, R.; Gaipl, U.S.; Frey, B. Modulation of the peripheral immune system after low-dose radon spa therapy: Detailed longitudinal immune monitoring of patients within the RAD-ON01 study. Autoimmunity 2017, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Aslankoca, R.; Savranb, M.; Ozmenc, O.; Ascid, S. Hippocampus and cerebellum damage in sepsis induced bylipopolysaccharide in aged rats-Pregabalin can prevent damage. Biomed. Pharmacother. 2018, 108, 384–1392. [Google Scholar]
- Ahmad, A.; Ali, T.; Rehman, S.U.; Kim, M. Phytomedicine-based potent antioxidant, Fisetin protects CNS-insult LPS-induced oxidative stress-mediated neurodegeneration and memory impairment. J. Clin. Med. 2019, 8, 850. [Google Scholar] [CrossRef]
- Mizunoe, S.; Shuto, T.; Suzuki, S.; Matsumoto, C.; Watanabe, K.; Ueno-Shuto, K.; Suico, M.A.; Onuki, K.; Gruenert, D.C.; Kai, H. Synergism between interleukin (IL)-17 and Toll-like receptor 2 and 4 signals to induce IL-8 expression in cystic fibrosis airway epithelial cells. J. Pharmacol. Sci. 2012, 118, 512–520. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. Res. 2021, 41, 294–303. [Google Scholar] [CrossRef]
- Saitoh, N.; Awaya, A.; Sakudo, A.; Wook, S.S.; Saeki, K.; Matsumoto, Y.; Onodera, T. Serum thymic factor prevents LPS-induced pancreatic cell damage in mice via up-regulation of Bcl-2 expression in pancreas. Microbiol. Immunol. 2004, 48, 629–638. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen sulfide attenuates LPS-induced acute kidney injury by inhibiting inflammation and oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef]
- Islam, M.S.; Miao, L.; Yu, H.; Han, Z.; Sun, H. Ethanol extract of illicium henryi attenuates LPS-induced acute kidney injury in mice via regulating inflammation and oxidative stress. Nutrients 2019, 11, 1412. [Google Scholar] [CrossRef]
- Kataoka, T.; Ishida, T.; Naoe, S.; Kanzaki, N.; Sakoda, A.; Tanaka, H.; Mitsunobu, F.; Yamaoka, K. Potential inhibitory effects of low-dose thoron inhalation and ascorbic acid administration on alcohol-induced hepatopathy in mice. J. Radiat. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, T.; Naoe, S.; Murakami, K.; Fujimoto, Y.; Yukimine, R.; Tanaka, A.; Yamaoka, K. Immunomodulatory Effects of Radon Inhalation on Lipopolysaccharide-Induced Inflammation in Mice. Int. J. Environ. Res. Public Health 2022, 19, 10632. https://doi.org/10.3390/ijerph191710632
Kataoka T, Naoe S, Murakami K, Fujimoto Y, Yukimine R, Tanaka A, Yamaoka K. Immunomodulatory Effects of Radon Inhalation on Lipopolysaccharide-Induced Inflammation in Mice. International Journal of Environmental Research and Public Health. 2022; 19(17):10632. https://doi.org/10.3390/ijerph191710632
Chicago/Turabian StyleKataoka, Takahiro, Shota Naoe, Kaito Murakami, Yuki Fujimoto, Ryohei Yukimine, Ayumi Tanaka, and Kiyonori Yamaoka. 2022. "Immunomodulatory Effects of Radon Inhalation on Lipopolysaccharide-Induced Inflammation in Mice" International Journal of Environmental Research and Public Health 19, no. 17: 10632. https://doi.org/10.3390/ijerph191710632
APA StyleKataoka, T., Naoe, S., Murakami, K., Fujimoto, Y., Yukimine, R., Tanaka, A., & Yamaoka, K. (2022). Immunomodulatory Effects of Radon Inhalation on Lipopolysaccharide-Induced Inflammation in Mice. International Journal of Environmental Research and Public Health, 19(17), 10632. https://doi.org/10.3390/ijerph191710632





