Acute Effects of High-Intensity Functional Training and Moderate-Intensity Continuous Training on Cognitive Functions in Young Adults
Abstract
:1. Introduction
1.1. High-Intensity Exercise
1.2. High-Intensity Exercise with Overloads
1.3. Moderate/Low-Intensity Exercise
2. Materials and Methods
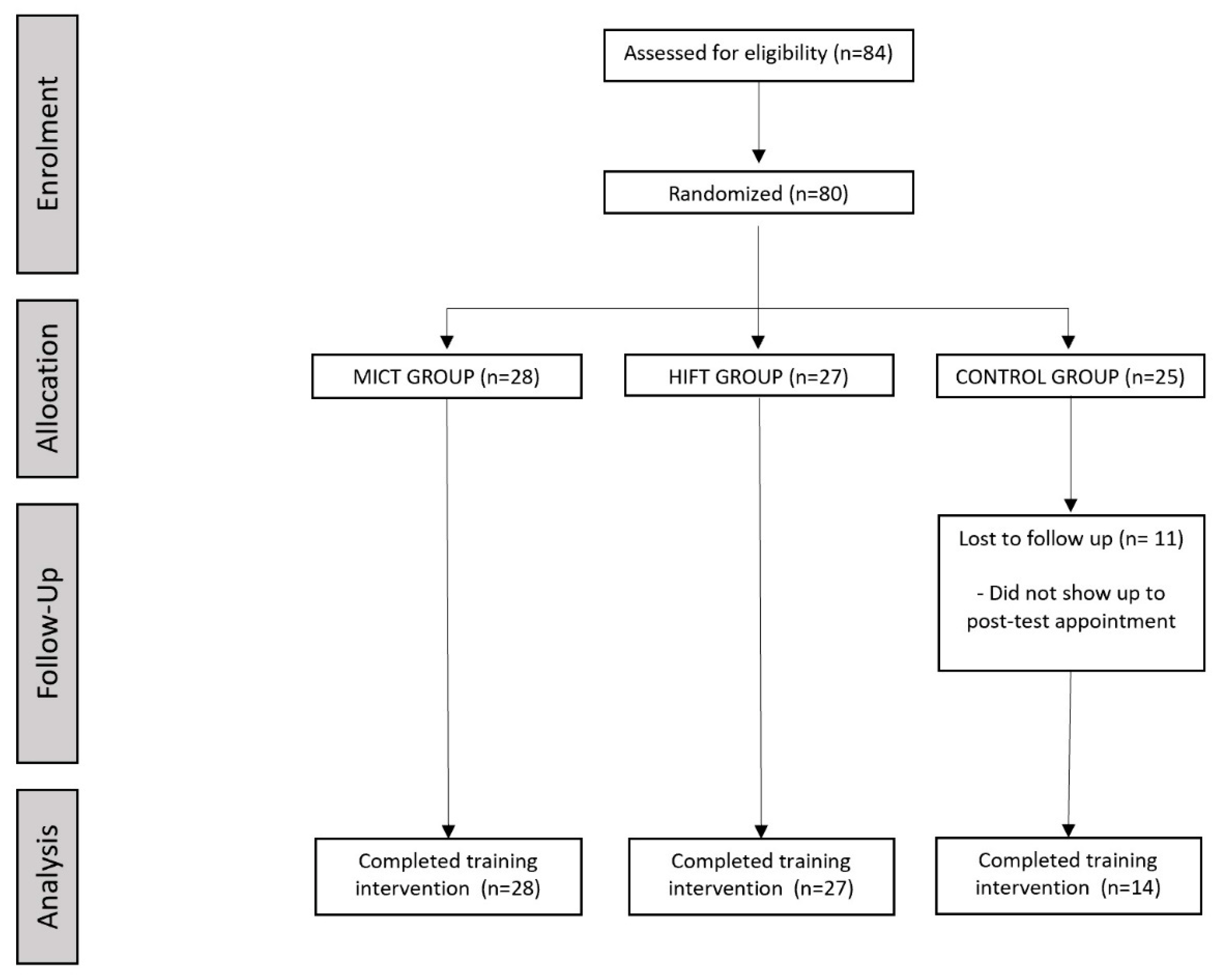
2.1. Study Design and Participants
2.2. Sample Size Calculation
2.3. Allocation to Intervention
2.4. Procedure
2.5. Instruments
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colcombe, S.; Kramer, A.F. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.; Sherwood, A. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bs, S.B.H.; Zelinski, E.M. Extended Practice and Aerobic Exercise Interventions Benefit Untrained Cognitive Outcomes in Older Adults: A Meta-Analysis. J. Am. Geriatr. Soc. 2012, 60, 136–141. [Google Scholar] [CrossRef]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Groot, C.; Hooghiemstra, A.; Raijmakers, P.; van Berckel, B.; Scheltens, P.; Scherder, E.; van der Flier, W.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic Exercise Improves Cognitive Functioning in People with Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2017, 43, 546–556. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Song, D.; Yu, D.S.; Li, P.W.; Lei, Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018, 79, 155–164. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Mekari, S.; Fraser, S.; Bosquet, L.; Bonnéry, C.; Labelle, V.; Pouliot, P.; Lesage, F.; Bherer, L. The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. Eur. J. Appl. Physiol. 2015, 115, 2189–2197. [Google Scholar] [CrossRef]
- Smith, M.; Tallis, J.; Miller, A.; Clarke, N.; Guimarães-Ferreira, L.; Duncan, M. The effect of exercise intensity on cognitive performance during short duration treadmill running. J. Hum. Kinet. 2016, 51, 27–35. [Google Scholar] [CrossRef]
- Moreau, D.; Chou, E. The Acute Effect of High-Intensity Exercise on Executive Function: A Meta-Analysis. Perspect. Psychol. Sci. 2019, 14, 734–764. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C.; Huang, T.-H.; Chou, F.-Y. Executive function and endocrinological responses to acute resistance exercise. Front. Behav. Neurosci. 2014, 8, 262. [Google Scholar] [CrossRef]
- Chang, H.; Kim, K.; Jung, Y.-J.; Kato, M. Effects of Acute High-Intensity Resistance Exercise on Cognitive Function and Oxygenation in Prefrontal Cortex. J. Exerc. Nutr. Biochem. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- Dietrich, A.; Audiffren, M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci. Biobehav. Rev. 2011, 35, 1305–1325. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ibañez, J.; Calbet, J.A.L.; Navarro-Amezqueta, I.; González-Izal, M.; Idoate, F.; Häkkinen, K.; Kraemer, W.J.; Palacios-Sarrasqueta, M.; Almar, M.; et al. Cytokine and hormone responses to resistance training. Eur. J. Appl. Physiol. 2009, 107, 397–409. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chu, C.-H.; Chu, I.-H.; Chan, K.-H.; Chang, Y.-K. Executive function during acute exercise: The role of exercise intensity. J. Sport Exerc. Psychol. 2013, 35, 358–367. [Google Scholar] [CrossRef]
- Browne, S.E.; Flynn, M.J.; O’Neill, B.V.; Howatson, G.; Bell, P.G.; Haskell-Ramsay, C.F. Effects of acute high-intensity exercise on cognitive performance in trained individuals: A systematic review. Prog. Brain Res. 2017, 234, 161–187. [Google Scholar] [CrossRef]
- French, D.N.; Kraemer, W.J.; Volek, J.S.; Spiering, B.A.; Judelson, D.A.; Hoffman, J.R.; Maresh, C.M. Anticipatory responses of catecholamines on muscle force production. J. Appl. Physiol. 2007, 102, 94–102. [Google Scholar] [CrossRef]
- Anders, J.P.V.; Kraemer, W.J.; Newton, R.U.; Post, E.M.; Caldwell, L.K.; Beeler, M.K.; DuPont, W.H.; Martini, E.R.; Volek, J.S.; Häkkinen, K.; et al. Acute Effects of High-intensity Resistance Exercise on Cognitive Function. J. Sports Sci. Med. 2021, 20, 391–397. [Google Scholar] [CrossRef]
- Audiffren, M.; Tomporowski, P.D.; Zagrodnik, J. Acute aerobic exercise and information processing: Energizing motor processes during a choice reaction time task. Acta Psychol. 2008, 129, 410–419. [Google Scholar] [CrossRef]
- Audiffren, M.; Tomporowski, P.D.; Zagrodnik, J. Acute aerobic exercise and information processing: Modulation of executive control in a Random Number Generation task. Acta Psychol. 2009, 132, 85–95. [Google Scholar] [CrossRef]
- Wilke, J.; Giesche, F.; Klier, K.; Vogt, L.; Herrmann, E.; Banzer, W. Acute Effects of Resistance Exercise on Cognitive Function in Healthy Adults: A Systematic Review with Multilevel Meta-Analysis. Sports Med. 2019, 49, 905–9166. [Google Scholar] [CrossRef]
- Querido, J.S.; Sheel, A.W. Regulation of Cerebral Blood Flow during Exercise. Sports Med. 2007, 37, 765–782. [Google Scholar] [CrossRef]
- Ogoh, S.; Ainslie, P.N. Regulatory mechanisms of cerebral blood flow during exercise: New concepts. Exerc. Sport Sci. Rev. 2009, 37, 123–129. [Google Scholar] [CrossRef]
- Grooms, D.; Appelbaum, G.; Onate, J. Neuroplasticity Following Anterior Cruciate Ligament Injury: A Framework for Visual-Motor Training Approaches in Rehabilitation. J. Orthop. Sports Phys. Ther. 2015, 45, 381–393. [Google Scholar] [CrossRef]
- Wilkerson, G.B. Neurocognitive Reaction Time Predicts Lower Extremity Sprains and Strains. Int. J. Athl. Ther. Train. 2012, 17, 4–9. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Hillman, C.H.; Fernhall, B.; Thompson, K.M.; Valentini, T.A. The Effect of Acute Aerobic and Resistance Exercise on Working Memory. Med. Sci. Sports Exerc. 2009, 41, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Addamo, P.K.; Raj, I.S.; Borkoles, E.; Wyckelsma, V.; Cyarto, E.; Polman, R. An Acute Bout of Exercise Improves the Cognitive Performance of Older Adults. J. Aging Phys. Act. 2016, 24, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E. Cognitive Psychology: Connecting mind, Research and Everyday Experience, 4th ed.; Wadsworth Publishing: Belmont, CA, USA, 2014. [Google Scholar]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. Gen. 1992, 121, 15–23. [Google Scholar] [CrossRef]
- Sibley, B.A.; Etnier, J.L.; Le Masurier, G.C. Effects of an Acute Bout of Exercise on Cognitive Aspects of Stroop Performance. J. Sport Exerc. Psychol. 2006, 28, 285–299. [Google Scholar] [CrossRef]
- Crawford, J.R.; Parker, D. A Handbook of Neuropsychological Assessment; McKinlay, W.W., Ed.; Psychology Press: London, UK, 1992. [Google Scholar]
- Vaynman, S.; Gomez-Pinilla, F. License to Run: Exercise Impacts Functional Plasticity in the Intact and Injured Central Nervous System by Using Neurotrophins. Neurorehabilit. Neural Repair 2005, 19, 283–295. [Google Scholar] [CrossRef]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The Effects of Aerobic Activity on Brain Structure. Front. Psychol. 2012, 3, 86. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Kandola, A.; Hendrikse, J.; Lucassen, P.J.; Yücel, M. Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment. Front. Hum. Neurosci. 2016, 10, 373. [Google Scholar] [CrossRef]
- Kim, Y. The effect of regular Taekwondo exercise on Brain-derived neurotrophic factor and Stroop test in undergraduate student. J. Exerc. Nutr. Biochem. 2015, 19, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Berg, V.V.D.; Saliasi, E.; Jolles, J.; De Groot, R.H.M.; Chinapaw, M.; Singh, A.S. Exercise of Varying Durations: No Acute Effects on Cognitive Performance in Adolescents. Front. Neurosci. 2018, 12, 672. [Google Scholar] [CrossRef]
- Coles, K.; Tomporowski, P.D. Effects of acute exercise on executive processing, short-term and long-term memory. J. Sports Sci. 2008, 26, 333–344. [Google Scholar] [CrossRef]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Etsai, C.-L.; Wang, C.-H.; Epan, C.-Y.; Echen, F.-C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Hsu, C.L.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef]

| Group | N | Age (m ± sd) | IMC (m ± sd) |
|---|---|---|---|
| HIFT | 27 | 21.62 ± 3.83 | 22.58 ± 1.97 |
| MICT | 28 | 20.25 ± 1.23 | 22.94 ± 0.80 |
| CTRL | 14 | 21.35 ± 2.43 | 22.93 ± 1.06 |
| Variable | Pre-Test (m ± sd) | Post-Test (m ± sd) | Difference | |
|---|---|---|---|---|
| Stroop | N° Corrects | 8.71 ± 2.09 | 9.07 ± 3.42 | 0.36 |
| Fast Response (s) | 9.68 ± 2.42 | 8.76 ± 2.32 | −0.92 | |
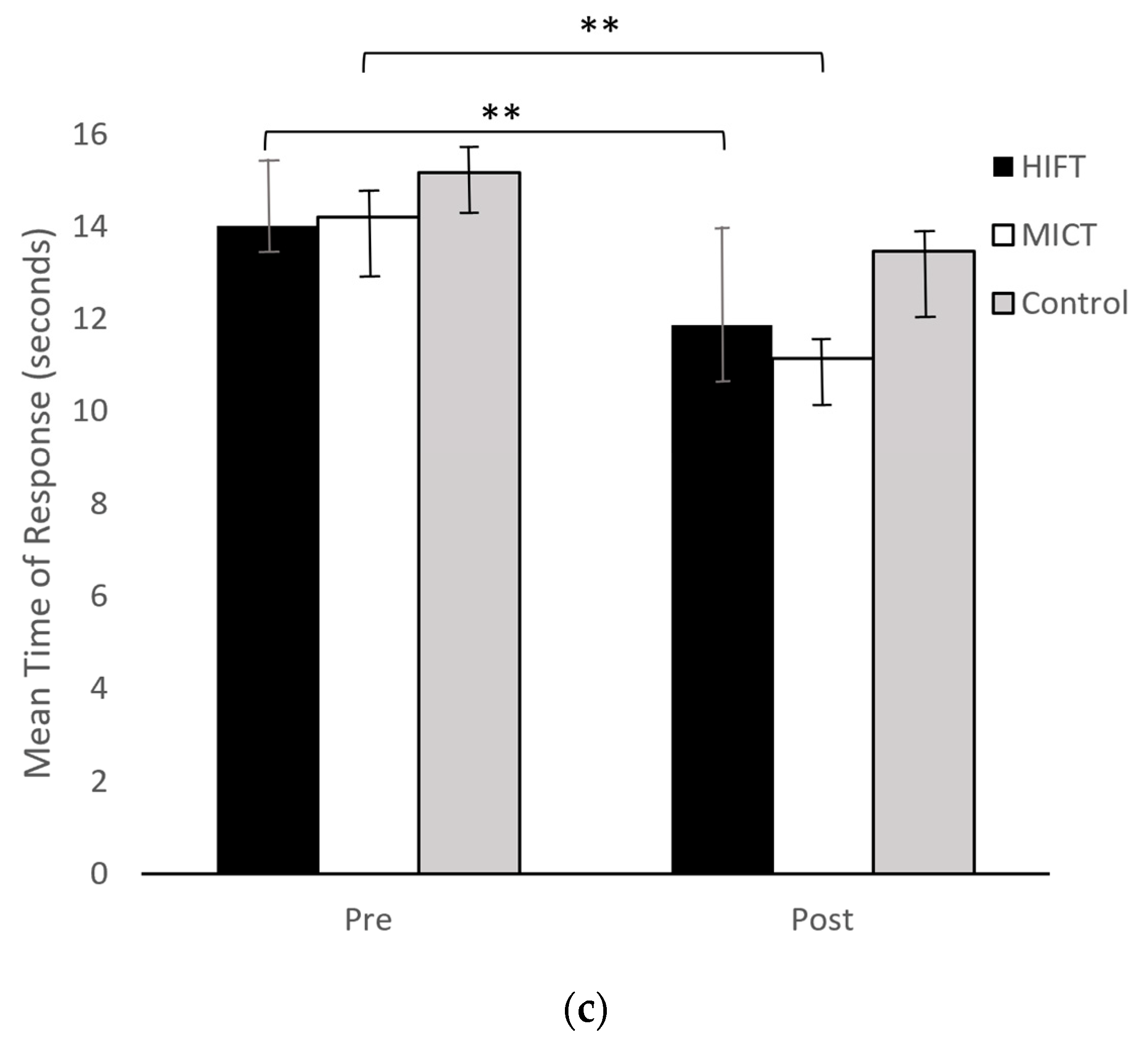
| Medium Response (s) | 15.16 ± 3.77 | 13.47 ± 4.30 | −1.69 | |
| Word Recall | % corrects | 67.85 ± 12.51 | 67.14 ± 15.40 | −0.71 |
| N-Back | 1-Back (% corrects) | 91.9 ± 11.14 | 93.32 ± 8.67 | 1.42 |
| 2-Back (% corrects) | 80.47 ± 15.11 | 82.27 ± 16.40 | 1.8 | |
| 3-Back (% corrects) | 76.17 ± 16.88 | 75.20 ± 15.58 | −0.97 | |
| Variable | Pre-Test (m ± sd) | Post-Test (m ± sd) | Difference | |
|---|---|---|---|---|
| Stroop | N° Corrects | 8.42 ± 2.58 | 9.96 ± 2.83 | 1.54 * |
| Fast Response (s) | 9.62 ± 2.17 | 7.83 ± 1.76 | −1.79 ** | |
| Medium Response (s) | 14.20 ± 3.08 | 11.13 ± 2.03 | −3.07 ** | |
| Word Recall | % corrects | 67.14 ± 13.29 | 66.78 ± 13.34 | −0.36 |
| N-Back | 1-Back (% corrects) | 93.56 ± 10.65 | 93.08 ± 8.41 | −0.48 |
| 2-Back (% corrects) | 82.64 ± 12.30 | 88.09 ± 9.47 | 5.45 * | |
| 3-Back (% corrects) | 72.17 ± 15.40 | 75.00 ± 14.47 | 2.83 | |
| Variable | Pre-Test (m ± sd) | Post-Test (m ± sd) | Difference | |
|---|---|---|---|---|
| Stroop | N° Corrects | 8.62 ± 2.35 | 9.70 ± 2.38 | 1.08 * |
| Fast Response (s) | 8.87 ± 1.70 | 7.73 ± 1.57 | −1.14 ** | |
| Medium Response (s) | 14.02 ± 3.48 | 11.86 ± 2.77 | −2.16 ** | |
| Word Recall | % corrects | 64.07 ± 13.08 | 68.51 ± 10.99 | 4.4 |
| N-Back | 1-Back (% corrects) | 91.35 ± 11.37 | 90.87 ± 12.68 | −0.48 |
| 2-Back (% corrects) | 77.80 ± 13.18 | 80.48 ± 14.89 | 2.68 | |
| 3-Back (% corrects) | 72.10 ± 13.29 | 71.60 ± 13.78 | −0.50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Diego-Moreno, M.; Álvarez-Salvago, F.; Martínez-Amat, A.; Boquete-Pumar, C.; Orihuela-Espejo, A.; Aibar-Almazán, A.; Jiménez-García, J.D. Acute Effects of High-Intensity Functional Training and Moderate-Intensity Continuous Training on Cognitive Functions in Young Adults. Int. J. Environ. Res. Public Health 2022, 19, 10608. https://doi.org/10.3390/ijerph191710608
de Diego-Moreno M, Álvarez-Salvago F, Martínez-Amat A, Boquete-Pumar C, Orihuela-Espejo A, Aibar-Almazán A, Jiménez-García JD. Acute Effects of High-Intensity Functional Training and Moderate-Intensity Continuous Training on Cognitive Functions in Young Adults. International Journal of Environmental Research and Public Health. 2022; 19(17):10608. https://doi.org/10.3390/ijerph191710608
Chicago/Turabian Stylede Diego-Moreno, Manuel, Francisco Álvarez-Salvago, Antonio Martínez-Amat, Carmen Boquete-Pumar, Antonio Orihuela-Espejo, Agustín Aibar-Almazán, and José Daniel Jiménez-García. 2022. "Acute Effects of High-Intensity Functional Training and Moderate-Intensity Continuous Training on Cognitive Functions in Young Adults" International Journal of Environmental Research and Public Health 19, no. 17: 10608. https://doi.org/10.3390/ijerph191710608
APA Stylede Diego-Moreno, M., Álvarez-Salvago, F., Martínez-Amat, A., Boquete-Pumar, C., Orihuela-Espejo, A., Aibar-Almazán, A., & Jiménez-García, J. D. (2022). Acute Effects of High-Intensity Functional Training and Moderate-Intensity Continuous Training on Cognitive Functions in Young Adults. International Journal of Environmental Research and Public Health, 19(17), 10608. https://doi.org/10.3390/ijerph191710608










