A Multilevel Analysis of the Associated and Determining Factors of TB among Adults in South Africa: Results from National Income Dynamics Surveys 2008 to 2017
Abstract
1. Introduction
2. Materials and Methods
2.1. The NIDS Data
2.2. Model Formulation
- is a column vector of responses for available observations,
- the desgn matrix for the fixed effect with ,
- is a column vector of unknown regression coefficients for the fixed effects,
- and design matrix for the random effects with ,
- is a vector of unknown subject-specific random effects representing all levels of single factor where, and is a positive-definite matrix.
3. Results
3.1. Multicollinearity Results
3.2. Frequentist MLM Results
3.3. Bayesian MLM Results
3.4. Comparison of Posterior Distributions for Informative and Non-Informative Priors
3.5. Comparison of Frequentist and Bayesian MLM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coef. (Std. Err) | 95% CI | Coef. (Std. Err.) | 95% CI | Coef. (Std. Err) | 95% CI | Coef. (Std. Err) | 95% CI | Coef. (Std. Err) | 95% CI |
| Intercept | −3.42(0.43) | (−3.990; −2.303) | −3.192(0.715) | (−4.59; −1.792) | −1.889(0.341) | (−2.557; −1.220) | −2.15(0.322) | (−2.782; −1.519) | −2.488(0.351) | (−3.175; −1.801) |
| Marital status: Single | 0.25(0.09) | (0.068; 0.438) | 0.175(0.114) | (−0.049; 0.399) | 0.249(0.078) | (0.096; 0.403) | 0.359(0.07) | (0.221; 0.497) | 0.276(0.073) | (0.133; 0.420) |
| Age: 30−44 | 1.11(0.13) | (0.852; 1.369) | 1.185(0.137) | (0.916; 1.454) | 1.228(0.103) | (1.025; 1.430) | 1.149(0.089) | (0.975; 1.323) | 1.251(0.095) | (1.064; 1.438) |
| 45−59 | 0.94(0.15) | (0.648; 1.227) | 0.935(0.164) | (0.613; 1.257) | 1.165(0.116) | (0.938; 1.393) | 1.169(0.101) | (0.972; 1.367) | 1.293(0.108) | (1.082; 1.504) |
| 60+ | 0.41(0.17) | (0.073; 0.749) | 0.468(0.214) | (0.048; 0.888) | 0.707(0.136) | (0.440; 0.974) | 0.534(0.122) | (0.295; 0.773) | 0.787(0.128) | (0.536; 1.039) |
| Gender: female | −0.52(0.1) | (−0.719; −0.329) | −0.033(0.116) | (−0.260; 0.195) | −0.273(0.08) | (−0.430; −0.116) | −0.269(0.07) | (−0.407; −0.132) | −0.513(0.074) | (−0.657; −0.369) |
| Race: non-black-African | −0.28(0.16) | (−0.591; 0.042) | −0.445(0.172) | (−0.783; −0.107) | −0.135(0.124) | (−0.378; 0.108) | −0.118(0.108) | (−0.331; 0.094) | −0.26(0.11) | (−0.475; −0.044) |
| Education: Primary | 0.11(0.15) | (−0.184; 0.402) | 0.412(0.534) | (−0.635; 1.459) | 0.071(0.138) | (−0.200; 0.342) | 0.132(0.139) | (−0.141; 0.405) | 0.268(0.153) | (−0.033; 0.569) |
| Secondary | −0.41(0.17) | (−0.744; −0.074) | 0.087(0.539) | (−0.969; 1.145) | −0.275(0.149) | (−0.567; 0.016) | −0.194(0.150) | (−0.487; 0.099) | 0.034(0.163) | (−0.287; 0.353) |
| Tertiary | −0.42(0.26) | (−0.937; 0.098) | −0.2(0.579) | (−1.336; 0.935) | −0.818(0.216) | (−1.242; −0.394) | −0.438(0.187) | (−0.805; −0.070) | −0.245(0.200) | (−0.638; 0.148) |
| Home language literacy: cannot read | −0.05(0.15) | (−0.350; 0.250) | 0.146(0.309) | (−0.460; 0.752) | −0.06(0.147) | (−0.348; 0.229) | 0.541(0.138) | (−0.217; 0.326) | 0.227(0.151) | (−0.068; 0.522) |
| Employment status: not employed | 0.19(0.11) | (−0.019; 0.418) | 0.223(0.128) | (−0.028; 0.473) | 0.169(0.087) | (−0.002; 0.339) | 0.154(0.074) | (0.009; 0.298) | 0.031(0.076) | (−0.119; 0.180) |
| Regular smoking: No | −0.50(0.11) | (−0.704; −0.287) | −0.659(0.132) | (−0.917; −0.400) | −0.488(0.09) | (−0.663; −0.312) | −0.337(0.079) | (−0.492; −0.182) | −0.308(0.082) | (−0.469; −0.147) |
| Other disease: No | −0.28(0.12) | (−0.521; −0.047) | −0.571(0.184) | (−0.933; −0.210) | −1.07(0.099) | (−1.264; −0.876) | −0.895(0.084) | (−1.059; −0.732) | −1.051(0.084) | (−1.216; −0.886) |
| Regular exercise; No | 0.35(0.12) | (0.110; 0.591) | 0.162(0.128) | (−0.089; 0.413) | 0.142(0.089) | (−0.033; 0.317) | 0.105(0.075) | (−0.042; 0.252) | 0.185(0.078) | (0.031; 0.338) |
| Consult with health practitioner: No | −1.09(0.14) | (−1.357; −0.819) | −0.09(0.137) | (−1.358; −0.822) | −0.625(0.092) | (−0.806; −0.444) | −0.939(0.103) | (−1.141; −0.736) | −0.644(0.102) | (−0.843; −0.445) |
| Diagnosed asthma: No | −0.45(0.79) | (−0.788; −0.115) | −0.219(0.234) | (−0.678; 0.240) | −0.768(0.130) | (−1.024; −0.513) | −0.622(0.119) | (−0.854; −0.389) | −0.633(0.129) | (−0.887; −0.380) |
| Diagnosed diabetes: No | 0.19(0.21) | (−0.222; 0.604) | −0.146(0.234) | (−0.604; 0.313) | 0.096(0.148) | (−0.194; 0.385) | 0.059(0.13) | (−0.196; 0.314) | 0.238(0.149) | (−0.055; 0.528) |
| Social grant: No | −0.15(0.10) | (−0.353; 0.053) | −0.282(0.121) | (−0.519; −0.045) | −0.125(0.083) | (−0.288; 0.038) | −0.106(0.072) | (−0.247; 0.034) | −0.144(0.075) | (−0.292; 0.004) |
| Perceived household Income: No | 0.36(0.10) | (0.165; 0.546) | 0.047(0.107) | (−0.163; 0.256) | 0.112(0.074) | (−0.033; 0.258) | 0.161(0.064) | (0.036; 0.287) | 0.153(0.068) | (0.019; 0.286) |
| Access to better housing: No | −0.25(0.13) | (−0.508; 0.012) | −0.067(0.116) | (−0.294; 0.160) | −0.128(0.082) | (−0.289; 0.033) | −0.068(0.07) | (−0.206; 0.00) | −0.222(0.078) | (−0.374; −0.070) |
| Income below median: No | 0.13(0.11) | (−0.084; 0.344) | 0.211(0.117) | (−0.018; 0.440) | −0.03(0.086) | (−0.198; 0.139) | 0.187(0.077) | (0.035; 0.338) | 0.034(0.083) | (−0.127; 0.196) |
| Expenditure below median: No | 0.07(0.11) | (−0.142; 0.273) | 0.062(0.116) | (−0.165; 0.289) | 0.12(0.083) | (−0.043; 0.283) | 0.002(0.075) | (−0.144; 0.149) | 0.273(0.082) | (0.113; 0.434) |
| Geotype: Urban | 0.21(0.13) | (−0.049; 0.461) | 0.482(0.131) | (0.225; 0.738) | 0.309(0.095) | (0.123; 0.496) | 0.689(0.084) | (−0.097; 0.234) | 0.147(0.086) | (−0.021; 0.314) |
| Farms | 0.29(0.154) | (−0.014; 0.591) | −0.145(0.235) | (−0.606; 0.315) | 0.188(0.146) | (−0.098; 0.474) | 0.004(0.136) | (−0.262; 0.270) | 0.035(0.142) | (−0.244; 0.313) |
| Province Var(_cons) | 0.17(0.099) | (0.051; 0.532) | 0.108(0.069) | (0.031; 0.375) | 0.148(0.081) | (0.051; 0432) | 0.18(0.95) | (0.064; 0.505) | 0.216(0.111) | (0.079; 0.589) |
| ICC | 0.048(0.027) | (0.015; 0.139) | 0.032(0.02) | (0.009; 0.102) | 0.043(0.023) | (0.015; 0.116) | 0.052(0.026) | (0.019; 0.133) | 0.062(0.03) | (0.024; 0.152) |
| L.R test | 40.57 | 20.14 | 64.68 | 81.65 | 116.17 | |||||
| Wald Chi2(24) | 416.9 | 296.94 | 638.03 | 736.27 | 774.54 | |||||
| L.L | −2213.58 | −1733.031 | −3355.32 | −4237.021 | −3902.859 | |||||
| AIC | 4479.16 | 3518.013 | 6762.641 | 8526.042 | 7857.78 | |||||
| BIC | 4674.293 | 3712.263 | 6965.646 | 8733.214 | 8065.605 | |||||
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | val2.5pc | val97.5pc | Mean | val2.5pc | val97.5pc | Mean | val2.5pc | val97.5pc | Mean | val2.5pc | val97.5pc | Mean | 2.50% | 97.50% | |
| Intercept | −3.405 | −4.3 | −2.489 | −3.609 | −5.054 | −2.22 | −2.116 | −2.786 | −1.445 | −2.385 | −3.053 | −1.702 | −2.728 | −3.409 | −2.031 |
| Marital status: Single | 0.252 | 0.066 | 0.438 | 0.177 | −0.046 | 0.404 | 0.249 | 0.096 | 0.403 | 0.361 | 0.221 | 0.5 | 0.277 | 0.134 | 0.421 |
| Age: 30−44 | 1.113 | 0.856 | 1.374 | 1.19 | 0.921 | 1.461 | 1.226 | 1.023 | 1.43 | 1.151 | 0.977 | 1.327 | 1.254 | 1.068 | 1.441 |
| 45−59 | 0.94 | 0.651 | 1.231 | 0.938 | 0.614 | 1.26 | 1.163 | 0.935 | 1.391 | 1.17 | 0.969 | 1.37 | 1.294 | 1.084 | 1.505 |
| 60+ | 0.408 | 0.067 | 0.747 | 0.454 | 0.029 | 0.871 | 0.701 | 0.433 | 0.97 | 0.532 | 0.291 | 0.772 | 0.785 | 0.532 | 1.037 |
| Gender: female | −0.525 | −0.72 | −0.331 | −0.032 | −0.263 | 0.198 | −0.276 | −0.433 | −0.119 | −0.269 | −0.406 | −0.131 | −0.514 | −0.658 | −0.37 |
| Race: non-black-African | −0.334 | −0.655 | −0.017 | −0.519 | −0.86 | −0.182 | −0.175 | −0.417 | 0.066 | −0.144 | −0.356 | 0.068 | −0.28 | −0.496 | −0.067 |
| Education: Primary | 0.11 | −0.182 | 0.404 | 0.487 | −0.478 | 1.654 | 0.062 | −0.204 | 0.334 | 0.13 | −0.147 | 0.411 | 0.268 | −0.032 | 0.571 |
| Secondary | −0.406 | −0.738 | −0.072 | 0.165 | −0.809 | 1.342 | −0.283 | −0.569 | 0.007 | −0.195 | −0.494 | 0.108 | 0.033 | −0.287 | 0.357 |
| Tertiary | −0.423 | −0.955 | 0.085 | −0.124 | −1.194 | 1.109 | −0.829 | −1.256 | −0.412 | −0.441 | −0.816 | −0.067 | −0.248 | −0.643 | 0.146 |
| Home language literacy: cannot read | −0.05 | −0.354 | 0.25 | 0.119 | −0.528 | 0.706 | −0.067 | −0.358 | 0.221 | 0.051 | −0.224 | 0.325 | 0.226 | −0.072 | 0.519 |
| Employment status: not employed | 0.201 | −0.016 | 0.421 | 0.227 | −0.023 | 0.48 | 0.17 | 0.001 | 0.34 | 0.155 | 0.01 | 0.301 | 0.032 | −0.117 | 0.182 |
| Regular smoking: No | −0.498 | −0.71 | −0.287 | −0.658 | −0.918 | −0.396 | −0.487 | −0.663 | −0.311 | −0.337 | −0.493 | −0.182 | −0.308 | −0.47 | −0.147 |
| Other disease: No | −0.285 | −0.52 | −0.042 | −0.562 | −0.922 | −0.192 | −1.077 | −1.271 | −0.883 | −0.899 | −1.063 | −0.733 | −1.056 | −1.22 | −0.891 |
| Regular exercise; No | 0.345 | 0.105 | 0.59 | 0.162 | −0.089 | 0.421 | 0.147 | −0.028 | 0.324 | 0.106 | −0.04 | 0.255 | 0.187 | 0.034 | 0.342 |
| Consult with health practitioner: No | −1.096 | −1.371 | −0.83 | −1.091 | −1.367 | −0.827 | −0.631 | −0.816 | −0.453 | −0.942 | −1.148 | −0.744 | −0.646 | −0.849 | −0.449 |
| Diagnosed asthma: No | −0.444 | −0.777 | −0.1 | −0.183 | −0.629 | 0.304 | −0.764 | −1.014 | −0.505 | −0.618 | −0.852 | −0.379 | −0.629 | −0.878 | −0.37 |
| Diagnosed diabetes: No | 0.209 | −0.194 | 0.641 | −0.113 | −0.556 | 0.364 | 0.103 | −0.178 | 0.394 | 0.065 | −0.188 | 0.329 | 0.245 | −0.04 | 0.543 |
| Social grant: No | −0.151 | −0.356 | 0.053 | −0.28 | −0.521 | −0.045 | −0.128 | −0.292 | 0.035 | −0.108 | −0.249 | 0.032 | −0.146 | −0.296 | 0 |
| Perceived household Income: No | 0.361 | 0.17 | 0.553 | 0.038 | −0.172 | 0.25 | 0.112 | −0.034 | 0.257 | 0.16 | 0.034 | 0.287 | 0.155 | 0.021 | 0.288 |
| Access to better housing: No | −0.243 | −0.503 | 0.017 | −0.044 | −0.272 | 0.183 | −0.125 | −0.286 | 0.036 | −0.068 | −0.207 | 0.07 | −0.223 | −0.376 | −0.072 |
| Income below median: No | 0.131 | −0.082 | 0.345 | 0.212 | −0.017 | 0.44 | −0.027 | −0.194 | 0.143 | 0.187 | 0.036 | 0.339 | 0.032 | −0.13 | 0.194 |
| Expenditure below median: No | 0.062 | −0.145 | 0.272 | 0.061 | −0.168 | 0.287 | 0.117 | −0.047 | 0.28 | 0.001 | −0.146 | 0.148 | 0.274 | 0.113 | 0.435 |
| Geotype: Urban | 0.194 | −0.062 | 0.448 | 0.469 | 0.207 | 0.729 | 0.304 | 0.118 | 0.491 | 0.066 | −0.1 | 0.233 | 0.145 | −0.022 | 0.311 |
| Farms | 0.281 | −0.027 | 0.582 | −0.162 | −0.641 | 0.288 | 0.183 | −0.109 | 0.465 | −0.003 | −0.273 | 0.26 | 0.027 | −0.258 | 0.301 |
| Var(_cons) Tau.prov | 3.688 | 0.916 | 8.823 | 3.493 | 2.458 | 12.87 | 6.541 | 1.63 | 15.62 | 6.335 | 1.543 | 15.24 | 6.609 | 1.633 | 15.88 |
| Wave 1 Posterior | Wave 2 Posterior | Wave 3 Posterior | Wave 4 Posterior | Wave 5 Informative Priors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TB | Mean | Sd | Mean | Sd | Mean | Sd | Mean | Sd | Mean | Variance |
| Intercept | −3.405 * | 0.461 | −3.609 * | 0.732 | −2.116 * | 0.343 | −2.385 * | 0.345 | 0.260 | 0.223 |
| Marital status: Single | 0.252 * | 0.095 | 0.177 | 0.115 | 0.249 * | 0.078 | 0.361 * | 0.071 | 1.163 | 0.007 |
| Age: 30−44 | 1.113 * | 0.132 | 1.190 * | 0.138 | 1.226 * | 0.104 | 1.151 * | 0.090 | 1.053 | 0.013 |
| 45−59 | 0.940 * | 0.148 | 0.938 * | 0.165 | 1.163 * | 0.116 | 1.170 * | 0.102 | 0.524 | 0.017 |
| 60+ | 0.408 * | 0.173 | 0.454 * | 0.215 | 0.701 * | 0.137 | 0.532 * | 0.123 | −0.276 | 0.026 |
| Gender: female | −0.525 * | 0.099 | −0.032 | 0.117 | −0.276 * | 0.080 | −0.269 * | 0.070 | −0.218 | 0.007 |
| Race: non-black-African | −0.334 * | 0.163 | −0.519 * | 0.173 | −0.175 | 0.123 | −0.144 | 0.108 | 0.298 | 0.028 |
| Education: Primary | 0.110 | 0.150 | 0.487 | 0.541 | 0.062 | 0.138 | 0.130 | 0.142 | 1.000 | 0.000 |
| Secondary | −0.406 * | 0.171 | 0.165 | 0.546 | −0.283 | 0.148 | −0.195 | 0.154 | −0.423 | 0.171 |
| Tertiary | −0.423 | 0.265 | −0.124 | 0.586 | −0.829 * | 0.216 | −0.441 * | 0.191 | −0.008 | 0.042 |
| Home language literacy: cannot read | −0.050 | 0.154 | 0.119 | 0.315 | −0.067 | 0.148 | 0.051 | 0.140 | 0.188 | 0.037 |
| Employment status: not employed | 0.201 | 0.112 | 0.227 | 0.128 | 0.170 * | 0.087 | 0.155 * | 0.074 | −0.412 | 0.007 |
| Regular smoking: No | −0.498 * | 0.108 | −0.658 | 0.134 | −0.487 * | 0.090 | −0.337 * | 0.079 | −0.706 | 0.010 |
| Other disease: No | −0.285 * | 0.122 | −0.562 * | 0.186 | −1.077 * | 0.099 | −0.899 * | 0.084 | 0.190 | 0.015 |
| Regular exercise; No | 0.345 * | 0.124 | 0.162 | 0.130 | 0.147 | 0.089 | 0.106 | 0.075 | −1.096 | 0.124 |
| Consult with health practitioner: No | −1.096 * | 0.138 | −1.091 * | 0.138 | −0.631 * | 0.092 | −0.942 * | 0.104 | −0.502 | 0.014 |
| Diagnosed asthma: No | −0.444 * | 0.172 | −0.183 | 0.238 | −0.764 * | 0.130 | −0.618 * | 0.120 | 0.094 | 0.020 |
| Diagnosed diabetes: No | 0.209 | 0.212 | −0.113 | 0.236 | 0.103 | 0.146 | 0.065 | 0.132 | 1.000 | 0.000 |
| Social grant: No | −0.151 | 0.104 | −0.280 * | 0.121 | −0.128 | 0.083 | −0.108 | 0.072 | 0.038 | 0.121 |
| Perceived household Income: No | 0.361 * | 0.097 | 0.038 | 0.108 | 0.112 | 0.074 | 0.160 * | 0.064 | −0.156 | 0.006 |
| Access to better housing: No | −0.243 | 0.132 | −0.044 | 0.116 | −0.125 | 0.082 | −0.068 | 0.071 | 1.000 | 0.000 |
| Income below median: No | 0.131 | 0.109 | 0.212 | 0.117 | −0.027 | 0.086 | 0.187 * | 0.077 | 0.001 | 0.077 |
| Expenditure below median: No | 0.062 | 0.106 | 0.061 | 0.116 | 0.117 | 0.084 | 0.001 | 0.075 | 1.000 | 0.000 |
| Geotype: Urban | 0.194 | 0.130 | 0.469 * | 0.133 | 0.304 * | 0.095 | 0.066 * | 0.085 | 0.010 | 0.013 |
| Farms | 0.281 | 0.155 | −0.162 | 0.238 | 0.183 | 0.146 | −0.003 | 0.136 | 1.000 | 0.000 |
| Province Var(_cons) | 3.688 | 2.071 | 3.493 | 4.562 | 6.541 | 3.693 | 6.335 | 3.581 | 5.521 | 11.143 |
Appendix B
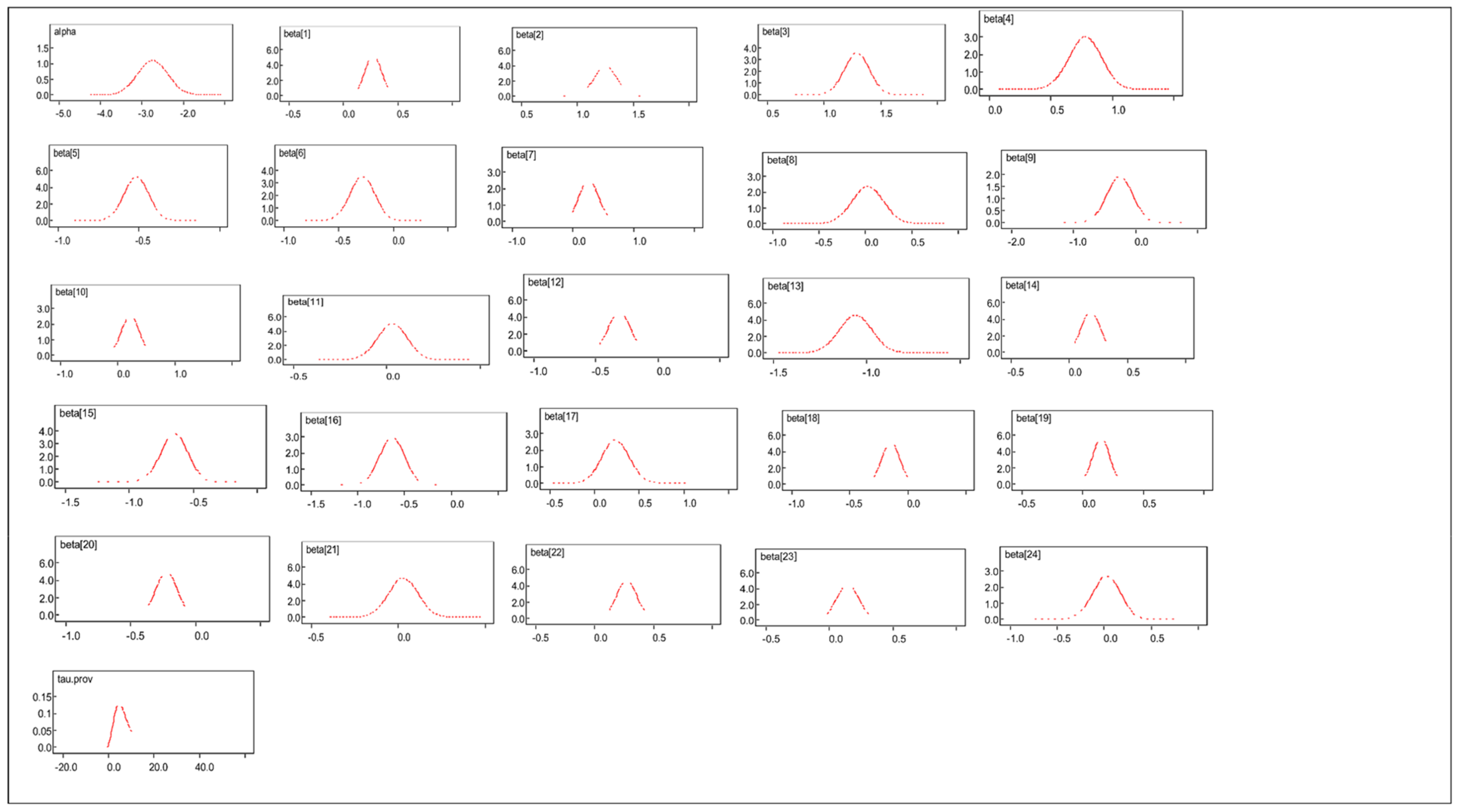
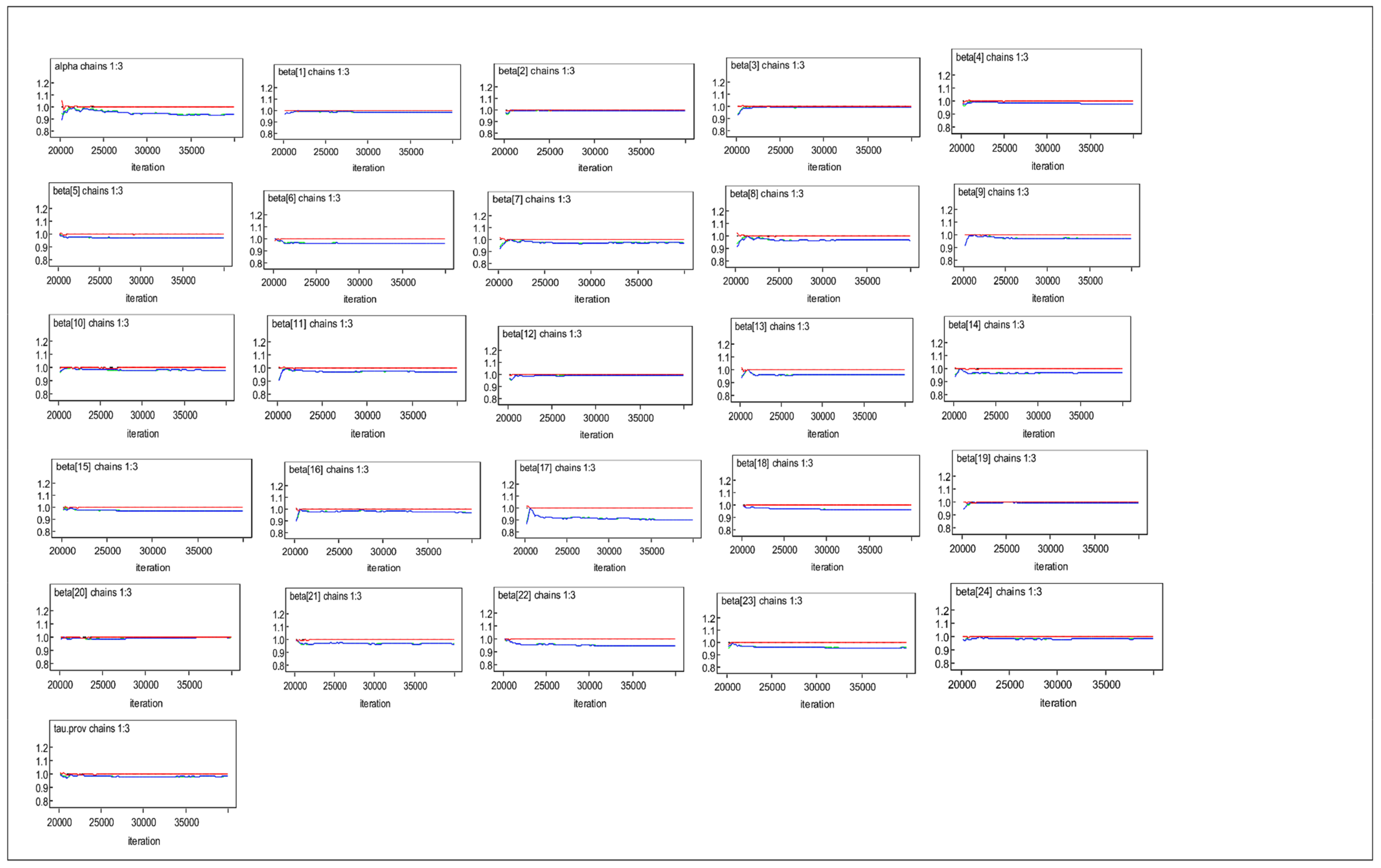
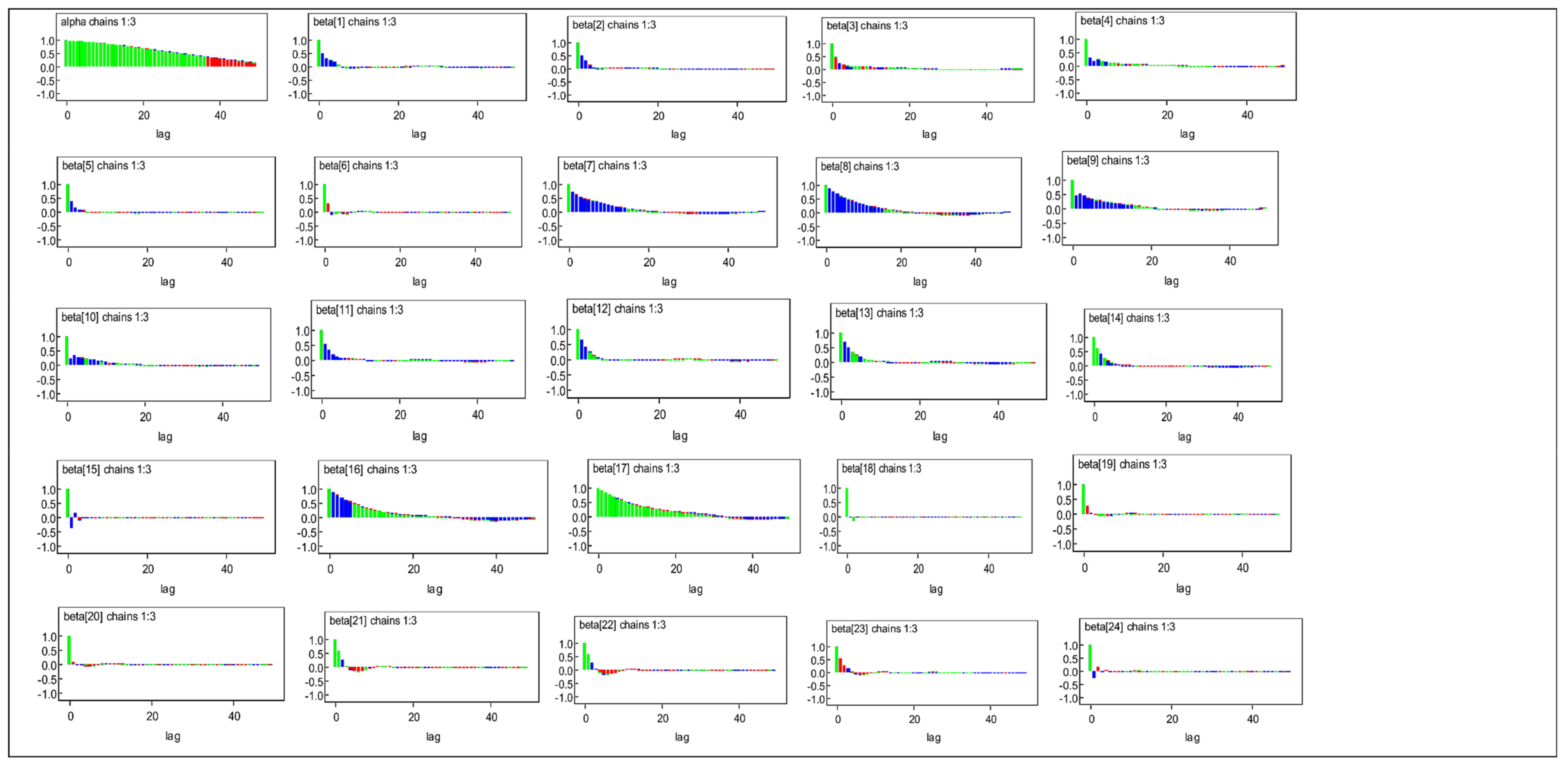
References
- WHO. Tuberculosis Fact Sheets. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 23 May 2019).
- WHO. Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015. End TB Strategy. 2015. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 24 May 2019).
- USAID. USAID: Tuberculosis Project South Africa, National TB Control Programme, Goals and Strategies. 2015. Available online: http://pdf.usaid.gov/pdf_docs/PNADR735.pdf (accessed on 22 May 2019).
- Harling, G.; Ehrlich, R.; Myer, L. The social epidemiology of tuberculosis in South Africa: A multilevel analysis. Soc. Sci. Med. 2008, 66, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Yach, D. Tuberculosis in the Western Cape health region of South Africa. Soc. Sci. Med. 1988, 27, 683–689. [Google Scholar] [CrossRef]
- Adjei, I.A.; Karim, R. An Application of Bootstrapping in Logistic Regression Model. Open Access Libr. J. 2016, 3, 1. [Google Scholar] [CrossRef][Green Version]
- Dhlakama, H.; Lougue, S. Bayesian Modelling of Tuberculosis Risk Factors in South Africa 2014. Int. J. Stat. Med. Res. 2017, 6, 34–48. [Google Scholar] [CrossRef]
- Young, B.N.; Rendón, A.; Rosas-Taraco, A.; Baker, J.; Healy, M.; Gross, J.M.; Long, J.; Burgos, M.; Hunley, K.L. The effects of socioeconomic status, clinical factors, and genetic ancestry on pulmonary tuberculosis disease in northeastern Mexico. PLoS ONE 2014, 9, e94303. [Google Scholar]
- Stoesslé, P.; González-Salazar, F.; Santos-Guzmán, J.; Sánchez-González, N. Risk Factors and Current Health-Seeking Patterns of Migrants in Northeastern Mexico: Healthcare Needs for a Socially Vulnerable Population. Front. Public Health 2015, 3, 191. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4526788/ (accessed on 31 January 2019). [CrossRef] [PubMed]
- Cramm, J.M.; Koolman, X.; Møller, V.; Nieboer, A.P. Socio-Economic Status and Self-Reported Tuberculosis: A Multilevel Analysis in a Low-Income Township in the Eastern Cape, South Africa. J. Public Health Afr. 2011, 2, e34. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5345507/ (accessed on 31 January 2019). [CrossRef]
- Knight, G.M.; Dodd, P.J.; Grant, A.D.; Fielding, K.L.; Churchyard, G.J.; White, R.G. Tuberculosis prevention in South Africa. PLoS ONE 2015, 10, e0122514. [Google Scholar] [CrossRef] [PubMed]
- Cramm, J.M.; Nieboer, A.P. The influence of social capital and socio-economic conditions on self-rated health among residents of an economically and health-deprived South African township. Int. J. Equity Health 2011, 10, 51. [Google Scholar] [CrossRef]
- Leibbrandt, M.; Woolard, I.; de Villiers, L. Methodology: Report on NIDS Wave 1. N.I.D.S. Natl. Income Dyn. Study 2009, 1, 34. [Google Scholar]
- Pfeffermann, D. Modelling of complex survey data: Why model? Why is it a problem? How can we approach it. Surv. Methodol. 2011, 37, 115–136. [Google Scholar]
- Carle, A.C. Fitting multilevel models in complex survey data with design weights: Recommendations. BMC Med. Res. Methodol. 2009, 9, 49. [Google Scholar] [CrossRef]
- Goldstein, H. Multilevel Statistical Models; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Goldstein, H.; Rasbash, J.; Plewis, I.; Draper, D.; Brown, W.; Yang, M. Multi-Level Models Project; Institute of Education, University of London: London, UK, 1995. [Google Scholar]
- Hox, J.J.; Mahwah, N.J. Multilevel Analysis: Techniques and Applications; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2002. [Google Scholar]
- Ghosh, S.; Pahwa, P. Design-based versus model-based methods: A comparative study using longitudinal survey data. In Proceedings of the Statistical Society of Canada Survey Methods Section, London, ON, Canada, 28 May 2006. [Google Scholar]
- Snijders, T.A.; Bosker, R.J. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling; Sage: Newcastle, UK, 2011. [Google Scholar]
- Molenberghs, G.; Verbeke, G. Models for Discrete Longitudinal Data. Springer-Verlag: New York, NY, USA, 2005; Available online: http://link.springer.com/10.1007/0−387−28980−1 (accessed on 12 February 2019).
- Lesaffre, E.; Lawson, A.B. Bayesian Biostatistics; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bruce, P.; Bruce, A.; Gedeck, P. Practical Statistics for Data Scientists: 50+ Essential Concepts Using R and Python; O’Reilly Media: Newton, MA, USA, 2020. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ojo, O.B.; Lougue, S.; Woldegerima, W.A. Bayesian generalized linear mixed modeling of Tuberculosis using informative priors. PLoS ONE 2017, 12, e0172580. [Google Scholar] [CrossRef] [PubMed]
- Harling, G.; Castro, M.C. A Spatial Analysis of Social and Economic Determinants of Tuberculosis in Brazil. Health Place; Elsevier: Amsterdam, The Netherlands, 2014; Volume 25, pp. 56–67. [Google Scholar]
- Altet, N.; Latorre, I.; Jiménez-Fuentes, M.Á.; Maldonado, J.; Molina, I.; González-Díaz, Y.; Milà, C.; García-García, E.; Muriel, B.; Villar-Hernández, R.; et al. Assessment of the influence of direct tobacco smoke on infection and active TB management. PLoS ONE 2017, 12, e0182998. [Google Scholar] [CrossRef] [PubMed]
| Variable | VIF | 1/VIF |
|---|---|---|
| Marital status: Single | 1.3 | 0.769004 |
| Age: 30–44 | 1.48 | 0.6761 |
| 45–59 | 1.7 | 0.588023 |
| 60+ | 1.74 | 0.576259 |
| Gender: female | 1.27 | 0.788557 |
| Race: non-black-African | 1.38 | 0.725238 |
| Education: Primary | 4.73 | 0.211383 |
| Secondary | 7.46 | 0.134047 |
| Tertiary | 3.98 | 0.251132 |
| Home language literacy: cannot read | 1.98 | 0.504168 |
| Employment status: not employed | 1.35 | 0.740186 |
| Regular smoking: No | 1.25 | 0.79856 |
| Other disease: No | 1.04 | 0.963998 |
| Regular exercise; No | 1.16 | 0.865169 |
| Consult with health practitioner: No | 1.07 | 0.934643 |
| Diagnosed asthma: No | 1.01 | 0.985332 |
| Diagnosed diabetes: No | 1.08 | 0.922889 |
| Social grant: No | 1.18 | 0.844367 |
| Perceived household Income: No | 1.14 | 0.879005 |
| Access to better housing: No | 1.3 | 0.769982 |
| Income below median: No | 1.49 | 0.671186 |
| Expenditure below median: No | 1.55 | 0.647064 |
| Geotype: Urban | 1.46 | 0.684569 |
| Farms | 1.2 | 0.836529 |
| Mean VIF | 1.85 |
| Variables | Non-Informative | Informative | ||||
|---|---|---|---|---|---|---|
| TB | Mean | MC Error | 95% Cred. Interval | Mean | MC Error | 95% Cred. Interval |
| Intercept | −2.728 | 0.004 | (−3.409: −2.031) | −2.813 | 0.023 | (−4.275:−1.396) |
| Marital status: Single | 0.277 | 0.000 | (0.134: 0.421) | 0.276 | 0.000 | (0.1337: 0.420) |
| Age: 30–44 | 1.254 | 0.000 | (1.068: 1.441) | 1.254 | 0.001 | (1.068: 1.443) |
| 45–59 | 1.294 | 0.000 | (1.084: 1.505) | 1.296 | 0.001 | (1.085: 1.508) |
| 60+ | 0.785 | 0.001 | (0.532: 1.037) | 0.788 | 0.001 | (0.536: 1.039) |
| Gender: female | −0.514 | 0.000 | (−0.658: −0.370) | −0.513 | 0.000 | (−0.657: −0.369) |
| Race: non-black-African | −0.280 | 0.000 | (−0.496: −0.067) | −0.287 | 0.000 | (−0.502: −0.071) |
| Education: Primary | 0.268 | 0.001 | (−0.032: 0.571) | 0.269 | 0.002 | (−0.025: 0.569) |
| Secondary | 0.033 | 0.001 | (−0.287: 0.357) | 0.038 | 0.002 | (−0.273: 0.357) |
| Tertiary | −0.248 | 0.001 | (−0.643: 0.146) | −0.242 | 0.002 | (−0.631: 0.148) |
| Home language literacy: cannot read | 0.226 | 0.001 | (−0.072: 0.519) | 0.228 | 0.001 | (−0.068: 0.520) |
| Employment status: not employed | 0.032 | 0.000 | (−0.117: 0.182) | 0.031 | 0.001 | (−0.118: 0.182) |
| Regular smoking: No | −0.308 | 0.000 | (−0.470: −0.147) | −0.307 | 0.001 | (−0.467: −0.144) |
| Other disease: No | −1.056 | 0.000 | (−1.220: −0.891) | −1.056 | 0.001 | (−1.221: −0.889) |
| Regular exercise; No | 0.187 | 0.000 | (0.034: 0.342) | 0.186 | 0.000 | (0.033: 0.340) |
| Consult with health practitioner: No | −0.646 | 0.000 | (−0.849: −0.449) | −0.647 | 0.000 | (−0.849: −0.451) |
| Diagnosed asthma: No | −0.629 | 0.001 | (−0.878: −0.370) | −0.626 | 0.002 | (−0.875: −0.365) |
| Diagnosed diabetes: No | 0.245 | 0.001 | (−0.040: 0.543) | 0.248 | 0.002 | (−0.038: 0.564) |
| Social grant: No | −0.146 | 0.000 | (−0.296: 0.000) | −0.143 | 0.000 | (−0.293: 0.005) |
| Perceived household Income: No | 0.155 | 0.000 | (0.021: 0.288) | 0.152 | 0.000 | (0.020: 0.285) |
| Access to better housing: No | −0.223 | 0.000 | (−0.376: −0.072) | −0.221 | 0.000 | (−0.374: −0.070) |
| Income below median: No | 0.0318 | 0.000 | (−0.130: 0.1940 | 0.033 | 0.000 | (−0.128: 0.196) |
| Expenditure below median: No | 0.274 | 0.000 | (0.113: 0.435) | 0.272 | 0.000 | (0.111: 0.434) |
| Geotype: Urban | 0.145 | 0.000 | (−0.022: 0.311) | 0.142 | 0.001 | (−0.027: 0.311) |
| Farms | 0.027 | 0.000 | (−0.258: 0.301) | 0.027 | 0.001 | (−0.257: 0.304) |
| Province Var(_cons) Tau.prov | 6.609 | 0.014 | (1.633: 15.880) | 0.587 | 0.001 | (0.231: 1.110) |
| Prior Type | Dbar | Dhat | pD | DIC |
|---|---|---|---|---|
| Non-informative | 7801.7 | 7771.82 | 29.88 | 7831.58 |
| Informative | 7802.73 | 7769.83 | 32.894 | 7835.62 |
| Variables | Frequentist MLM | Bayesian MLM (Non-Informative) | ||
|---|---|---|---|---|
| TB | Coef. | 95% Conf. Interval | Mean (MC. Error) | 95% Cred. Interval |
| Intercept | 0.276 * | (0.133; 0.420) | 0.277 (0.000) * | (0.134: 0.421) |
| Marital status: Single | 1.251 * | (1.064; 1.438) | 1.254 (0.000) * | (1.068: 1.441) |
| Age: 30–44 | 1.293 * | (1.082; 1.504) | 1.294 (0.000) * | (1.084: 1.505) |
| 45–59 | 0.787 * | (0.536; 1.039) | 0.785 (0.000) * | (0.532: 1.037) |
| 60+ | −0.513 * | (−0.657; −0.369) | −0.514 (0.000) * | (−0.658: −0.370) |
| Gender:Female | −0.260 * | (−0.475; −0.044) | −0.280 (0.000) * | (−0.496: −0.067) |
| Race: non-black-African | 0.268 | (−0.033; 0.569) | 0.268 (0.001) | (−0.032: 0.571) |
| Education: Primary | 0.034 | (−0.287; 0.353) | 0.033 (0.001) | (−0.287: 0.357) |
| Secondary | −0.245 | (−0.638; 0.148) | −0.248 (0.001) | (−0.643: 0.146) |
| Tertiary | 0.227 | (−0.068; 0.522) | 0.226 (0.001) | (−0.072: 0.519) |
| Home language literacy: cannot read | 0.031 | (−0.119; 0.180) | 0.032 (0.000) | (−0.117: 0.182) |
| Employment status: not employed | −0.308 * | (−0.469; −0.147) | −0.308 (0.000) * | (−0.470: −0.147) |
| Regular smoking: No | −1.051 * | (−1.216; −0.886) | −1.056 (0.000) * | (−1.220: −0.891) |
| Other disease: No | 0.185 * | (0.031; 0.338) | 0.187 (0.000) * | (0.034: 0.342) |
| Regular exercise; No | −0.644 * | (−0.843; −0.445) | −0.646 (0.000) * | (−0.849: −0.449) |
| Consult with health practitioner: No | −0.633 * | (−0.887; −0.380) | −0.629 (0.001) * | (−0.878: −0.370) |
| Diagnosed asthma: No | 0.238 | (−0.055; 0.528) | 0.245 (0.001) | (−0.040: 0.543) |
| Diagnosed diabetes: No | −0.144 | (−0.292; 0.004) | −0.146 (0.000) | (−0.296: 0.000) |
| Social grant: No | 0.153 * | (0.019; 0.286) | 0.155 (0.000) * | (0.021: 0.288) |
| Perceived household Income: No | −0.222 * | (−0.374; −0.070) | −0.223 (0.000) * | (−0.376: −0.072) |
| Access to better housing: No | 0.034 | (−0.127; 0.196) | 0.0318 (0.000) | (−0.130: 0.194) |
| Income below median: No | 0.273 * | (0.113; 0.434) | 0.274 (0.000) * | (0.113: 0.435) |
| Expenditure below median: No | 0.147 | (−0.021; 0.314) | 0.145 (0.000) | (−0.022: 0.311) |
| Geotype: Urban | 0.035 | (−0.244; 0.313) | 0.027 (0.000) | (−0.258: 0.301) |
| Farms | 0.216 * | (0.079; 0.589) | 6.609 (0.014) * | (1.633: 15.880) |
| LR test | 116.17 | |||
| Wald Chi2(24) | 774.54 | |||
| LL | −3902.859 | |||
| AIC | 7857.78 | |||
| BIC | 8065.605 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhlakama, H.; Lougue, S.; Mwambi, H.G.; Ogunsakin, R.E. A Multilevel Analysis of the Associated and Determining Factors of TB among Adults in South Africa: Results from National Income Dynamics Surveys 2008 to 2017. Int. J. Environ. Res. Public Health 2022, 19, 10611. https://doi.org/10.3390/ijerph191710611
Dhlakama H, Lougue S, Mwambi HG, Ogunsakin RE. A Multilevel Analysis of the Associated and Determining Factors of TB among Adults in South Africa: Results from National Income Dynamics Surveys 2008 to 2017. International Journal of Environmental Research and Public Health. 2022; 19(17):10611. https://doi.org/10.3390/ijerph191710611
Chicago/Turabian StyleDhlakama, Hilda, Siaka Lougue, Henry Godwell Mwambi, and Ropo Ebenezer Ogunsakin. 2022. "A Multilevel Analysis of the Associated and Determining Factors of TB among Adults in South Africa: Results from National Income Dynamics Surveys 2008 to 2017" International Journal of Environmental Research and Public Health 19, no. 17: 10611. https://doi.org/10.3390/ijerph191710611
APA StyleDhlakama, H., Lougue, S., Mwambi, H. G., & Ogunsakin, R. E. (2022). A Multilevel Analysis of the Associated and Determining Factors of TB among Adults in South Africa: Results from National Income Dynamics Surveys 2008 to 2017. International Journal of Environmental Research and Public Health, 19(17), 10611. https://doi.org/10.3390/ijerph191710611






